High-flow nasal cannula (HFNC) therapy is being increasingly used in adult and paediatric patients. It delivers a heated and humidified mixture of air and oxygen utilising a flow superior than the patient's demand, thus providing a quite stable fraction of inspired oxygen. The mode of action is not fully understood, and some mechanisms suggested include dead space carbon dioxide washout and ‘some’ positive end-expiratory pressure (PEEP).1
Asthmatic exacerbation is a frequent cause of admission in paediatric wards, and also in paediatric intensive care units (PICUs). Non-invasive ventilation (NIV) has been suggested as a useful tool in order to improve patients with severe asthmatic exacerbations, thus avoiding intubation. Although this use is still controversial, many intensive care units worldwide have included NIV as part of the cornerstone treatment in refractory status asthmaticus.2,3 External PEEP or expiratory positive airway pressure, may decrease the dynamic expiratory collapse, reducing the occurrence of air trapping, and therefore, of intrinsic PEEP. This may relieve the uploading of respiratory muscles while maintaining patency of smaller airways. Inspiratory positive airway pressure, ideally delivered as synchronised pressure support, may help inspiratory muscles to counteract airflow limitation and chest wall overstretching, improving tidal volumes.4,5
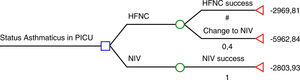
The study by Pilar et al.6 suggests that NIV is far more effective than HFNC in severe asthmatic exacerbations: 22/22 were successfully treated using NIV while 8/20 who were previously being treated with HFNC then had to be treated with NIV, and those 8 patients also avoided tracheal intubation with NIV. According to these results, HFNC seems to be cost-ineffective and therefore, the use of HFNC in severe asthmatic exacerbations would be inacceptable. We have had the opportunity to look at the economic data from Cruces University Hospital in order to analyse cost-effectiveness of HFNC and NIV according to Pilar et al. results.6 The cost of a mean admission per day of an asthmatic patient in the PICU is € 1575.85 plus the cost of consumables (we considered the cost per patient of the multiuser total face mask – reused up to 10 times – and that children who fail HFNC will have required both materials; HFNC and NIV). Considering the HFNC rate of failure in the present study (40%), we have elaborated a decision tree using the rolling-back method (Fig. 1). According to this decision tree, if NIV is used as the first option, the expected cost is € 2804, while if HFNC is chosen; the expected cost is € 4167. The cost of each patient treated successfully with HFNC is € 2970 (60% of the patients), while the cost of a patient who had to be treated with NIV after failing on HFNC is € 5963.
Decision-tree analysis based on Pilar et al. study.7
Several paediatric publications have suggested that HFNC oxygenation is a promising tool. Heikkila et al. have recently published an interesting cost-effectiveness paper about the use of HFNC in bronchiolitis, reporting that this therapy was cost-effective mainly due to a reduction in the need for PICU admissions.7 These authors used earlier published retrospective studies in order to know the admission rate to PICU. This is a source of possible bias, as admission criteria may differ significantly among PICUs as well as intubation criteria, suggested by a strikingly high intubation rate of 37% in one of the studies.8
Chisti et al. reported in a randomised controlled trial in children with pneumonia and hypoxaemia a mortality of 4% in children receiving bubble CPAP, 15% in children receiving low-flow oxygen therapy, and 13% in children receiving HFNC.9 The subgroup analysis of this study has shown that these differences in mortality were significant when comparing CPAP and HFNC groups.10
In regards to the use of HFNC after extubation in adult patients, two recent studies by Hernández et al. compared HFNC and conventional oxygen therapy in patients at low risk of reintubation,11 and HFNC and NIV in patients at high risk of reintubation.12 Authors conclude that HFNC is more effective than conventional oxygen therapy11 and non-inferior to NIV in terms of rates of reintubation.12
In the first study, several intubations were prevented with the use of HFNC (NNT=14), although there were no differences regarding length of stay and mortality11; furthermore, the number of patients who would not have needed reintubation with the use of NIV is not known. Performing a decision analysis according to costs, the most adequate decision would be to use standard low-flow oxygen. Considering the NNT (NNT=14; 95%CI: 8–40) and that the cost gap of € 123.88 per patient (€ 125 per HFNC, minus € 1.12 per nasal prongs), we can estimate that the Incremental Cost-Effectiveness Ratio is € 1734.32 (95%CI: 991 to € 4955.2) per avoided intubation, which would imply an economic burden.
The second study by Hernández et al.,12 concludes non inferiority of HFNC compared to NIV. We performed a robust Bayesian analysis, through 10,000 Monte Carlo Markov Chain simulations in a conjugated beta-binomial model, based on the data used by these authors in order to calculate the sample size for their study: NIV reintubation rate ranging from 9 to 32%; noninferiority margin for HFNC group 10%; and baseline intubation rate 20–25%. Bayesian priors used in our analysis are as follows: (a) reference (objective): Jeffrey's prior (Beta [0.5; 0.5]) for both groups; (b) sceptic on HFNC: it considers 9% reintubation rate with NIV (Beta [27; 273]) and 20% reintubation rate with HFNC (Beta [60; 240]); and (c) enthusiastic on HFNC: it considers 32% reintubation rate with NIV (Beta [96; 204]) and 20% reintubation rate with HFNC (Beta [60; 240]). Interpreting the results within the original framework of Freedman et al. discussed in the work by Spiegelhalter et al.,13 we have 3 different scenarios:
- -
Reference scenario: there is a 2.8% probability that NIV is superior, 97.2% probability of equivalence, and 0% probability that HFNC is superior.
- -
Sceptic on HFNC scenario: there is a 9.9% probability that NIV is superior, 90.1% probability of equivalence, and 0% probability that HFNC is superior.
- -
Enthusiastic on HFNC scenario: there is a 0% probability that NIV is superior, 99.4% probability of equivalence, and 0.6% probability that HFNC is superior.
Therefore, in all 3 scenarios, the most probable interval is equivalence, with 99.4% of posterior credibility in the enthusiastic on HFNC scenario, 97.2% in the reference scenario, and 90.1% in the sceptic on HFNC scenario. So, robust Bayesian analysis reveals that only the enthusiastic prior of HFNC may consider that HFNC is superior to NIV (Fig. 2), but with a scarce 0.6% probability.
Indifference ROPE zone [δL, δU] and corresponding conclusions for Hernández et al.12 clinical trial based on the location of the 95% posterior credible difference interval for each scenario.
As a conclusion, considering that most PICUs and adult intensive care units have NIV devices, and that HFNC does not seem to be superior to NIV in many clinical scenarios, the cost of acquiring HFNC devices is not currently justified. Further clinical and cost-effectiveness studies are warranted.






![Indifference ROPE zone [δL, δU] and corresponding conclusions for Hernández et al.12 clinical trial based on the location of the 95% posterior credible difference interval for each scenario.](https://static.elsevier.es/multimedia/21735727/0000004100000007/v1_201709220119/S2173572717301492/v1_201709220119/en/main.assets/thumbnail/gr2.jpeg?xkr=1dZuESKpnCAWr3yCSGZ24A==)


