There is a significant risk of hepatitis B transmission from HBsAg (−), HBcAb (+) donors in liver transplantation, but there is little information about hepatitis B transmission from HBcAb heart donors. The present study examines the influence of HBcAb presence in relation to heart donor acceptance and offers an update of the published studies.
DesignSurvey and medical database update from 1994 to October 2011.
SettingSpanish heart transplantation teams.
PatientsNot applicable.
Study variablesAcceptance of heart transplant from an HBcAb (+) organ donor.
ResultsTwelve out of 15 surveyed teams were seen to vaccinate against HBV, and two quantify HBsAb titers. Seven teams specifically request donor HBcAb status. If the latter proves positive, two do not accept transplantation, two accept if the donor is also HBsAb (+), one selects the receptor under emergency conditions, and three use drug prophylaxis separately or complementary to the above. Only one case of hepatitis B has been reported in HBcAb (−) and HBsAb (−) receptors that did not receive prophylactic measures. There have been reports of seroconversion of the HBcAb and HBsAb markers, though with an uncertain etiology.
ConclusionsHBcAb seropositivity influences acceptance of a heart donor, but agreement is lacking. There is limited information on receptor evolution. To date there has been one reported case of hepatitis B after heart transplant. Although rare, an HBcAb (+) donor can harbor occult HBV infection. The risk of infection can be prevented with appropriate HBsAb titers following vaccination or by pharmacological measures.
Existe alto riesgo de transmitir una infección por virus B con el hígado de donantes AgHBs (−), anti-HBc, sin embargo, este riesgo está poco estudiado en el trasplante cardiaco. Los objetivos son conocer la influencia del anti-HBc (+) en la aceptación del corazón para trasplante y hacer una puesta al día de los trabajos publicados.
DiseñoEncuesta y revisión bibliográfica en bases médicas desde 1994 hasta octubre del 2011.
ÁmbitoEquipos españoles de trasplante cardiaco.
PacientesNo aplicable.
IntervencionesNinguna.
Variables de interésAceptación para trasplante del corazón de donantes anti-Hbc (+).
ResultadosDoce de 15 equipos encuestados vacunan contra el VHB y 2 cuantifican los títulos anti-HBs. Siete solicitan el anti-HBc del donante. En caso de positividad, 2 no aceptan la oferta, 2 aceptan si el donante es también anti-HBs (+), uno selecciona el receptor en situación de urgencia y 3 emplean profilaxis farmacológica de forma aislada o complementaria a las anteriores. Solo se ha publicado un caso de hepatitis B en un receptor anti-HBc (−), anti-HBs (−) que no recibió medidas profilácticas. Hay descritas seroconversiones de los marcadores anti-HBc y anti-HBs de dudosa etiología.
ConclusionesEl anti-HBc (+) del donante influye para aceptar el corazón aunque hay disparidad de criterios. Existe escasa información publicada sobre la evolución de los receptores. Hasta el momento se ha descrito un caso de infección postrasplante. Aunque poco frecuente, un donante anti-HBc (+) puede albergar infección oculta por VHB. El riesgo puede prevenirse con títulos anti-HBs adecuados o con medidas farmacológicas.
In heart transplant patients, hepatitis B tends to take an aggressive course, and probably evolves toward cirrhosis or liver failure.1 For this reason, potential heart donors who prove serologically positive for hepatitis B virus (HBV) surface antigen (i.e., HBsAg (+) individuals) are rejected in view of the high risk of transmitting the infection to the recipient.2 However, there is controversy regarding the potential for transmission through organs from HBsAg (−) donors who are positive for antibodies targeted to the core antigen of the virus (i.e., HBcAb positive individuals). The risk appears to differ according to the organ implanted, being very high in the case of the liver3,4 and low for the rest of organs. According to some authors, the risk of transmission via the heart is comparable to that associated with kidney transplantation and lower than in the case of lung transplants.5
HBcAb positivity with HBsAg (−) status can reflect a number of situations6: (1) it may indicate a false-positive result; (2) it may represent past and currently healed infection, which supposes a high risk of transmission through liver donation; and (3) it may constitute the sole marker of occult HBV infection, which is thus potentially transmissible, as has been demonstrated by contagion occurring through blood transfusion from donors who are only HBcAb (+).7 The importance of limiting the cold ischemia time in heart transplantation usually precludes the performance of additional tests that could serve to delimit this risk. Moreover, in the recipient, the risk varies depending on the patient immune status with respect to HBV, as conditioned by either previous infection or vaccination.
According to some authors, HBcAb (+) potential heart donors are regarded as expanded criteria donors,8 only suitable for patients in extreme life-threatening situations, while other investigators consider such donors to be perfectly valid, since they pose very little or no risk of infection for the recipient.9 The consensus document “Organ donor selection criteria regarding the transmission of infections”, drafted in 2004 by the Spanish National Transplant Organization (ONT) and the Transplant Infections Study Group (GESITRA), concluded that when donors with this serological profile are used, the recipients preferably should be immunized or present HBsAb (+) or HBsAg (+) status, and due informed consent should be obtained in all cases. The document specifies that the risk of transmission through the liver is high. However, in reference to other organs there are few data on which to base the decision – though the risk appears to be small or minimal.10 In the heart transplantation guidelines published by the Spanish Society of Cardiology in 1999,11 no recommendations are made in this respect, and the section referred to donors and infections of the consensus conference of the Spanish heart transplantation groups, published in 2007,12 only make reference to the ONT-GESITRA consensus document.
Between 8% and 10% of all organ donors in Spain in recent years have presented this serological profile.13 The present study examines the influence of donor HBcAb positivity upon acceptance of the organ by the different Spanish adult transplantation teams, and offers an update on the studies published in this field, with a view to establishing a series of recommendations.
Materials and methodsIn order to analyze the influence of HBcAb positivity upon acceptance of the heart for transplantation, in January 2011 we forwarded a multiple-response questionnaire by e-mail to the supervisors of the 16 adult heart transplantation teams:
- (1)
Do you routinely vaccine patients on the waiting list for transplantation against HBV?
- a.
Yes, it is part of routine assessment of the recipient in those patients with negative HBsAb markers.
- b.
In addition to vaccination, we regularly monitor the protection status.
- c.
We have never considered such practice.
- a.
- (2)
When a donor becomes available, do you specifically request HBcAb testing?
- (3)
How do you proceed in the case of a HBcAb (+) donor? (You can chose more than one answer; specify other type of decision.)
- a.
It does not influence the recipient selection decision.
- b.
The donor is ruled out.
- c.
Acceptance is decided only if the donor is moreover also HBsAb (+).
- d.
We select a recipient vaccinated against hepatitis B or with positive HBV markers.
- e.
It does not influence the decision, though we administer lamivudine and/or gammaglobulin.
- f.
Another strategy is used.
- a.
- (4)
When you accept an HBcAb (+) donor, is use made of some specific consent model?
- (5)
Have you detected cases of acute hepatitis due to HBV or seroconversion of uncertain origin among the transplant patients of your unit?
- a.
No.
- b.
Isolated cases.
- c.
More than 5 cases.
- a.
- (6)
Add any other comments which you consider important in relation to the influence of donor HBcAb status upon acceptance of the organ or selection of the recipients.
The literature update was carried out by means of a Medline/PubMed and EMBASE search of articles published from 1994 to 30 October 2011, based on the terms “hepatitis B core” or “HBcAb” or “occult hepatitis B” and “heart transplantation” or “transplant”, or “heart donor”, or “organ donor”, without restrictions referred to the language of publication. The two authors reviewed the selected articles and their references, conducting a manual search of the latter to identify other potentially relevant articles or reviews.
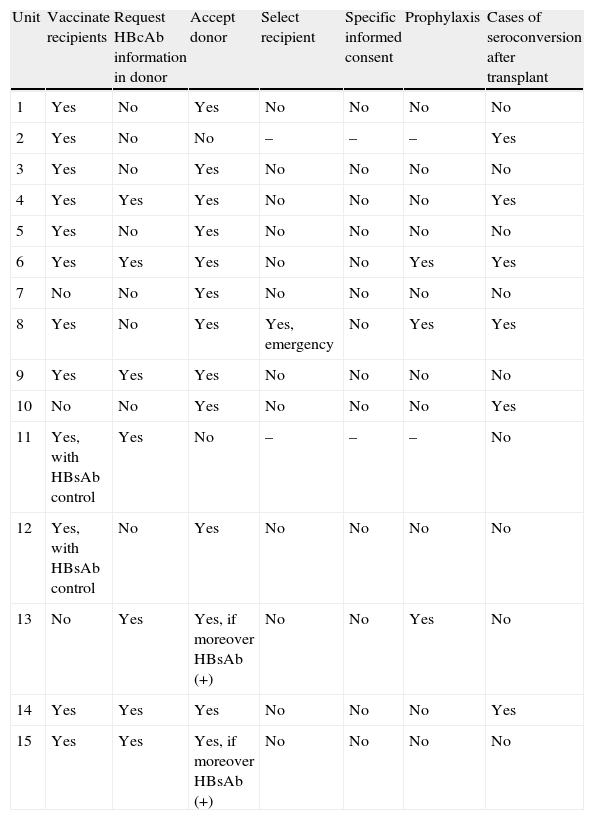
ResultsFifteen of the 16 transplant units answered the questionnaire (Table 1). Twelve of them routinely performed vaccination against HBV among the patients on the transplant waiting list, and two of them quantified the HBsAg titers. Seven units specifically requested the evaluation of donor HBcAb status. In the case of positivity, two rejected the donor; two accepted the organ if the donor was moreover also HBsAb (+); one of the units adopted prophylactic measures in the recipient; one accepted the organ and adopted prophylactic measures; and another selected a recipient in an emergency situation, likewise with the addition of prophylactic measures. Of the three units that did not perform vaccination on a routine basis, two did not take donor HBcAb status into account, while one unit accepted the organ if the donor was moreover HBsAb (+). Six transplantation units reported having evidenced occasional seroconversion of uncertain origin. None of the units made use of a specific informed consent document for donors of this kind. Regarding the requested comments, one unit reported that they are not usually provided with the HBcAb status of the potential heart donor.
Responses to the study questionnaire.
| Unit | Vaccinate recipients | Request HBcAb information in donor | Accept donor | Select recipient | Specific informed consent | Prophylaxis | Cases of seroconversion after transplant |
| 1 | Yes | No | Yes | No | No | No | No |
| 2 | Yes | No | No | – | – | – | Yes |
| 3 | Yes | No | Yes | No | No | No | No |
| 4 | Yes | Yes | Yes | No | No | No | Yes |
| 5 | Yes | No | Yes | No | No | No | No |
| 6 | Yes | Yes | Yes | No | No | Yes | Yes |
| 7 | No | No | Yes | No | No | No | No |
| 8 | Yes | No | Yes | Yes, emergency | No | Yes | Yes |
| 9 | Yes | Yes | Yes | No | No | No | No |
| 10 | No | No | Yes | No | No | No | Yes |
| 11 | Yes, with HBsAb control | Yes | No | – | – | – | No |
| 12 | Yes, with HBsAb control | No | Yes | No | No | No | No |
| 13 | No | Yes | Yes, if moreover HBsAb (+) | No | No | Yes | No |
| 14 | Yes | Yes | Yes | No | No | No | Yes |
| 15 | Yes | Yes | Yes, if moreover HBsAb (+) | No | No | No | No |
The order of the units corresponds to the order of the reception date of the questionnaire.
HBcAb: anti-core antibodies; HBsAb: anti-surface antigen antibodies.
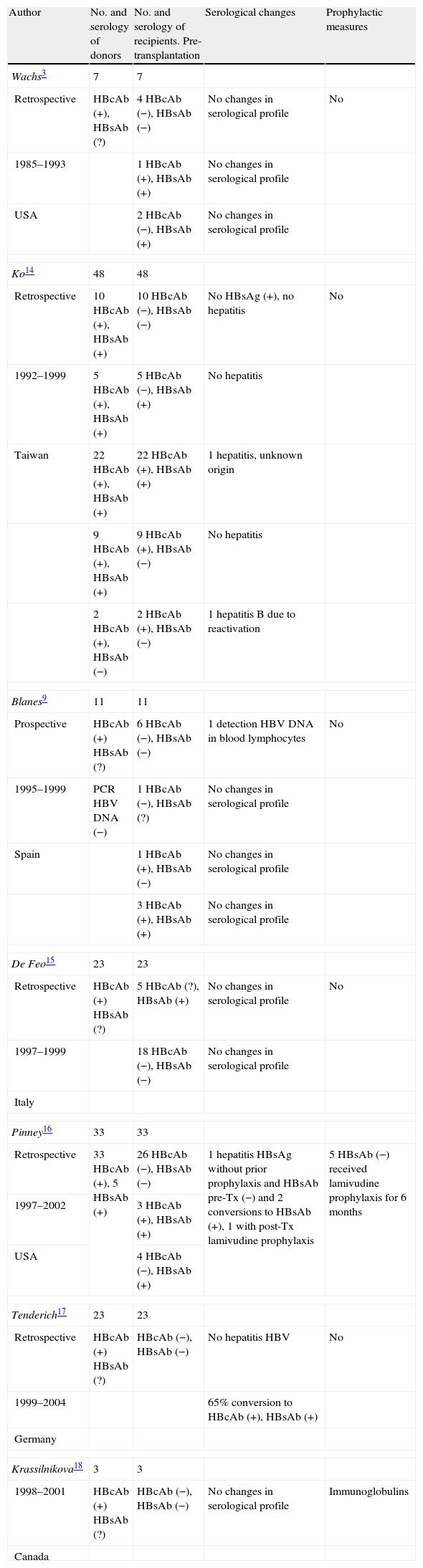
In the study of the literature we reviewed 7 publications specifically mentioning the serological evolution of recipients with hearts from HBcAb (+) donors, documenting the experience gained in 148 patients, of which 95 presented a HBcAb (−), HBsAb (−) serological profile before transplantation (Table 2).3,9,14–18 One patient with negative serology before transplantation and who received no prophylactic measures developed acute hepatitis B,16 two showed HBsAb conversion,16 and viral particles were detected in the lymphocytes in one patient.9 One of the 45 HBsAb (+) recipients developed hepatitis of uncertain origin, not attributed to HBV.14 Tenderich et al.17 described a high incidence of HBcAb and HBsAb seroconversion, but attributed it to passive antibody transfer through the immunoglobulins administered during the transplant period.
Published studies on the serological evolution of heart transplant recipients involving HBcAb (+) donors.
| Author | No. and serology of donors | No. and serology of recipients. Pre-transplantation | Serological changes | Prophylactic measures |
| Wachs3 | 7 | 7 | ||
| Retrospective | HBcAb (+), HBsAb (?) | 4 HBcAb (−), HBsAb (−) | No changes in serological profile | No |
| 1985–1993 | 1 HBcAb (+), HBsAb (+) | No changes in serological profile | ||
| USA | 2 HBcAb (−), HBsAb (+) | No changes in serological profile | ||
| Ko14 | 48 | 48 | ||
| Retrospective | 10 HBcAb (+), HBsAb (+) | 10 HBcAb (−), HBsAb (−) | No HBsAg (+), no hepatitis | No |
| 1992–1999 | 5 HBcAb (+), HBsAb (+) | 5 HBcAb (−), HBsAb (+) | No hepatitis | |
| Taiwan | 22 HBcAb (+), HBsAb (+) | 22 HBcAb (+), HBsAb (+) | 1 hepatitis, unknown origin | |
| 9 HBcAb (+), HBsAb (+) | 9 HBcAb (+), HBsAb (−) | No hepatitis | ||
| 2 HBcAb (+), HBsAb (−) | 2 HBcAb (+), HBsAb (−) | 1 hepatitis B due to reactivation | ||
| Blanes9 | 11 | 11 | ||
| Prospective | HBcAb (+) HBsAb (?) | 6 HBcAb (−), HBsAb (−) | 1 detection HBV DNA in blood lymphocytes | No |
| 1995–1999 | PCR HBV DNA (−) | 1 HBcAb (−), HBsAb (?) | No changes in serological profile | |
| Spain | 1 HBcAb (+), HBsAb (−) | No changes in serological profile | ||
| 3 HBcAb (+), HBsAb (+) | No changes in serological profile | |||
| De Feo15 | 23 | 23 | ||
| Retrospective | HBcAb (+) HBsAb (?) | 5 HBcAb (?), HBsAb (+) | No changes in serological profile | No |
| 1997–1999 | 18 HBcAb (−), HBsAb (−) | No changes in serological profile | ||
| Italy | ||||
| Pinney16 | 33 | 33 | ||
| Retrospective | 33 HBcAb (+), 5 HBsAb (+) | 26 HBcAb (−), HBsAb (−) | 1 hepatitis HBsAg without prior prophylaxis and HBsAb pre-Tx (−) and 2 conversions to HBsAb (+), 1 with post-Tx lamivudine prophylaxis | 5 HBsAb (−) received lamivudine prophylaxis for 6 months |
| 1997–2002 | 3 HBcAb (+), HBsAb (+) | |||
| USA | 4 HBcAb (−), HBsAb (+) | |||
| Tenderich17 | 23 | 23 | ||
| Retrospective | HBcAb (+) HBsAb (?) | HBcAb (−), HBsAb (−) | No hepatitis HBV | No |
| 1999–2004 | 65% conversion to HBcAb (+), HBsAb (+) | |||
| Germany | ||||
| Krassilnikova18 | 3 | 3 | ||
| 1998–2001 | HBcAb (+) HBsAb (?) | HBcAb (−), HBsAb (−) | No changes in serological profile | Immunoglobulins |
| Canada | ||||
HBcAb: anti-core antibodies; HBsAb: anti-surface antigen antibodies; HBsAg: surface antigen; HBV: hepatitis B virus; PCR: polymerase chain reaction; Tx: transplant.
The scarcity of organ donors produces changes in the classical donation criteria, with the introduction of so-called expanded criteria donors.19 The demonstrated capacity to transmit HBV infection through the liver in donors with HBsAg (−) but HBcAb (+) serological findings has led many authors to regard these individuals as expanded criteria donors—though in the case of heart donations this opinion is not uniformly accepted. The acceptance by the different transplant teams of expanded criteria donors varies and depends on a series of considerations, whether of a logistic nature or dependent upon other donor or recipient factors, or on the pressure of the transplant waiting list. The present study reflects an important diversity of protocols among the 15 adult heart transplantation units when considering the offer of a heart from a HBcAb (+) donor. Six units clearly modify their protocol in such cases: two do not accept the organ; two accept it only if the donor is moreover also HBsAb (+) (one of them also administering prophylaxis); one unit accepts the organ with the administration of prophylactic measures; and one only selects those recipients which are in an emergency situation, with the addition of prophylactic measures. On the other hand, 7 units accept such organs taking into account that their patients have been vaccinated—though only one of them quantifies the post-vaccination HBsAb levels. In contrast, two of the three units that do not vaccinate their recipients do not take the HBcAb status of the donor into account.
The number of heart donors is scarce. Consequently, potential donors should not be rejected without due justification—though recipients also should not be exposed to unnecessary risks. HBcAb positivity in an HBsAg (−) individual can reflect different situations:
- (1)
Patients who have cleared HBsAg but have acute infection in the resolution phase, or who have chronic infection, or present a HBsAg mutation preventing its detection by means of the usual techniques. In other words, these patients have occult HBV infection, and are potentially capable of transmitting the disease, as has been demonstrated by transmission of the virus through blood transfusion from donors who are only HBcAb (+).7 This situation could explain the single described case of acute hepatitis B after heart transplantation involving a HBcAb (+) donor.16 Carriers of this kind could also account for the seroconversion of other markers such as HBsAb16 or HBcAb,17 though in most of the reported cases it is suggested that seroconversion was due to the passive administration of antibodies through immunoglobulins administered in the context of transplantation, and not to contact with viral particles.17 In delimiting infection risk, the serum determination of HBV DNA using polymerase chain reaction (PCR) techniques could prove very useful. The presence of HBV DNA in patients who are only HBcAb (+) varies according to the population studied—exceeding 35% in hepatitis C or human immunodeficiency virus (HIV) carriers, and reaching 0–7.4% in blood donors.20,21 In transplantation, Cirocco et al. described an incidence of 0% in 53 organ donors,22 while Challine et al. reported an incidence of 3.1% (4 of 129 organ and/or tissue donors),23 and Solves et al., in a Spanish study, recorded an incidence of 1.2% (1 of 82 organ and/or tissue donors).24
- (2)
False-positive test results. This is infrequent with the enzyme-linked immunosorbent assay (ELISA) techniques commonly used for the detection of this marker, and which according to some authors can be observed in 20–30% of all determinations.2 The use of more specific techniques such as radioimmunoassay (RIA) clearly lowers this incidence.
- (3)
The condition may represent a past and healed infectious process, in which case it is not always accompanied by the HBsAb marker. In such cases transmission of the infection through the liver is common, probably due to the hepatotropic nature of the virus,3–5 and is infrequent through other organs.10,25
On the other hand, transmission is dependent not only upon the serological status of the donor and of the transplanted organ but also on the HBsAg immune status of the recipient and the prophylactic measures taken. There is unanimous agreement on the advisability of vaccinating patients on the transplant waiting list against hepatitis B,10,12,26 though as we have seen, this criterion is not followed by all the transplantation units. Vaccination does not guarantee immunization, since the immune response tends to be impaired in patients with chronic illnesses or in critical condition26; quantitation of the HBsAb titers is therefore advisable. Madayag et al. recommend that patients on the renal transplant waiting list should not receive organs from HBcAb (+) donors if they present HBsAb titers of <100IU/l.27 In lung transplantation, some authors suggest that there is a lesser risk of HBcAb conversion if the HBsAb titers are >100IU/l compared with titers of >10IU/l.28 In the review of the studies referred to heart transplantation, we only found a description of the evolution of 11 patients with markers typically indicative of having been vaccinated, i.e., HBcAb (−), HBsAb (+), and in which no post-transplantation serological changes were reported. Unfortunately, however, the HBsAb titer prior to transplantation was not specified.3,14,16 Probably, with the low risk of transmission of the infection through the heart, HBsAb titers of >10IU/l can prove protective against the infection.26
In some cases, the patient either has not been vaccinated, or there has not been or there is not enough time to complete the recommended vaccination period. These patients could receive an organ from an HBcAb (+) donor, provided drug prophylactic measures are also adopted. This aspect has been widely studied in liver transplantation, where the risk of transmission of the infection is considerably greater than in any other type of transplant. Two reviews published in the year 201029,30 suggest that the administration of lamivudine via the oral route in recipients of livers from HBcAb (+) donors is as effective as the administration of immunoglobulins in maintaining HBsAb titers of >100IU/l or as the combination of both strategies. There are no publications in this respect referred to heart transplantation, except for the study published by Krassilnikova et al.,18 in which immunoglobulin administration in three HBcAb (−), HBsAb (−) recipients proved effective in avoiding the infection.
The importance of limiting the cold ischemia time in heart transplantation usually precludes additional tests capable of delimiting the mentioned risk in the moment when the organ is offered. The dilemma, therefore, is to determine the best approach when a heart from a HBcAb (+) donor becomes available, considering the current gradual decrease in the number of “ideal” heart donors.
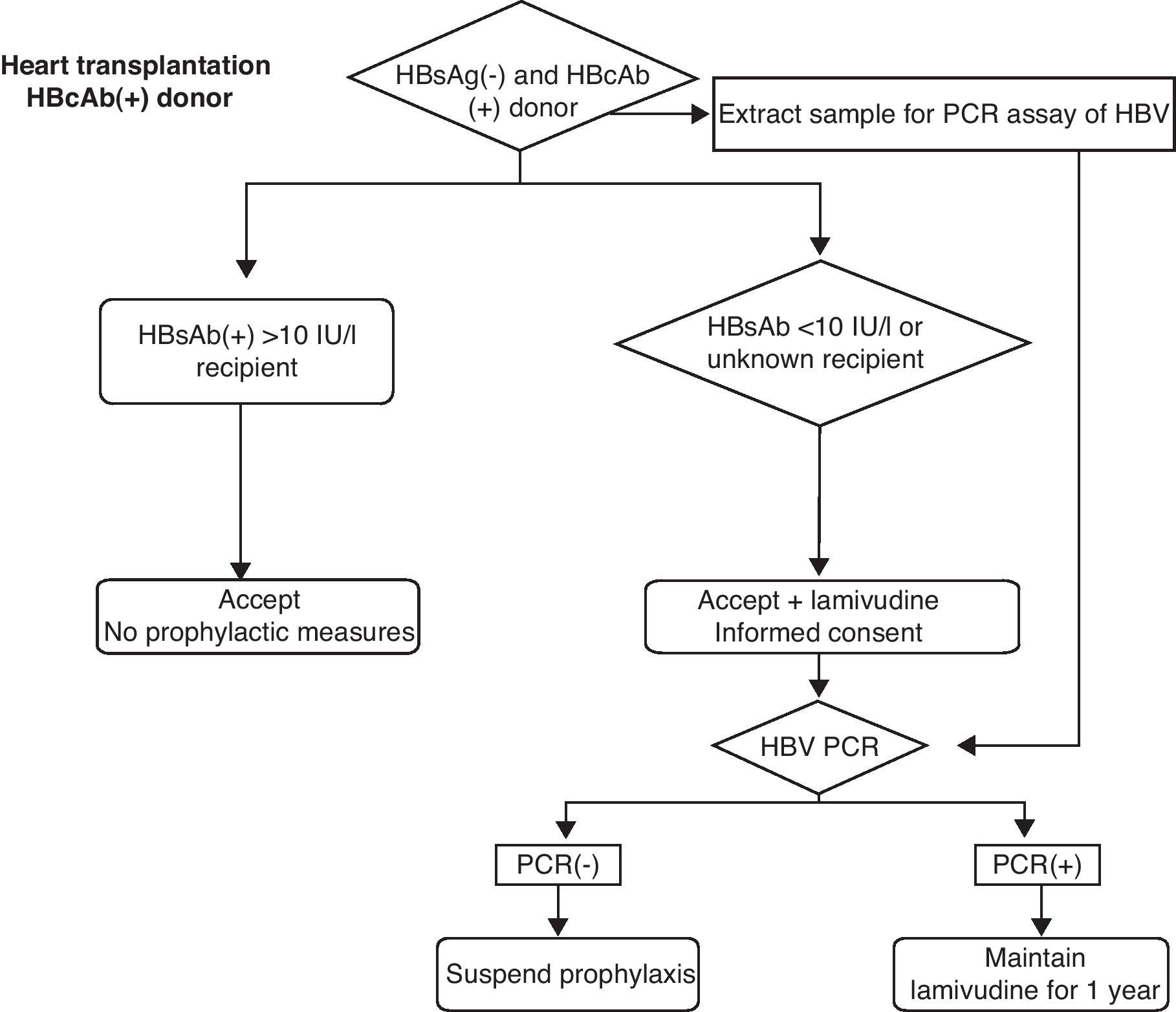
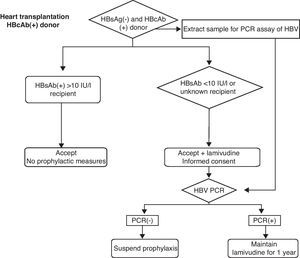
Knowing that the risk will never be reduced to zero, and with the due risk-benefit considerations, the following measures could be adopted (Fig. 1).
- (1)
Routine vaccination against HBV for all patients on the transplant waiting list, and quantification of the HBsAb response are mandatory. Probably, patients with titers of >10IU/l could receive these organs, while patients with lower titers, non-vaccinated patients or patients with unknown post-vaccination levels could receive these organs provided prophylactic measures are adopted, with the obtainment of specific informed consent. If the HBsAb titers are not known, a sample should be collected at the time of transplantation, since the resulting information—probably received after the transplant—can contribute to the decision to either continue or suspend the prophylactic measures.
- (2)
Donor viral PCR testing, with extraction of the sample by the heart transplantation team and performance of the test in the hospital recipient. As in the previous case, the resulting information—probably received after the transplant—can contribute to the decision to either continue or suspend the prophylactic measures. Negative PCR results imply a clearly lessened risk, and the prophylactic measures could be suspended accordingly.31,32
- (3)
Determination of the type of HBcAb immunoglobulin, i.e., whether IgM or IgG, is not recommended. Although it would help to differentiate between acute infection in the resolution phase and chronic infection, such testing is not performed on a routine basis, and there are no studies demonstrating that the management approach should be changed according to the type of HBcAb immunoglobulin involved. On the other hand, the risk is better quantified on the basis of the PCR findings.
- (4)
The routine determination of donor HBsAb titers is not recommended. Although positive values in HBcAb (+) donors would support antecedents of past and healed infection, and not a false-positive result, positivity does not rule out potential infection, as has been demonstrated in liver transplantation, and likewise it would not discard occult HBV infection. High HBsAb titers probably reduce or eliminate the risk of infection, but as has been commented above, the result of the PCR test offers more information. Moreover, the determination of HBsAb likewise is not carried out on an emergency basis in the assessment of organ donors.
- (5)
In patients at risk, as described above, the recommendation is to provide prophylactic treatment in the form of oral lamivudine or some other specific antiviral agent, from the moment of transplantation.33 The use of immunoglobulins or the combination of immunoglobulins and lamivudine does not appear to offer comparatively greater benefit than the isolated administration of lamivudine.
It must be taken into account that these recommendations are only theoretical, and that controlled studies are needed to delimit the risk and define the HBsAb titers and drug measures most effective in terms of prevention.
ConclusionsThere is important variability among the Spanish heart transplantation units regarding the approach to HBcAb (+) donors, ranging from rejection of such donors to non-evaluation of this serological marker. At present, little information is available on the serological evolution of heart transplant patients who have received organs from HBcAb (+) donors. While very infrequent, an HBcAb (+) donor can harbor occult HBV infection; the risk therefore seems low but not zero. A case has been reported of acute hepatitis B, and in some recipients there have been changes in the HBsAb and HBcAb markers, though the underlying causes are not clear. The risk of infection can be prevented by securing adequate post-vaccination or post-infection HBsAb titers, or by using drug measures.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Chamorro C, Aparicio M. Influencia de la positividad del anti-HBc en el donante de órganos en el trasplante cardiaco. Med Intensiva. 2012;36:563–570.