Cognitive impairment after intensive care unit (ICU) admission is becoming increasingly recognized. High-dose deep sedation has been suggested to play an important role in the development of cognitive impairment. However, the impact of heavy sedation as a single cause in the development of cognitive impairment in ICU patients remains unclear. In this study we investigated whether a three-day deep sedation protocol could reduce cognitive function in mechanically ventilated non-critical patients.
DesignA prospective observational study was carried out.
PatientsA total of 17 surgical patients were studied.
InterventionNone.
Variables of interestCognitive function before and after ICU admission.
ResultsThirty-one patients requiring three days of sedation after microvascular reconstruction were initially enrolled in the study. Sedation in the ICU was maintained with propofol and dexmedetomidine combined with fentanyl. Cognitive function was assessed using a battery of 6 neuropsychological tests two days before surgery and three weeks after surgery. Finally, a total of 17 patients were included in the analysis. Cognitive impairment (defined as a decline of >20% from the pre-admission cognitive evaluation scores in at least two of 6 tests) was observed in 5 of the 17 patients (29%). However, there were no significant differences between the pre- and post-admission cognitive evaluations in 6 tests.
ConclusionsMiddle-term cognitive function can be impaired in some patients subjected to deep sedation during several days following maxillary–mandibular oral surgery with microvascular reconstruction.
Cada vez existe un mayor consenso sobre la afectación cognitiva tras el ingreso en la unidad de cuidados intensivos (UCI). Se ha sugerido que la sedación profunda con dosis elevada desempeña un papel importante en el desarrollo de la alteración cognitiva. Sin embargo, todavía existen dudas sobre el impacto de este tipo de sedación como causa única del desarrollo de alteraciones cognitivas en pacientes ingresados en la UCI. En este estudio, investigamos si la aplicación de un protocolo de sedación profunda durante 3 días disminuía la función cognitiva en pacientes no críticos bajo ventilación mecánica.
DiseñoSe llevó a cabo un estudio observacional prospectivo.
PacientesSe estudió a un total de 17 pacientes quirúrgicos.
IntervencionesNinguna.
Variables de interésFunción cognitiva antes y después del ingreso en la UCI.
ResultadosEn este estudio se incluyó inicialmente a 31 pacientes que requerían 3 días de sedación tras una reconstrucción microvascular. Se mantuvo la sedación en la UCI con propofol y dexmedetomidina en combinación con fentanilo. Se evaluó la función cognitiva mediante un grupo de 6 pruebas neurofisiológicas antes de la intervención y 3 días después de esta. Por último, se incluyó a un total de 17 pacientes en el análisis. Se observó alteración cognitiva (definida como una reducción>20% frente a las puntuaciones de la evaluación cognitiva previa al ingreso en al menos 2 de las 6 pruebas) en 5 de los 17 pacientes (29%). Sin embargo, no se observaron diferencias significativas entre las evaluaciones previas y posteriores al ingreso en 6 pruebas.
ConclusionesLa función cognitiva a medio plazo puede verse afectada en algunos pacientes sometidos a sedación profunda durante varios días tras una cirugía oral maxilar-mandibular con reconstrucción microvascular.
Cognitive impairment after intensive care unit (ICU) admission has been recognized as one of symptoms of post-intensive care syndrome (PICS).1 High-dose deep sedation has been suggested to play an important role of development of cognitive impairment.2,3 It was also suggested that strategies for reducing sedation levels in the ICU do not seem to be associated with worse cognitive and psychological status among ICU survivors.4 However, multiple factors should contribute to its development. It has been suggested that critical illness per se may develop brain damage by complicated status and situations such as increased level of cytokines in the brain, hypoxemia, hypotension, hyper- and hypo-glycemia, and sedatives, etc.5 Thus, the impact of heavy sedation as a single cause on development of cognitive impairment in ICU patients remains unclear.
Despite the evidence suggesting beneficial effects of lighter sedation strategy, many ICUs still continue to use high dose sedation practices for managing patient on mechanical ventilation.6,7 One of the reasons of such practices is because postoperative management for specific surgeries, especially reconstruction with microvascular anastomosis, conventionally immobilization with deep sedation is considered necessary for a certain period to avoid possible mechanical strain to the transplanted tissues although there is still no evidence available to support this practice.8,9 It is supposed that these patients are the population whose cognitive function can be affected by deep sedation itself rather than co-existing critical illness and ICU admission.
In this study, we investigated whether 3 days deep sedation protocol could decline cognitive function in mechanically ventilated non-critical patients, who underwent surgery for oral and maxillofacial cancer and microvascular reconstruction.
Patients and methodsThis observational cohort study was approved (August 19, 2013) by the Ethics Committee of Nara Medical University Hospital (Kashihara, Japan; study number 715) and the revision was approved on August 31, 2016. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000019914) on August 30, 2013 and the revision was registered on August 9, 2016. Written informed consent was obtained from all individual participants included in this study. The reason why the revision was required is because participant's eligibility was changed midway through. We initially recruited participants from both Departments of Otorhinolaryngology, which required 2 days sedation, and Oral and Maxillofacial Surgery, which required 3 days sedation and intended to compare the two groups. However, we quit recruiting participants from Department of Otorhinolaryngology because otolaryngologists modified and have not yet determined their postoperative care for this kind of surgery. Consequently, all patients from Departments of Otorhinolaryngology that had participated until then were excluded from the study.
Patients were eligible for inclusion in the study if they were scheduled for elective oral and maxillofacial surgery and were 40–85 yr of age, which was changed to 50–85 yr of age in the revised protocol. Additional inclusion criteria were American Society of Anesthesiologists physical status I–III, fluency in Japanese, ability to read, and absence of serious hearing or visual impairments that would preclude neuropsychological testing. The following exclusion criteria were applied: a history of neurological or mental illness, baseline Mini Mental State Examination (MMSE) score below 24.
Complete medical history, physical examination findings, electrocardiograms, chest X-ray, blood examination, and pulmonary function test were performed for all patients. All the operations and postoperative managements were performed by the same surgical and intensive care team. Cognitive function was assessed two days preoperatively and three weeks postoperatively using a battery of seven neuropsychological tests: the MMSE, Trail Making Test (Parts A and B), Digit Span (forward and backward), and Grooved Pegboard Test (dominant).10,11 Regarding Grooved Pegboard Test, significant impairment in the non-dominant hand test was predicted because the radial forearm flap was usually harvested from the non-dominant arm, which may impair a smooth movement of the hand. Therefore, non-dominant hand Grooved Pegboard Test was not included. As described previously, cognitive impairment was defined as a decline of 20% from preoperative test scores in at least two of these tests.12 Cognitive function was assessed by the same intensivists. All cognitive tests were performed in the same hospital room.
Methods of anesthetic induction and maintenance and tracheal intubation were not standardized for each patient. Usually, general anesthesia was induced with intravenous propofol (1–2.5mgkg−1) and fentanyl (1–2μgkg−1) or remifentanil (0.2–0.3μgkg−1min−1). Tracheal intubation was facilitated using rocuronium (0.6–0.9mgkg−1) with laryngoscopy. Anesthesia was maintained with sevoflurane (1–1.5%) and 40% oxygen and air mixture. Fentanyl (1–2μgkg−1h−1) or remifentanil (0.1–0.2μgkg−1min−1) were used for analgesia. Rocuronium (0.2–0.3mgkg−1h−1) was used for muscle relaxation. Fluid management was also at the discretion of the attending anesthetist. Usually, 500–1000ml of colloid (6% hydroxyethyl starch) was infused in the first 1h followed by 2–4mlkg−1h−1 of crystalloid. Transfusion was performed if necessary, in which hemoglobin level was maintained between 7 and 10g/gL. Tracheostomy was performed prior to tumor resection. Most patients had unilateral or bilateral neck dissections and excision of the primary tumor with immediate reconstruction. The radial forearm flap was usually harvested usually from the non-dominant arm. After completion of microvascular anastomosis, continuous administration of prostaglandin E1 (10ngkg−1min−1) was started through a central venous line.
After surgery, patients were transferred to the ICU and remained sedated under mechanical ventilation with the head maintained in a neutral position and elevated 30–40 degree. Patients were sedated with propofol (2–3mgkg−1h−1), fentanyl (0.2–0.3μgkg−1h−1), and dexmedetomidine (0.4–0.7μgkg−1h−1 to maintain deep sedation (Richmond Agitation-Sedation Scale [RASS]-4 or -5).13 We combined different agents to maximize efficacy and minimize adverse effects, with expecting that these combinations have significant benefits over single agents in spite of the absence of strong evidence. Basically, a protocol-based sedation in consideration of pre-emptive analgesia was provided by trained nurses. Dobtamine was used to keep systolic blood pressure>110mmHg. Patients received hydrocortisone 100mg on anesthetic induction and several further doses at 12h intervals. The reconstructive surgeons monitored the color of transferred flaps every 2h and cleaned the oral cavity every 6h until the morning of postoperative day 3. When the target sedation level was not maintained, midazolam (5mg) was temporally administered. On the morning of postoperative day 3, sedative administration was terminated but fentanyl was still administered for postoperative pain management although the dose was decreased (0.04–0.06μgkg−1h−1). Patients were weaned from mechanical ventilation after patients regained consciousness and discharged from the ICU.
StatisticsCognitive impairment after ICU sedation has been reported to vary approximately between 20 and 60%.2,3 For the purpose of a sample-size calculation, we assumed the incidence of cognitive impairment as high as 25% and its 95% confident interval as wide as 50%. The reason of its wide setting of the confident interval was because there was a possibility of no incidence of cognitive impairment. We estimated that 12 patients were required to satisfy this assumption. Considering misestimation of the baseline confident interval, we added 5 participants. Consequently, 17 participants were recruited.
Depending on the data distribution, results are presented as mean (standard deviation [SD]) or median [interquartile range]. Comparisons of change between pre- and post-evaluation were made using the paired t-test or the Wilcoxon test. A correlation between the number of declined cognitive test and age was assessed using Spearman's rank correlation test. A p-value of <0.05 was considered statistically significant for all analyses. Analyses were computed using R (version 3.0.3, R Foundation for Statistical Computing, Vienna, Austria).
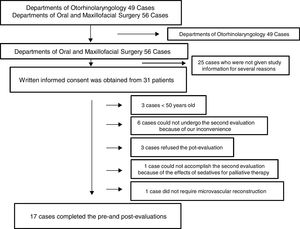
ResultsOne hundred and five consecutive patients (>20 years old) who underwent surgery for oral and maxillofacial cancer and microvascular reconstruction under general anesthesia between September 2013 and August 2016 at Nara Medical University Hospital were initially enrolled. Of those, several patients from Departments of Otorhinolaryngology participated this study; however, 49 patients in all were finally excluded from the study because of the above reason. Of 56 patients from Departments of Oral and Maxillofacial Surgery, written informed consent was obtained from 31 patients; however, 3 patients refused the second evaluation, 6 could not undergo the second evaluation because of our inconvenience, 1 could not accomplish the second evaluation because of the effects of sedatives for palliative therapy, 1 was excluded because microvascular reconstruction was not necessary, and 3 was excluded because they were younger than 50 years old although they accomplished the pre- and post-evaluations. Eventually, 17 patients completed both cognitive evaluations and were included in this study (Fig. 1).
Patient characteristics, duration of sedation, and doses of analgosedatives are shown in Table 1. One patient required a revision of microvascular anastomosis and additional 3 days sedation. All the remaining patients had uneventful intraoperative and postoperative courses. The pre-cognitive evaluation was performed on preoperative day 2 (range 1–2). The post-cognitive evaluation was conducted on postoperative day 23 (range 21–30).
Patient characteristics, intraoperative data, duration of sedation, and doses of analgosedatives.
| Overall population (n=17) | Cognitive impairment (n=5) | No cognitive impairment (n=12) | |
|---|---|---|---|
| Age (yr) | 62±9 | 55±2 | 64±10 |
| Sex (F/M) | 5/12 | 0/5 | 5/7 |
| Hight (cm) | 163.3±10.1 | 171±2.7 | 160±10.4 |
| Weight (kg) | 58.3±13.2 | 66.8±7.6 | 54.8±13.7 |
| BMI (kg/m2) | 21.7±3.3 | 22.9±2.7 | 21.2±3.5 |
| Duration of anesthesia (min) | 929 [808–1022] | 929 [911–941] | 932 [770–1022] |
| Duration of surgery (min) | 786 [665–893] | 786 [759–848] | 782 [645–895] |
| Intraoperative fentanyl (μg) | 1406±492 | 1310±261 | 1446±567 |
| Duration of sedation in ICU (h) | 55.5 [54–59] | 54 [53.5–55] | 56.5 [54.4–63.3] |
| Duration of mechanical ventilation in ICU (h) | 56.5 [55.9–60.4] | 56.5 [56–56.5] | 58 [55.9–65.6] |
| Fentanyl for sedation (μg) | 3449±961 | 3037±169 | 3620±1106 |
| Propofol for sedation (mg) | 6450±2247 | 7950±1444 | 5825±2266 |
| Dexmedetomidine for sedation (μg) | 2003±652 | 2148±620 | 1943±682 |
| Midazolam for sedation (mg) | 10 [5–20] | 15 [10–30] | 10 [5–16.3] |
Data are presented as median [interquartile range] or mean (standard deviation) as indicated.
BMI=body mass index
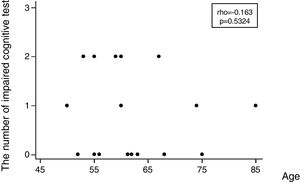
Regarding the incidence of cognitive impairment, 5 of 17 patients (29%) showed the defined cognitive impairment (Table 2A). In this connection, the patient, who required additional sedation, showed no decline in any tests. Patients data with dividing into patients who developed and no developed cognitive impairment are shown on the right side of Table 1. In regard with performing statistical tests, it was likely that our population was too small to satisfy any statistical power for comparing patients who developed and no developed cognitive impairment. Consequently, statistical tests were not conducted. Table 2B shows the number of patients showing a decline of each cognitive test. Except MMSE test, several patients showed a decline of each test. Regarding the association between the number of impaired cognitive test and patient's age, a significant correlation was not observed (rho=−0.163, 95% CI=−0.597 to 0.345, p=0.5324) (Fig. 2).
The number of patients showing a decline of 20% from pre-cognitive evaluation scores.
| Number of tests | Number of patients |
|---|---|
| 0 test | N=8 |
| 1 test | N=4 |
| 2 tests | N=5 |
In this study, cognitive impairment was defined as a decline of 20% from pre-cognitive evaluation scores in at least two of seven tests.
The number of patients showing a decline of each cognitive test.
| Name of the test | Number of patients showing a decline |
|---|---|
| Mini Mental State Examination | N=0 |
| Trail Making Test Part A | N=4 |
| Trail Making Test Part B | N=3 |
| Digit Span Forward Test | N=1 |
| Digit Span Backward Test | N=3 |
| Dominant hand Pegboard Test | N=2 |
The median scores on all six cognitive tests are shown in Table 3. No significant difference between pre- and post-cognitive evaluations was observed in any tests.
Cognitive test results at baseline and three weeks postoperatively.
| Baseline | 3 weeks after surgery | p value | |
|---|---|---|---|
| MMSE | 29 [28–30] | 30 [29–30] | 0.4258 |
| TMT A (s) | 39 [29–52] | 42 [33–56] | 0.5171 |
| TMT B (s) | 102 [87–118] | 89 [70–120] | 0.7119 |
| Digit Span Forward | 7 [6–7] | 7 [6–7] | 0.8203 |
| Digit Span Backward | 5 [4–5] | 4 [4–6] | 0.8984 |
| Dominant hand Pegboard (s) | 73 [63–81] | 77 [62–89] | 0.2069 |
Data are presented as median [interquartile range].
MMSE=Mini Mental State Examination; TMT A=Trail Making Test Part A; TMT B=Trail Making Test Part B.
On the whole, scores in any cognitive tests did not significantly differ after discharge from the ICU compared with before admission. Looking at each patient result, however, the rate of patients showed a decline of >20% from pre-cognitive evaluation scores in at least two of six tests was 29% of all the patients. These results indicate that deep sedation during several days even under non-critical status can impair the medium term cognitive function in some patients.
The strength of this study is to conduct and compare neurophysiological test performance before ICU admission and after discharge. A major limitation of the studies investigating the effect of ICU admission on cognitive outcome is that a baseline assessment of cognitive status before admission is lacking.5 We used multiple cognitive tests probing different domains, including MMSE (screening), Trail Making Test Part A (attention), Trail Making Test Part B (executive function), Digit Span Test (verbal working memory), and Grooved Pegboard Test (motor function). According to a consensus statement, the Rey Auditory Verbal Learning Test, Trail Making Test A/B, and Grooved Pegboard Test are recommended as core components of a comprehensive neuropsychological battery.10 In our preliminary studies, we included the Rey Auditory Verbal Learning Test; however, it was excluded from the main trial as it took a long time to complete and was stressful for some postoperative patients. In current results, MMSE as a dementia screening test was intact. However, it has been suggested that screening tests such as the MMSE do not have the sensitivity to examine for Postoperative cognitive dysfunction (POCD).14 On the contrary, cognitive function was broadly impaired such as domains of attention, executive function, verbal working memory, and motor function. It has been suggested that even minor mood changes may have a negative impact on motivation and ability to complete neuropsychological testing.15 If important changes in mood or anxiety occur after surgery, it is possible that the performance in the neuropsychological tests was adversely affected. However, the fact that almost 30% of patient showed cognitive impairment demands close attention even if the above concern is considered. In this study, non-dominant hand Grooved Pegboard Test was not included for the reason mentioned before. However, more patients might have shown cognitive impairment if this test had been included if the flap had been harvested from the other part of the body, because taking one more test would increase opportunities to show a decline of more than 20% from preoperative test scores.
Although several mechanisms for cognitive impairment after ICU admission have been postulated as mentioned before,5 the etiology of this type of cognitive impairment is still unclear. The results of most studies reviewed suggested that critical illness and ICU treatment are associated with cognitive impairment.5 Of those, high-dose deep sedation has been suggested to play an important role of development of cognitive impairment2,3; however, it is still unclear deep sedation status with critical illness or deep sedation status by exposure to analgosedatives plays a main role. Several pre-clinical studies have shown that anesthetic or sedative agents have toxic effects on the central nerve system.16–18 In addition, considering that our participants were in relatively non-critical status, it is likely that exposure to analgosedatives and immobilization adversely, at least partially, affected cognitive outcome in our population. According to the report of the Second International Perioperative Neurotoxicity Workshop, there is still considerable controversy over whether specific anesthetic agents increase the risk of cognitive impairment.19,20 Besides, sedation was provided by a protocol-based sedation using co-administration of three analgosedatives. Therefore, it is difficult to determine which specific analgosedative agent did affect the cognitive outcome. On the other hand, a combination of sedatives caused a greater increase in the rate of neuronal damage than either drug alone.21 A sedation protocol based on a single analgosedative agent might have had less impact on cognitive outcome. Looking back on the backgrounds of patients who developed cognitive impairment, the doses of propofol and midazolam used seems larger although the dose of dexmedetomidine seems similar compared with patients who did not develop cognitive impairment. It is concluded that benzodiazepine administration is an important and potentially modifiable risk factor for transitioning into delirium.2 Additionally, some, but not all, clinical studies demonstrated that propofol led to delirium.2 Considering that increasing duration of delirium was an independent predictor of worse cognitive impairment,5 these drags might have contributed to development of cognitive impairment in our patients. As for dexmedetomidine, this drug has been reported to have ability to attenuate propofol-induced neurotoxicity.22 Different doses of dexmedetomidine might have produced a different result. However, these comments may be too speculative.
POCD is common following non-cardiac surgery. In a prospective multicenter trial of as many as a thousand patients undergoing non-cardiac surgery, POCD was present in 26% of patients one week postoperatively and in 10% of patients three months postoperatively.23 Therefore, our results might have been an identical phenomenon of POCD. To distinguish whether our findings were derived from POCD or cognitive impairment by high dose deep sedation in the ICU, we should have needed same surgical patients who had not required sedation and immobilization in the ICU. However, it has been reported that cognitive impairment after ICU discharge is correlated with duration of mechanical ventilation and psychophysiological disorder is positively associated with more days of sedation in the ICU.24,25 Therefore, it is possible that the additional days of sedation under mechanical ventilation in the ICU contributed to cognitive impairment after ICU discharge in our patients. Regarding the long-term cognitive outcome like 3 months or 1 year later, we should have evaluated it; however, it is regrettable that we did not for personal reasons.
There are several limitations to this study. First, our study was a single arm trial missing the control group, which means patients who underwent surgery without additional days of sedation under mechanical ventilation. If we had set a control group, we might have made a more appropriate judgment on the effect of additional days of sedation on cognitive outcome. However, it would have required to change the postoperative management protocol at the commencement of the study. As another limitation, age was not associated with cognitive impairment after ICU discharge in our study in spite of several previous suggestions. Our study population consisted of patients over 50 years old with the mean age of 62. Relatively older age in our population might have masked the effect of age on cognitive outcome. Otherwise, for patients over a certain age, the effect of high dose deep sedation on cognitive outcome may depend on patient's susceptibility rather than age. Lastly, the considerable number of patients were excluded from the study. In addition, it has been suggested that plausible assumptions regarding outcomes of patients lost to follow-up could change the interpretation of results of relevant randomized controlled trials.26 Especially, patients who refused the second evaluation might have showed cognitive impairment because such patients may have wished to conceal their cognitive impairment.15 Therefore, more percentage of participants might have showed cognitive impairment after discharge from ICU.
In conclusion, the medium-term cognitive function was impaired in some patients, who underwent deep sedation during several days continuing from maxillomandibular oral surgery with microvascular reconstruction. However, it is still unclear whether this cognitive impairment was due to an epiphenomenon of POCD or additional several days sedation under mechanical ventilation in the ICU.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionDr. Terada helped to design the study and prepared the manuscript.
Dr. Konda helped to design the study and prepared the manuscript.
Dr. Inoue designed the study and prepared the manuscript.
Dr. Egawa helped to design the study and prepared the manuscript.
Dr. Ueda helped to design the study and prepared the manuscript.
Dr. Kirita helped to design the study and prepared the manuscript.
Dr. Kawaguchi helped to design the study and reviewed the manuscript.
All authors approved the final manuscript.
The authors declare that they have no conflict of interest.
None.