To describe the indications, diagnostic performance and safety of fiberoptic bronchoscopy (FOB) performed in a respiratory intensive care unit (RICU).
DesignA prospective, observational study was carried out.
SettingA 6-bed RICU in a tertiary university hospital.
PatientsPatients admitted to RICU who required FOB.
InterventionsNone.
Main measurementsFOB indications and complications, endoscopic procedures, time required to perform FOB.
ResultsSixty-nine out (23%) of the 297 patients admitted to the RICU underwent a total of 107 FOB. Sixty-eight percent of FOB were performed in patients on mechanical ventilation. FOB was performed for diagnostic and therapeutic purposes in 88 (82%) and 19 cases (18%), respectively. The study of pulmonary infiltrates was the main indication for diagnostic FOB (44 cases; 50%), particularly in immunocompromised patients (24 cases; 27%). In immunocompromised patients the diagnostic performance of FOB was significantly higher than in immunocompetent subjects (48% vs 30%; p<0.01). No major complications were recorded. Only a significant drop in PaO2/FiO2 ratio was observed (182±74 vs 163±79; p<0.005) in patients undergoing bronchoalveolar lavage (BAL). Overall mortality in patients in the RICU was 14%. In patients requiring a single FOB procedure, mortality was 25% vs 45% among those requiring more than one FOB procedure.
ConclusionsThese results show that FOB is used commonly in the RICU. It is a safe and fast procedure that contributes significantly to clinical management. Patients requiring additional FOB during admission to the RICU show high mortality.
Describir las indicaciones, rentabilidad diagnóstica y complicaciones de la fibrobroncoscopia (FBS) en una unidad de vigilancia intensiva respiratoria (UVIR).
DiseñoEstudio prospectivo observacional.
ÁmbitoUVIR de 6 camas en un hospital universitario de tercer nivel.
PacientesPacientes admitidos en una UVIR a los que se les realizó una FBS.
IntervencionesNinguna.
Variables de interésIndicaciones y complicaciones de la FBS, técnicas endoscópicas realizadas y tiempo empleado en la FBS.
ResultadosSe realizaron 107 (23%) FBS a 69 de los 297 pacientes admitidos en la UVIR. El 68% de las FBS se practicaron a pacientes con ventilación mecánica. La FBS se realizó con fines diagnósticos en 88 ocasiones (82%) y terapéuticos en 19 (18%). La indicación más frecuente para la FBS diagnóstica fue el estudio de infiltrados pulmonares (44 casos; 50%), particularmente en pacientes inmunodeprimidos (24 casos; 27%). Para esta indicación, la rentabilidad diagnóstica de la FBS fue significativamente mejor en los pacientes inmunodeprimidos, respecto a los inmunocompetentes (48% vs 30%; p<0,01). La FBS no causó complicaciones mayores; únicamente se observó un descenso significativo en la PaO2/FiO2 (182±74 vs 163±79; p<0,005) cuando se realizó un lavado broncoalveolar. La mortalidad global en la UVIR fue del 14%; del 25% en los pacientes que precisaron FBS y del 45% en aquellos que precisaron FBS adicionales.
ConclusionesLa FBS es un procedimiento seguro y rápido que se utiliza con frecuencia en la UVIR y que contribuye significativamente al manejo clínico. Los pacientes de la UVIR que requieren FBS adicionales tienen una elevada mortalidad.
FBS is commonly used in intensive care units (ICUs) for diagnostic and/or therapeutic purposes. To date, however, the available information on the indications, safety, diagnostic performance and influence of FBS upon the patient clinical course and prognosis is limited and is based on old series.1,2 In recent years, a series of improvements in the management of critical patients, with optimization of the ventilation modes and sedation techniques, have made it possible to significantly expand the criteria for admission to the ICU. Thus, it is now common to admit oncological and immune depressed individuals who until only a few years ago would not have been moved to the ICU,3,4 and who often suffer pulmonary complications with the need for FBS in order to establish the diagnosis.
Although FBS may be considered safe, a number of studies have shown that insertion of the fiberoptic bronchoscope in the orotracheal tube can alter ventilation parameters and cause changes in hemodynamic parameters and gas exchange.5–7 Although BAL is generally well tolerated, in intubated patients a worsening of arterial oxygenation is often seen.8 On the other hand, the indications for FBS and the incidence and type of possible adverse effects can vary depending on the type of patient population involved.9 In this sense, in principle, the high prevalence of patients with lung disease admitted to RICUs should imply increased risk and a greater rate of complications. To date, no studies have described the use of FBS in the RICU.
A prospective observational study has been made of the indications, safety, diagnostic performance and influence of FBS upon the patient clinical course in a 6-bed RICU belonging to a third level hospital, with inclusion of all the bronchoscopic procedures carried out in the Unit over a one-year period.
MethodsA prospective observational study was designed, including all the patients subjected to FBS in the RICU between June 2008 and June 2009. The indication of FBS was established by the supervising physician in each case. The contraindications of FBS included coagulation alterations not corrected following plasma transfusion, a platelet count of <50×106/μl, severe hypoxemia refractory to oxygen therapy (PaO2<60mmHg with FiO2≥0.8), hemodynamic instability despite the use of vasoactive drugs, acute ischemia, and uncontrolled cardiac arrhythmias. The study was carried out following the diagnostic FBS guidelines.10
Procedure and monitorizationBronchoscopes with an external diameter of 4.9mm were used (FB15-V Pentax Europe GMBH). All patients had a venous catheter and were subjected to continuous monitorization of blood pressure, heart rate and oxyhemoglobin saturation determined by pulsioxymetry, during and until 1h after the end of the procedure. Arterial blood samples were collected and assessed in situ using a blood gas analyzer (Radiometer ABL 30) 5min before and 1h after the procedure. FBS was performed in the RICU and was supervised by one of the senior endoscopists (CA, AX). The endoscopic exploration was carried out under sedation with intravenous propofol (1–2mg/kg) or midazolam (0.1mg/kg)–the use of fentanyl and/or pancuronium proving necessary in some cases. The medication doses were varied on an individualized basis with the purpose of ensuring correct oxygenation and adaptation of the patient to ventilation. The bronchoscope was inserted through the nasopharynx, tracheostomy, orotracheal tube (8mm in diameter), or the laryngeal mask. In the latter three cases we used an adaptor to maintain ventilation with positive pressure. Patients requiring mechanical ventilation were maintained in volume control mode. The ventilator pressure limit was increased to ensure an adequate tidal volume during each respiratory cycle. FiO2 was increased to 100% from 20min before premedication and was maintained during the study period and in the immediate recovery period. Where present, the positive end-expiratory pressure (PEEP) was maintained at 5mmHg during the procedure. In patients with noninvasive ventilation (BiPAP vision, Respironics, Murrysville, PA, USA), bilevel positive airway pressure (BiPAP) was maintained.11
The endoscopic techniques performed included the following: diffuse bronchoaspiration (BAS), BAL (150ml of physiological saline solution divided into 3 aliquots of 50ml each, instilled in the lobe of the pulmonary infiltrate or in the middle lobe or lingula in the case of diffuse infiltration), bronchial biopsies and transbronchial biopsies. The samples obtained during the endoscopic study were individualized in each case. The techniques for microbiological sample processing and interpretation of the results have been described elsewhere.12
Data collection and analysisThe indications of FBS were divided into diagnostic and therapeutic. The impact of FBS upon patient management was evaluated on the basis of the results obtained with the procedure. An endoscopic study was considered positive when it resulted in a definitive diagnosis. We documented both global mortality in the RICU and mortality among the patients subjected to FBS.
Complications of FBS were defined as those events occurring during the procedure and/or 24h after its conclusion. The adverse effects were documented prospectively. In this context we assessed fever, oxyhemoglobin desaturation with a need for increased oxygenation in the 4h after the procedure, the presence of bleeding requiring termination of the study, hypotension with or without the need for volume expansion or the use of vasoactive drugs, the development of hypertensive crises requiring the start of treatment and/or suspension of the procedure, and the evidence of cardiac arrhythmias during and 1h after conclusion of the technique. We also documented the time from the start to the end of the endoscopic exploration, and divided the explorations according to whether they had taken less than 5min, between 5 and 10min, and over 10min.
To the effects of the statistical analysis of the results obtained, quantitative variables were expressed as the mean±standard deviation, while qualitative variables were expressed as percentages and absolute frequencies. Proportions were compared using the chi-squared test. A p-value of <0.05 was regarded as statistically significant. The SPSS statistical package was used (Microsoft; Redmond, WA, USA).
ResultsPatient characteristicsDuring the inclusion period, a total of 297 patients were admitted to the RICU, and 107 FBS procedures were performed in 69 patients (23% of the total subjects admitted to the RICU)–of which 46 were males. The mean patient age was 60±14 years. A total of 47 patients underwent a single FBS procedure, 19 underwent two, one patient underwent three, another underwent 5, and two patients were subjected to 7 procedures each.
Tables 1 and 2 show the indications of admission to the RICU, and the comorbidities of the patients who required FBS.
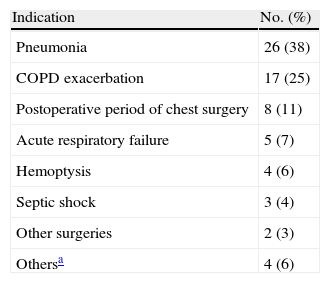
Criteria for admission to the respiratory intensive care unit (RICU) among the 69 patients subjected to fiberoptic bronchoscopy (FBS).
| Indication | No. (%) |
| Pneumonia | 26 (38) |
| COPD exacerbation | 17 (25) |
| Postoperative period of chest surgery | 8 (11) |
| Acute respiratory failure | 5 (7) |
| Hemoptysis | 4 (6) |
| Septic shock | 3 (4) |
| Other surgeries | 2 (3) |
| Othersa | 4 (6) |
Other indications were: pulmonary embolism, empyema, endocarditis and cardiogenic shock.
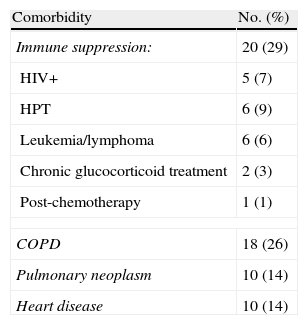
Comorbidities among the patients subjected to fiberoptic bronchoscopy.
| Comorbidity | No. (%) |
| Immune suppression: | 20 (29) |
| HIV+ | 5 (7) |
| HPT | 6 (9) |
| Leukemia/lymphoma | 6 (6) |
| Chronic glucocorticoid treatment | 2 (3) |
| Post-chemotherapy | 1 (1) |
| COPD | 18 (26) |
| Pulmonary neoplasm | 10 (14) |
| Heart disease | 10 (14) |
COPD: chronic obstructive pulmonary disease; HPT: hematopoietic precursor transplantation; HIV: human immunodeficiency virus.
Bronchoscopy was carried out for diagnostic purposes in 88 cases (82%), and with therapeutic intent in 19 cases (18%). The chest X-ray study proved normal in 25 of the 88 cases (28%) in which FBS was carried out for diagnostic purposes, while unilateral and bilateral infiltrates were observed in 24 (27%) and 39 cases (44%), respectively.
The clinical indications of diagnostic FBS are presented in Table 3. All therapeutic FBS procedures were carried out as treatment for secretion retention and atelectasis, with the exception of one patient in which a Fogarty catheter had to be placed in the context of a bronchopleural fistula.
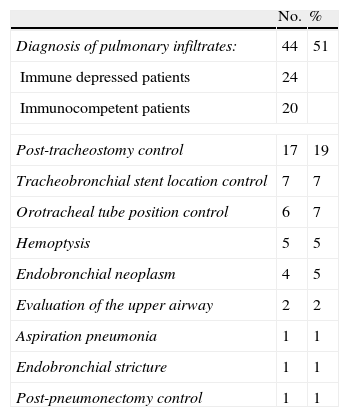
Clinical indications of diagnostic fiberoptic bronchoscopy.
| No. | % | |
| Diagnosis of pulmonary infiltrates: | 44 | 51 |
| Immune depressed patients | 24 | |
| Immunocompetent patients | 20 | |
| Post-tracheostomy control | 17 | 19 |
| Tracheobronchial stent location control | 7 | 7 |
| Orotracheal tube position control | 6 | 7 |
| Hemoptysis | 5 | 5 |
| Endobronchial neoplasm | 4 | 5 |
| Evaluation of the upper airway | 2 | 2 |
| Aspiration pneumonia | 1 | 1 |
| Endobronchial stricture | 1 | 1 |
| Post-pneumonectomy control | 1 | 1 |
Seventy-three (68%) of the 107 FBS procedures were carried out in patients with mechanical ventilation, and 34 (32%) in patients with spontaneous respiration and oxygen therapy. In 6 cases (6%) the procedure was performed in patients subjected to noninvasive ventilation.
FBS was performed by residents in Pneumology (always under supervision by a pneumologist) in 72 cases (67%), by endoscopists in 28 cases (26%), and by pneumologists in 7 cases (6%). In 53% of the FBS techniques the duration was under minutes, while in 31% it lasted between 5 and 10min, and in only 16% of the cases did the procedure take more than 10min.
Diagnostic performanceIn 68 cases (64%) different diagnostic bronchoscopic methods were used. In this context, BAL and BAS were the most common techniques (both performed in 34 cases, 32%). In only 7 cases (7%) were biopsy samples obtained.
In 45 of the 88 diagnostic FBS procedures (51%) the objective was to identify the causal agent in a patient with pneumonia. FBS proved able to yield a specific diagnosis in 19 of the 45 cases (42%). Specifically, 20 FBS procedures were performed in 18 immunocompetent patients (10 in patients with community-acquired pneumonia and 8 in patients with ventilation associated pneumonia), while 25 procedures were carried out in 15 patients with different types of immune suppression (Table 4). In the group of immunocompetent patients, a specific diagnosis was obtained in 6 of the 20 explorations carried out (30%). In contrast, the diagnostic performance of FBS in the immune depressed patients was significantly better – reaching a specific diagnosis in 12 of the 25 explorations performed (48%) (p<0.01) (Tables 4 and 5).
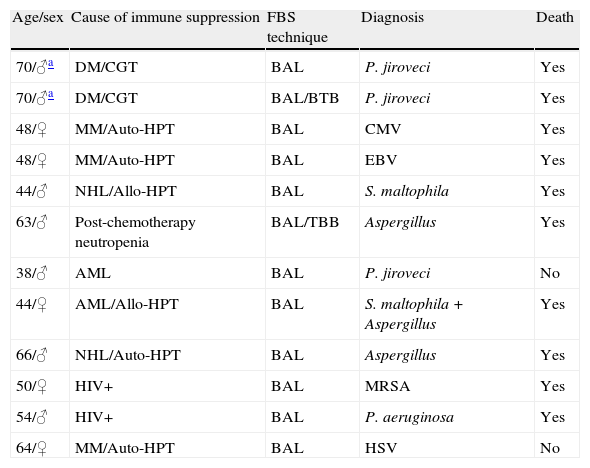
Immune depressed patients with a specific diagnosis (n=12).
| Age/sex | Cause of immune suppression | FBS technique | Diagnosis | Death |
| 70/♂a | DM/CGT | BAL | P. jiroveci | Yes |
| 70/♂a | DM/CGT | BAL/BTB | P. jiroveci | Yes |
| 48/♀ | MM/Auto-HPT | BAL | CMV | Yes |
| 48/♀ | MM/Auto-HPT | BAL | EBV | Yes |
| 44/♂ | NHL/Allo-HPT | BAL | S. maltophila | Yes |
| 63/♂ | Post-chemotherapy neutropenia | BAL/TBB | Aspergillus | Yes |
| 38/♂ | AML | BAL | P. jiroveci | No |
| 44/♀ | AML/Allo-HPT | BAL | S. maltophila+Aspergillus | Yes |
| 66/♂ | NHL/Auto-HPT | BAL | Aspergillus | Yes |
| 50/♀ | HIV+ | BAL | MRSA | Yes |
| 54/♂ | HIV+ | BAL | P. aeruginosa | Yes |
| 64/♀ | MM/Auto-HPT | BAL | HSV | No |
TBB: transbronchial biopsy; CMV: cytomegalovirus; DM: dermatomyositis; AML: acute myeloid leukemia; BAL: bronchoalveolar lavage; NHL: non-Hodgkin lymphoma; MM: multiple myeloma; MRSA: methicillin-resistant S. aureus; CGT: chronic glucocorticoid treatment; HPT: hematopoietic precursor transplantation; EBV: Epstein-Barr virus; HSV: herpes simplex virus; HIV: human immunodeficiency virus.
Two explorations in the same patient.
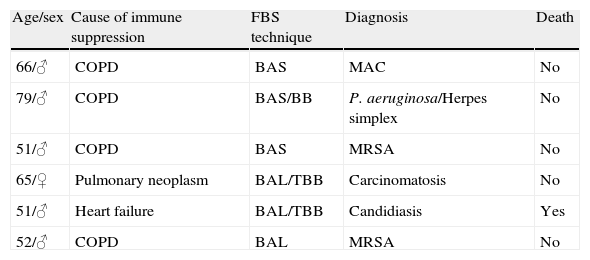
Immunocompetent patients with a specific diagnosis (n=6).
| Age/sex | Cause of immune suppression | FBS technique | Diagnosis | Death |
| 66/♂ | COPD | BAS | MAC | No |
| 79/♂ | COPD | BAS/BB | P. aeruginosa/Herpes simplex | No |
| 51/♂ | COPD | BAS | MRSA | No |
| 65/♀ | Pulmonary neoplasm | BAL/TBB | Carcinomatosis | No |
| 51/♂ | Heart failure | BAL/TBB | Candidiasis | Yes |
| 52/♂ | COPD | BAL | MRSA | No |
BAS: bronchial aspirate; BB: bronchial biopsy; TBB: transbronchial biopsy; COPD: chronic obstructive pulmonary disease; BAL: bronchoalveolar lavage; MAV: M. avium complex; MRSA: methicillin-resistant S. aureus.
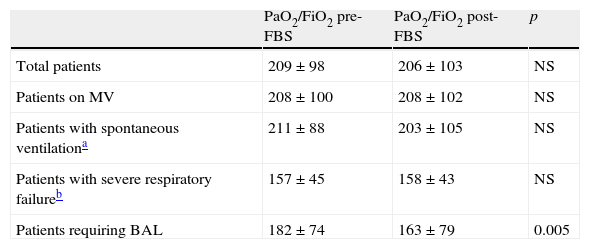
FBS did not trigger the need for mechanical ventilation in any patient under spontaneous respiration or subjected to noninvasive ventilation. There were no differences in PaO2/FiO2 before and after the procedure (Table 6). The changes in PaO2/FiO2 were not significant on dividing the patients into those subjected to mechanical ventilation and those with spontaneous respiration, or into those with unilateral or bilateral pulmonary infiltrates. In contrast, in those patients subjected to BAL, a significant decrease in PaO2/FiO2 was observed after the procedure (182±74 vs 163±79; p<0.005) in both the immune depressed patients (n=20) (203±79 vs 182±86; p<0.02) and in the immunocompetent individuals (n=14) (140±39 vs 125±42; p<0.02).
Pre- and post-FBS arterial blood gases in the studied population (n=69).
| PaO2/FiO2 pre-FBS | PaO2/FiO2 post-FBS | p | |
| Total patients | 209±98 | 206±103 | NS |
| Patients on MV | 208±100 | 208±102 | NS |
| Patients with spontaneous ventilationa | 211±88 | 203±105 | NS |
| Patients with severe respiratory failureb | 157±45 | 158±43 | NS |
| Patients requiring BAL | 182±74 | 163±79 | 0.005 |
BAL: bronchoalveolar lavage; NS: nonsignificant; MV: mechanical ventilation.
Spontaneous ventilation including patients with noninvasive mechanical ventilation.
Severe respiratory failure: PaO2/FiO2<250 (n=63).
In 13 (12%) of the 107 FBS procedures a transient increase in FiO2 proved necessary (with respect to the FiO2 value before the endoscopic exploration) due to worsening of the oxyhemoglobin values during the procedure. In 10 cases (9%) we recorded reversible cardiorespiratory events (isolated supraventricular extrasystoles and/or variations in blood pressure), without the need for drug treatment. No other significant complications were observed in the hours close to the procedure.
The global mortality rate among the patients admitted to the RICU during the study period was 14% (42 of the 297 admitted patients). In the group of patients who did not require FBS the mortality rate was 11% vs 25% in the group of patients subjected to at least one FBS procedure (17 of the 68 included patients). In turn, the mortality rate was 15% (7 of 46 patients) in those requiring one FBS procedure, and 45% in the patients requiring more than one procedure (10 of 22 patients).
The mortality rate among the immune depressed patients subjected to diagnostic FBS was 60% (9 of 15 patients)–this being significantly higher than the percentage seen in the immunocompetent patients, where the mortality rate was 30% (6 of 20 patients) (p<0.05). In 7 of 9 immune depressed patients who died, the specific diagnosis was obtained by FBS.
DiscussionThe present study shows FBS to be common practice in the RICU; it can be performed quickly in critical patients, without major complications, and offers good diagnostic performance. The need for repeated FBS procedures during admission to the RICU is an indicator of poor patient prognosis.
This is the first prospective observational study of its kind conducted in a specialized RICU characterized by a high prevalence of acute and chronic lung disease. The originality of this study is that it explores the indications of FBS and its performance or yield and potential deleterious effects in this clinical setting. Dunagan et al. found that only 0.5% of the patients admitted to a coronary unit underwent FBS.9 In contrast, in the present study, 23% of the patients required FBS at some point during admission to the RICU. The most frequent indication of FBS in our Unit was the study of pulmonary infiltrates, which curiously affected mainly patients with some underlying immunosuppressive condition (Table 3). In diagnostic terms, FBS proved more effective in the group of immune depressed patients than among the immunocompetent patients (48% vs 30%; p<0.01). Nevertheless, mortality in the former group was comparatively greater, in coincidence with the observations of other series.13–15 Although admission to the ICU of oncological patients with different forms of immune suppression has been questioned for years in view of the important mortality rate involved, recent studies have reported significant improvement in terms of survival. In this sense, Lecuyer et al., in a recent study evaluating 188 cancer patients requiring mechanical ventilation, reported a survival rate of 20%.16 In our study, 6 of 15 immune depressed patients subjected to FBS survived after their stay in the RICU (40% survival rate).
Safety is one of the most important issues when deciding to perform FBS in the ICU. Different studies have shown that the technique may produce adverse effects in the critically ill patient, including hemodynamic, blood gas or respiratory mechanical alterations.5–7 Although these effects may prove significant, in most cases they probably lack clinical relevance. In effect, Olopade and Prakash, after evaluating 1150 FBS procedures performed in the ICU, recorded only 7 transient complications.17 In our study, and considering the common presence of chronic pulmonary disease in the Unit, significant hypoxemia was not observed even in the subgroup of patients with greater respiratory problems (PaO2/FiO2 ratio<250) (Table 6). Likewise, we recorded a low frequency of cardiovascular events, in coincidence with previous observations.9,18 The brief period of time involved in performing the procedure possibly explains the absence of significant adverse effects in our patients.19 Residents in training supervised by a senior physician or experienced endoscopist can quickly perform FBS in a simple manner, particularly when using the technique for diagnostic purposes. In our series, 53% of the explorations were completed in under 5min.
The absence of significant adverse effects commented above contrasts with the experience of other authors. Trouillet et al., in a series of 107 ventilated patients, observed a decrease in PaO2 following FBS in 26% of the cases.20 However, as the authors themselves pointed out, the use of neuromuscular blockers in addition to sedation could have avoided asynchrony of the patient with the ventilator and the consequent significant decreases in PaO2.21
In our study the patients subjected to BAL experienced significant changes in PaO2/FiO2. Different authors, including our own group, have shown that BAL performed in ventilated patients can cause changes in gas exchange even 24h after the end of the procedure.8,22,23 Although this fact must be taken into account, it should be remembered that BAL is the best diagnostic option for the study of pulmonary infiltrates, particularly in immune depressed patients. Very recently, Azoulay et al. have published the first randomized controlled study in immune depressed patients with acute respiratory failure–showing FBS to be a profitable technique when performed soon after admission to the ICU, and without increasing the need for intubation.24 In this sense, the increased mortality recorded among the patients subjected to several bronchoscopy procedures should not be attributed to the technique itself but very probably to the notion that a need for repeated FBS reflects increased seriousness of the pulmonary disease.
A potential limitation of our study is the absence of objective criteria for indicating FBS. Since the technique was indicated according to the criterion of the supervising physician in each case, it cannot be discarded that patient selection may have partially influenced the results obtained. In this sense, performing FBS in the more seriously ill patients could increase the adverse effects. Nevertheless, the indication of FBS was established by pneumologists experienced with the use of the technique, and who followed the established guidelines for diagnosing respiratory failure.12
In conclusion, FBS is frequently used in the RICU, in most cases for diagnostic purposes. It is a rapid and safe technique that makes a considerable contribution to the management of critical patients. Those subjects who during admission to the RICU require additional FBS procedures show higher mortality rates–this probably being an indirect indicator of more serious lung problems.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Lucena CM, et al. Fibrobroncoscopia en una unidad de vigilancia intensiva respiratoria. Med Intensiva. 2012;36:389–95.