A study was made to validate two previously derived lung injury prediction scores (LIPS) for the prediction of acute respiratory distress syndrome (ARDS) in high risk intensive care patients, with the incorporation of C-reactive protein (CRP) for improving score accuracy.
DesignA prospective, observational cohort study was carried out.
PatientsA total of 200 patients with APACHE II score ≥15 and at least one ARDS risk factor upon ICU admission were included.
InterventionsCalculation of LIPS using formulas developed by Cartin-Ceba et al. (2009) and Trillo-Alvarez et al. (2011) (LIPS-2009 and LIPS-2011). C-reactive protein was measured upon admission (CRP-0) and after 48h (CRP-48).
Main variables of interestIndependent variables: LIPS-2009, LIPS-2011 and CRP values. Dependent variable: development of ARDS.
ResultsEighty-eight patients (44%) developed ARDS after a median (Q1–Q3) of 2.5 (1.3–6.8) days. The LIPS-2009 and LIPS-2011 scores were 4 (3–6) and 5 (3.6–6.5) in ARDS patients compared to 2 (1–4) and 3.5 (1.5–4.5) in non-ARDS patients (p<0.001). CRP-48 was 96 (67.5–150.3)mg/L and 48 (24–96)mg/L in the two groups, respectively (p<0.001). ΔCRP (i.e., CRP-48 minus CRP-0) was significantly higher in the ARDS patients (p<0.001). The AUC was 0.740 and 0.738 for LIPS-2011 and LIPS-2009, respectively – the difference being nonsignificant (p=0.9, 0.9 and 0.8 for pairwise comparison of the different ROC curves). Integrating ΔCRP with LIPS-2011 using binary logistic regression analysis identified a new score (LIPS-N) with AUC 0.803, which was significantly higher than the AUC of LIPS-2011 (p=0.01).
ConclusionsBoth LIPS scores are equally effective in predicting ARDS in high risk ICU patients. Integrating the change in CRP within the score might improve its accuracy.
Se llevó a cabo un estudio para validar 2 puntuaciones de predicción de la lesión pulmonar (LIPS) previamente obtenidas para la predicción del síndrome de dificultad respiratoria aguda (SDRA) en pacientes de alto riesgo ingresados en la unidad de cuidados intensivos, con la incorporación de la proteína C reactiva (PCR) para aumentar la precisión de la puntuación.
DiseñoSe llevó a cabo un estudio prospectivo y observacional de cohortes.
PacientesSe incluyó un total de 200 pacientes con una puntuación APACHE II≥15 y al menos un factor de riesgo de SDRA en el momento de su ingreso en la UCI.
IntervencionesSe calcularon las puntuaciones por medio de las fórmulas desarrolladas por Cartin-Ceba et al. (2009) y Trillo-Alvarez et al. (2011) (LIPS-2009 y LIPS-2011). La concentración de PCR se midió en el momento del ingreso (PCR-0) y al cabo de 48horas (PCR-48).
Principales variables de interésVariables independientes: LIPS-2009, LIPS-2011 y valores de PCR. Variable dependiente: desarrollo de SDRA.
ResultadosOchenta y ocho pacientes (44%) desarrollaron SDRA tras una mediana (Q1-Q3) de 2,5 (1,3-6,8) días. Las puntuaciones LIPS-2009 y LIPS-2011 fueron 4 (3-6) y 5 (3,6-6,5) en los pacientes con SDRA, frente a 2 (1-4) y 3,5 (1,5-4,5) en pacientes sin SDRA (p<0,001). El valor de PCR-48 fue 96mg/l (67,5-150,3) y 48mg/l (24-96) en los 2 grupos respectivamente (p<0,001). ΔPCR (esto es, RCR-48 menos PCR-0) fue significativamente mayor en los pacientes con SDRA (p<0,001). El AUC fue 0,740 y 0,738 para LIPS-2011 y LIPS-2009 respectivamente y la diferencia no fue significativa (p=0,9, 0,9 y 0,8 para la comparación por parejas de las distintas curvas ROC). La integración de ΔPCR con LIPS-2011 mediante un análisis de regresión logística binaria identificó una nueva puntuación (LIPS-N) con un AUC 0,803, el cual era significativamente mayor que el AUC de LIPS-2011 (p=0,01).
ConclusionesAmbas puntuaciones LIPS resultan igualmente eficaces en cuanto a la predicción del SDRA en pacientes de alto riesgo ingresados en la UCI. La integración en esta puntuación del cambio en la PCR podría aumentar su precisión.
The acute respiratory distress syndrome (ARDS) represents a well-known public health problem that was reported in 190,600 cases each year in the United States and to be associated with 74,500 deaths and 3.6 million hospital days.1
Despite advances in ARDS management, mortality rates remain high1,2 especially if associated with diffuse alveolar damage (DAD).3 Patients who even survive ARDS are at risk of diminished functional capacity, mental illness, and decreased quality of life.4
Until now, there are limited specific therapeutic options for ARDS.5 This lack of effective management strategies had directed the research to early identify patients at risk for the evaluation of preventive strategies before ARDS development.6
Early recognition of patients at high risk of ARDS is a prerequisite for conduction of these prevention studies. In the attempts for identifying patients at risk for ARDS, many investigators had derived and validated a lung injury prediction scores (LIPS).7,8 Despite using similar risk factors and risk modifiers, LIPS scores derived by Cartin-Ceba et al.7 and by Trillo-Alvarez et al.8 used different weights for every risk factors and risk modifiers present in the enrolled patients. Both scores were seen to be significantly higher in patients who subsequently develop ARDS.
Most of studies that evaluated these scores involved ED and ward patients with very low incidence of developing ARDS.7,9,10 Patients admitted to the ICU with higher APACHE-II scores had higher incidence of developing ARDS.10 Identifying patients at risk of ARDS development from those critically ill patients might help in structuring preventive studies.
This study was intended to validate and compare between two different lung injury prediction scores in predicting the occurrence of ARDS in high risk ICU patients and to improve the score accuracy by involving the serum CRP level.
Patients and methodsThis study was done as a prospective observational cohort study including all patients older than 18 years old who were admitted to critical care department at AL-Haram hospital, Egypt with APACHE-II score ≥15 and at least one of the predisposing risk factors or risk modifiers of ARDS within 6h from ICU admission during the period from January 2016 to May 2017. We used standardized definitions for the risk factors (Sepsis,11 Shock,12 shock index,13 High risk trauma,14 Pneumonia,14 Aspiration,14 Pancreatitis,15 and high risk surgery16) and risk modifiers (alcohol abuse,17 smoking,17 hypoalbuminemia,14 Diabetes,18 Chemotherapy use,19 Interstitial lung disease (ILD),20 and tachypnoea14). Patients with ARDS on admission, supposed cardiac cause for hypoxemia, and those with hospital readmission (within 7 days) were excluded from the study.
All included patients were subjected to complete history taking and clinical examination with special emphasis on risk factors and risk modifiers of ARDS, routine laboratory investigations included: complete blood count (CBC), serum sodium, serum potassium, serum creatinine, blood urea, random blood sugar, total protein, serum albumin, serum bilirubin, Chest X-ray on admission, every 24h and when needed, arterial blood gas analysis through direct arterial puncture or inserted arterial line for measurement of PaO2 to calculate (PaO2/FiO2 ratio), hemodynamic parameters including hourly monitoring of heart rate and non-invasive measurement of systolic and diastolic blood pressures (SBP and DBP) using bedside monitor. The Shock index was calculated as heart rate/systolic blood pressure.13
Sampling for CRP levels on admission (CRP-0) and 48h thereafter (CRP-48) were taken. The change in CRP (ΔCRP) was estimated as (CRP-48−CRP-0).
Six hours after admission, LIPS was calculated according to two different calculation formulas that use different weights for the variables; Cartin-Ceba et al. (2009)7 that will be referred in the text as LIPS-2009 and Trillo-Alvarez et al. (2011),8 that will be referred as LIPS-2011.
The outcome of interest was the development of ARDS according to Berlin definition (2012).21 The development of ARDS was determined by two independent experts who were blinded to the LIPS scores.
The study protocol was approved by the institutional review board at Cairo University together with representatives of study conduction site. Informed consent was obtained from patients or first degree relative.
Statistical analysisData were prospectively collected and coded using the statistical package of social science (SPSS version 22). Normal distribution of different dependent variables in relation to their independent variables was studied. A variable was considered normally distributed if the Shapiro–Wilk's test had a p>0.0522,23 and z-value of skewness and kurtosis between −1.96 and +1.96.24 Apart from LIPS-2011, all other variables were non-normally distributed. Continuous variables were accordingly expressed as median (25th–75th) percentiles [Median (Q1–Q3)]. Categorical variables were expressed as frequency and proportion. When two groups were studied, non-parametric test (Mann–Whitney U test) was used for comparison between two groups as regard quantitative variables. The confidence intervals of median difference across groups were derived by the Hodges–Lehmann estimate. Chi-Square Test (x2) was used for comparison between two groups regarding qualitative data. Exact test was used instead when the expected frequency is less than 5. Receiver operator characteristic (ROC) analysis was performed to define a cut-off value of a variable. We identified three cut-off values; one with a 100% sensitivity, one with a 100% specificity and the third with the best matched sensitivity and specificity according to the highest Youden index. Comparisons between the different area under curves (AUC) were done using the Z statistics calculation according to DeLong et al.25 MedCalc Statistical Software version 18.11 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018) was used for its calculation as it cannot be calculated using the SPSS.
New model was built using multivariate binary logistic regression analysis. The model contained only LIPS-2011 and ΔCRP which were associated with the ARDS prediction. The strength of the association was measured as the odds ratio (OR) and the 95% confidence interval (CI). The β-coefficient of different variables was estimated to formulate an equation of a new score (LIPS-N) that included constant value added to the sum of the variables multiplied by their β-coefficient. The AUC for the new model was compared with the LIPS-2011 by the DeLong test.25 The regression model and the odds ratio of the LIPS-N derived by univariate regression were validated using bootstrapping of 1000 sample.
Results were considered statistically significant if p≤0.05.
ResultsWe initially enrolled 280 patients in the study, 80 were subsequently excluded; 47 patients with cardiac cause of hypoxia, 25 with ARDS on admission and 8 patients had a history of previous admission. The remaining 200 patients represented the study population.
Eighty-eight of the study population (44%) developed ARDS during their ICU stay while 112 patients (56%) did not develop ARDS. ARDS developed after a median (Q1–Q3) of 2.5 (1.3–6.8) days.
The demographic data, co-morbidities, risk factors, risk modifiers, LIPS scores and CRP measures of our study are presented in Table 1.
The demographic data, co-morbidities, risk factors, risk modifiers, LIPS scores and CRP measures in the study groups.
| Risk factors and risk modifiers | The whole population(200 patients) | ARDS patients(88 patients) | Non-ARDS patients(112 patients) | p value |
|---|---|---|---|---|
| Age (years old) [(median (Q1–Q3)] | 63 (43–70) | 63 (44–70) | 63 (43–70) | 0.983 |
| Male gender [No. (%)] | 120 (60%) | 51 (58%) | 69 (61.6%) | 0.352 |
| APACHE II score [(median (Q1–Q3)] | 20 (18–24) | 21 (18–24) | 19 (18–22) | 0.004 |
| Risk modifiers | ||||
| Alcohol abuse [No. (%)] | 3 (1.5%) | 0 (0%) | 3 (2.7%) | 0.17 |
| Smoking [No. (%)] | 80 (40%) | 33 (37.5%) | 47 (42%) | 0.31 |
| Hypoalbuminemia [No. (%)] | 129 (64.5%) | 68 (77.3%) | 61 (54.5%) | 0.001 |
| Diabetes [No. (%)] | 82 (41%) | 38 (43.2%) | 44 (39.3%) | 0.34 |
| Chemotherapy [No. (%)] | 15 (7.5%) | 7 (7.9%) | 8 (7.1%) | 0.52 |
| Tachypnea [No. (%)] | 76 (38%) | 56 (62.9%) | 20 (18%) | <0.001 |
| Interstitial lung disease [No. (%)] | 5 (2.5%) | 2 (2.3%) | 3 (2.7%) | 0.613 |
| Risk factors | ||||
| Sepsis [No. (%)] | 99 (49.5%) | 56 (63.6%) | 43 (38.4%) | <0.001 |
| SBP (mmHg) [(median (Q1–Q3)] | 100 (80–120) | 90 (73–120) | 110 (90–120) | 0.003 |
| HR (bpm) [(median (Q1–Q3)] | 105 (90–110) | 110 (100–120) | 100 (90–110) | <0.001 |
| Shock index [(median (Q1–Q3)] | 1 (0.8–1.5) | 1.2 (0.9–1.8) | 0.9 (0.7–1.2) | <0.001 |
| Shock index score [No. (%)] | ||||
| <1 | 108 (54%) | 30 (34.1%) | 78 (69.6%) | <0.001 |
| 1–1.5 | 50 (25%) | 33 (37.5%) | 17 (15.2%) | |
| >1.5 | 42 (21%) | 25 (28.4%) | 17 (15.2%) | |
| High risk trauma [No. (%)] | 50 (25%) | 23 (26.1%) | 27 (24.1%) | 0.42 |
| Pneumonia [No. (%)] | 80 (40%) | 47 (53.4%) | 33 (29.5%) | 0.001 |
| Aspiration [No. (%)] | 49 (24.5%) | 28 (31.8%) | 21 (18.8%) | 0.02 |
| Pancreatitis [No. (%)] | 2 (1%) | 0 (0%) | 2 (1.8%) | 0.31 |
| High risk surgery [No. (%)] | ||||
| Elective | 25 (12.5%) | 5 (5.7%) | 20 (17.9%) | 0.03 |
| Emergent | 26 (13%) | 14 (15.9%) | 12 (10.7%) | |
| Total | 51 (25.5%) | 19 (22%) | 32 (28.6%) | |
| LIPS-2009 [(median (Q1–Q3)] | 3 (2–5) | 4 (3–6) | 2 (1–4) | <0.001 |
| LIPS-2011 [(median (Q1–Q3)] | 4 (2.5–5.5) | 5 (3.6–6.5) | 3.5 (1.5–4.5) | <0.001 |
| CRP-0, mg/L [(median (Q1–Q3)] | 48 (24–48) | 48 (24–48) | 48 (24–48) | 0.4 |
| CRP-48, mg/L [(median (Q1–Q3)] | 96 (48–96) | 96 (67.5–150.3) | 48 (24–96) | <0.001 |
| ΔCRP, mg/L [(median (Q1–Q3)] | 24 (0–61.5) | 48 (19.5–83.5) | 0 (0–48) | <0.001 |
SBP: systolic blood pressure, HR: heart rate, LIPS: lung injury prediction score, CRP-0: C-reactive protein on admission, CRP-48: C-reactive protein 48h after admission, ΔCRP: CRP-48−CRP-0.
The LIPS-2009, LIPS-2011, CRP-48 and ΔCRP but not CRP-0 were found to be significantly higher in patients who developed ARDS compared to non-ARDS patients (Table 1).
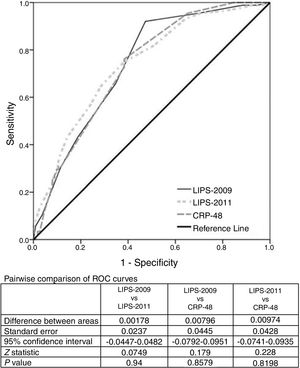
ROC analysis was used to evaluate the predictive value of the LIPS scores and CRP-48 for the prediction of ARDS development. The AUC was 0.74 [95% CI: 0.67–0.81)] for LIPS-2011 compared to 0.738 [95% CI: 0.67–0.81)] and 0.730 [95% CI: 0.67–0.8)] for LIPS-2009 and CRP-48 respectively. These AUCs were statistically significant when compared to the AUC of 0.5 (p=0.000 for the three AUCs) while comparing those AUCs together revealed a non-significant difference (p=0.9, 0.9, 0.8 for pairwise comparison of different ROC curves) (Fig. 1). Table 2 shows the cut-off values of the different variables with their sensitivity, specificity, positive likelihood ratio (LR+) and negative likelihood ratio (LR−).
The cut-off values of the different variables with their sensitivity, specificity, LR+, and LR−.
| Variable | Cut-off value | Sensitivity | 95% CI | Specificity | 95% CI | LR+ | 95% CI | LR− | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| LIPS-2009 | −1 | 100 | 95.9–100 | 0 | 0–3.2 | 1 | 1–1 | ||
| 2 | 92 | 84–97 | 53 | 43–62 | 1.95 | 1.6–2.4 | 0.15 | 0.07–0.3 | |
| 8 | 0 | 0–4.1 | 100 | 96.8–100 | 1 | 1–1 | |||
| LIPS-2011 | −1 | 100 | 95.9–100 | 0 | 0–3.2 | 1 | 1–1 | ||
| 3.5 | 75 | 65–84 | 62 | 52–71 | 1.95 | 1.5–2.5 | 0.41 | 0.3–0.6 | |
| 7.5 | 5.7 | 1.9–12.8 | 100 | 96.8–100 | 0.94 | 0.9–1 | |||
| CRP-48 (mg/L) | 17 | 100 | 95.9–100 | 15 | 9.1–23.2 | 1.18 | 1.1–1.3 | ||
| 48 | 76 | 66–85 | 62 | 52–71 | 1.98 | 1.5–2.6 | 0.39 | 0.3–0.6 | |
| 317 | 0 | 0–4.1 | 100 | 96.8–100 | 1 | 1–1 | |||
| LIPS-N | −2.9 | 100 | 95.9–100 | 4 | 1–9 | 1.04 | 1–1.1 | ||
| −0.418 | 78 | 68–87 | 69 | 59–77 | 2.5 | 1.9–3.4 | 0.31 | 0.2–0.5 | |
| 1.8 | 17 | 9.9–26.6 | 100 | 96.8–100 | 0.83 | 0.8–0.9 |
LIPS: lung injury prediction score, CRP: C-reactive protein, CI: confidence interval, LR+: positive likelihood ratio, LR−: negative likelihood ratio.
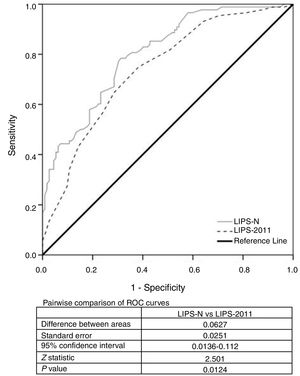
A multivariate binary logistic regression model involving the ΔCRP and LIPS-2011 was studied. The odds ratio (95% CI) of LIPS-2011 was 1.5 (1.3–1.8) and that of Δ CRP was 1.02 (1–1.02). Using their β-coefficient, the following equation was derived: −2.416+(0.41×LIPS-2011)+(0.016×ΔCRP). The new score was estimated according to this equation and was termed as LIPS-N. The LIPS-N was 0.31 (−0.37–1.4) in ARDS patients compared to −0.99 (−1.7–0.2) in non-ARDS patients [p=0.000, 95% CI for Hodges–Lehmann median difference −1.4 (−1.8,−1.1)]. We compared the new LIPS-N and LIPS-2011 scores using ROC analysis to evaluate their AUC. The AUC of the LIPS-N was 0.8 [95% CI (0.74–0.86)] which is significantly higher than AUC of 0.5 (p=0.000). The AUC of LIPS-N was seen to be significantly higher than that of LIPS-2011 (p=0.01) (Fig. 2). The cut-off of the LIPS-N and their sensitivity, specificity, LR+ and LR− are seen in Table 2. The odds ratio (95% CI) of patients with LIPS-N more than −0.418 compared to those with less LIPS-N was 7.989 (4.2–15.3).
DiscussionWe found in this study that the risk of progression to ARDS may be ascertained using the LIPS scores; either derived by Cartin-Ceba et al.7 (LIPS-2009) or by Trillo-Alvarez et al.8 (LIPS-2011) early in the course of illness. Both LIPS scores are significantly higher in patients who developed ARDS. We evaluated the accuracy of the LIPS for predicting ARDS using ROC curve. The AUC was 0.740 for LIPS-2011 compared to 0.738 for LIPS-2009 which is statistically insignificant. LIPS-2011 score of 3.5 was 75% sensitive and 62% specific for predicting ARDS while a LIPS-2009 score of 2 was found to have a sensitivity of 92% and specificity of 53%. In their derivation cohort for the LIPS-2009, Cartin-Ceba et al.7 found higher AUC of 0.85 for predicting ARDS in a population of 1431 patients which is nearly similar to the AUC derived by Trillo-Alvarez and colleagues8 which was 0.84 in their retrospective derivation and prospective validation cohorts using the LIPS-2011 score with a cut-off value of 3 to have 69% sensitive and 84% specific.
In surgically ventilated patients, Bauman et al. found that LIPS is predictive for ARDS with AUC of 0.7926 with 50% increase in the development of ARDS for every one-unit increase in LIPS. They however, used LIPS derived by Gajic et al.10 which we did not use in our study. In their study on 5584 patients, Gajic et al.10 derived their score on 2500 patients and validated it on the remaining 3084 patients. They identified AUC for the LIPS of 0.8 for predicting ARDS development in both the derivation and validation cohorts. They identified an optimum cut-off value of 4 to be 69% sensitive and 78% specific. Using this score in ICU patients, Soto et al.27 showed 31% increase in the likelihood of ARDS development for every point increase in LIPS with AUC of 0.7 which is close to ours; with a cut-off value of 4 to be 90% sensitive and 31% specific. Due to the higher mortality of ARDS patients with DAD, Lorente et al. derived a regression model including PaO2/FiO2 ratio, dynamic compliance and age to predict DAD within ARDS patients. The AUC for their model was 0.74 in derivation cohort and 0.73 in the validation cohort.28
The notion of using a biomarker reflecting the severity and course of alveolocapillary inflammation and increased permeability characterizing the ARDS is enthusiastic. Theoretically, the ideal biomarker would be involved in the disease pathogenesis, easy to measure, rapid results availability, and highly sensitive and specific in predicting the required outcome.29 Many biomarkers related to DAD and its association with distal airway pathologic changes characterizing ARDS might be seen of value30,31 and even, too specific biomarker at the molecular level had been studied.32 Many of these biomarkers are not commonly used in clinical practice. Despite being non-specific, C-reactive protein is a biomarker in common clinical use to delineate the activity of host inflammatory conditions such as sepsis, cardiovascular disease and rheumatological disorders.33 Patients with sepsis-induced ARDS have elevated levels of CRP in both plasma and the broncho-alveolar lavage.34 We measured the admission and 48h CRP levels in our study population.
In this study, CRP-48 and the change of the CRP over the first 48h following ICU admission were significantly higher in patients who developed ARDS. CRP-48 had an AUC of 0.730 for predicting patients who developed ARDS. The CRP-48 of 48mg/L was seen to be 76% sensitive and 62% specific. This was concordant with Zheng et al.35 who showed that the CRP can predict the occurrence of ARDS in trauma patients. Contrary to these results, In a study on community acquired pneumonia, Lee et al. concluded that the CRP does not predict patients who required mechanical ventilation and accordingly cannot predict ARDS development.36 Another study conducted by Komiya et al. showed that the CRP had an AUC of 0.831 for discriminating patients with ARDS compared to those with cardiogenic pulmonary edema and that this AUC was increased to 0.931 when CRP was added to the brain natriuretic peptide.37 This study was however used in patients admitted by respiratory failure for the diagnosis rather than for predicting ARDS as we intended to evaluate. Despite the specificity of 62% that we had for CRP-48, it is well-known that the CRP might be elevated in numerous inflammatory disorders and lack a specificity for the ARDS development.33
We incorporated the ΔCRP with the LIPS-2011 in a new score using binary logistic regression model forming the LIPS-N. This score was estimated by the formula of [−2.416+(0.41×LIPS-2011)+(0.016×ΔCRP)]. The AUC of this score was 0.803 which was significantly higher than that of LIPS-2011 alone. A result of this score of −0.418 was found to be 78% sensitive and 69% specific for predicting ARDS. This score however needs to be validated in another sample.
It is important to mention that the incidence of ARDS in this study was very high (44%) compared to other previous studies where the incidence did not exceed 20%8,38–40 and this could be due to using a sample population with APACHE-II score ≥15 representing high risk population who are admitted to the ICU rather than ED or ward admission. In Trillo-Alvarez's study,8 they found ARDS incidence to be 17% in their retrospective derivation cohort of ICU patients compared to 7% in the prospective validation cohort of all hospitalized patients.
Both previously derived LIPS scores were seen to be similarly functioning in terms of ARDS prediction. Both include readily available clinical information known to be associated with ARDS. They identify at-risk patients early during illness with fair sensitivity and specificity. Accordingly, both may be beneficial for segregating subsets of high-risk patients for enrollment in prevention strategies. Adding biomarker may, however, improve the score accuracy.
The lack of optimum sensitivity and specificity of these scores might be attributed to the absence of some well-known risk modifiers for ARDS; large volume transfusion of packed red blood cells and fluid balance are examples of these risk modifiers that was seen by many investigators to affect ARDS risk.14,41 The predictive value of these scoring systems could be accordingly improved using more additional variables including more risk modifiers in all patients. In addition, the exposure to risk factors and risk modifiers is a dynamic process that might develop during the hospital course rather than on admission and so, any predictive tool needs to be dynamic on daily bases. Adding more additional information and data that are unavailable on admission might however affect the simplicity of using these scores in real practice.
Our study is limited by the relatively small sample size that used for the validation of the LIPS and that it is a single center study. We recruited high risk ICU patients with high APACH-II score having higher incidence of ARDS that may explain the needed smaller sample. One of the important factors that should be considered during planning for a preventive study for ARDS is that many of those patients expose to their risk factors prior to their ICU admission. Despite being more difficult, the enrollment of all hospitalized patients might be more practical, yet the authors intended here to validate the LIPS scores in the more critically ICU patients. One of the draw-backs of this study is the validation of the new score derived from the study on the same study sample. The LIPS-N score should be validated in another ICU patients’ sample. The authors used bootstrapping for validation of the regression model.
Despite that the Berlin definition represents the gold standard for ARDS diagnosis, many of its items, like interpretation of portable chest X-ray, remain clinician dependent. Some authors concluded limited experts’ ability to accurately differentiate between ARDS and other causes of respiratory failure.42 On the histopathologic level, many of the clinically diagnosed ARDS patients showed subsequently normal lung after open lung biopsy or autopsy.28,43,44 Finally, many scores with different weighting power for the risk factors are established that limit the ability to compare different studies.
ConclusionsThis study concludes that both LIPS scores derived by Cartin-Ceba et al. on 2009 and Trillo-Alvarez et al. on 2011 are equally effective in predicting ARDS in risky ICU patients. The incorporation of change of CRP over the first 48h of ICU admission with the LIPS-2011 score may increase its accuracy in ARDS prediction.
FundingThe study is self-funded.
Author's contributionConcept: MEHA, KMT, SF, GH.
Design: MEHA, KMT, GH.
Definition of intellectual content: KMT, SF, GH.
Literature search: MEHA, KMT.
Data acquisition: MEHA, SF.
Data analysis and statistical analysis: KMT.
Manuscript preparation: KMT.
Manuscript editing: KMT.
Manuscript review: SF, GH.
Conflict of interestThe authors do not have a conflict of interest to declare.