To determine the risk of microbiological contamination with hospital use high- and low-flow bubbling humidifiers.
MethodsA systematic literature review was carried out in 6 databases. Observational or experimental studies published between 1990 and 2016 were selected, written in English or Spanish, and in which microbiological contamination with hospital use high- and low-flow bubbling humidifiers was investigated.
ResultsA total of 12 articles were included: 4 analyzed the water from reusable humidifiers, 4 analyzed the water from prefilled system humidifiers, and the rest compared samples from both models. Microbial contamination was observed in all studies in which reusable humidifiers were evaluated, usually involving common bacteria from the skin flora, while potential pathogenic species were notified in 2 studies. No microbial contamination was isolated from reusable humidifiers, regardless of whether they had been consecutively used over time by a single patient or by several patients.
ConclusionOn one hand, there seems to be a low risk of contamination during the first weeks of use of prefilled humidifiers, which allows multiple use in different patients, without a risk of cross-contamination. On the other hand, it should be underscored that handling reusable humidifiers without correct aseptic measures can increase the risk of contamination; replacing reusable humidifiers with prefilled models therefore could be the safest option.
Determinar el riesgo de contaminación microbiológica de los humidificadores de burbujeo para oxigenoterapia de alto o bajo flujo de uso hospitalario.
MétodosRevisión sistemática de la literatura a través de 6 bases de datos bibliográficas. Se seleccionaron estudios observacionales o experimentales publicados entre 1990 y 2016, en inglés o español, que analizaban la contaminación microbiana de los humidificadores de burbujeo de los dispositivos de oxigenoterapia hospitalaria de alto y bajo flujo.
ResultadosSe incluyeron 12 artículos: 4 analizaron el agua de humidificadores reutilizables, 4 de desechables y otros 4 compararon muestras procedentes de ambos modelos. Se observó la presencia de contaminación microbiana en todos los estudios que evaluaron humidificadores reutilizables (generalmente bacterias habituales de la flora cutánea). En 2 de ellos se notificaron aislamientos de especies potencialmente patogénicas. No se aisló contaminación microbiana en las muestras procedentes de modelos desechables, independientemente de si fueron utilizados por un único paciente o por varios de forma consecutiva a lo largo del tiempo.
ConclusiónParece existir bajo riesgo de contaminación en humidificadores desechables durante las primeras semanas de uso, pudiendo reutilizarse entre pacientes distintos sin riesgo de contaminación cruzada. Por otro lado, cabe destacar que la manipulación de los humidificadores reutilizables de forma no aséptica puede aumentar la probabilidad de contaminación, por lo que la sustitución de humidificadores reutilizables por modelos desechables podría ser la opción más segura.
Oxygen therapy is used to treat or prevent hypoxia through the supplementary administration of oxygen at concentrations higher than those found in room air. The flow and device used for administration depend on the clinical conditions and tolerance of the patient, and must be adapted to secure an increase in oxygen in arterial blood.1,2
The air inhaled by the patient is composed of room air plus the oxygen therapy supply. In low-flow oxygen therapy devices such as nasal cannulas or simple oxygen masks, the fraction of inspired oxygen varies according to the respiratory pattern of the patient and the selected flow rate. In contrast, in high-flow devices such as Venturi-type masks, the gases are mixed at constant concentration, independently of the respiratory pattern of the patient. If the fraction of inspired oxygen is modified, the percentage of oxygen supplied to the patient changes; however, if the flow is modified, only the total supply of mixed air reaching the patient changes.1
The problems often associated with oxygen therapy include nasal, oral and ocular dryness. In this regard, and to avoid the undesirable effects of medicinal oxygen dryness, it is currently advised to use humidifiers for the in-hospital administration of oxygen through high- and low-flow systems.2
The type of device most commonly used in our setting is the immersion or bubble humidifier without warming. These devices can be disposable or reusable, and are filled with water. Disposable or prefilled humidifiers are easy to use, require little handling specialization, and are ideal for short-stay Departments. On the other hand, reusable humidifiers are used in long-stay hospitalization areas, with the purpose of reducing costs by only requiring correct aseptic handling of the device and water for filling.
It has been postulated that humidifiers can be colonized by bacteria, and that the aerosols generated from contaminated humidifiers can contribute to the transmission of respiratory diseases.3,4 For this reason, and independently of the type of humidifier used, it is advisable to replace the system each time the device is used with a different patient, and to clean reusable models on a daily basis with disinfectant soap.2 Nevertheless, the indicated procedure may vary according to the protocols or practices found in each Department or center.5
The service life of the water in the humidifiers depends on the intensity and duration of use, and on the environmental conditions. Since it is not uncommon to use one same humidifier with several consecutive patients, concerns may arise as to the possibility of microbial contamination – thus compromising patient safety.
The aim of the present study is to determine the risk of microbiological contamination of bubble humidifiers used with in-hospital high- or low-flow oxygen therapy devices according to the time or mode of use (for a single patient or for several consecutive patients).
Material and methodsA systematic literature review was made according to the PRISMA recommendations.6 We selected articles published between January 1990 and November 2016, indexed in the Medline, Web of Science, Scopus, EMBASE, CINAHL and Cochrane Library plus databases, using the search strategy (adapted to each database) “oxygen” AND “humidifier” AND “contamina*”.
As secondary strategy, we reviewed the literature references of the identified articles in order to retrieve other possible relevant studies not obtained from the electronic databases.
We selected observational or experimental studies with available full text articles investigating the microbial contamination of bubble humidifiers used with high- and low-flow oxygen therapy devices. Studies referred to other types of humidifiers or involving home oxygen therapy systems were excluded. Narrative literature reviews, isolated clinical case descriptions, opinion articles or letters to the Editor were also excluded, in the same way as studies published in languages other than English or Spanish.
Two reviewers independently selected the potentially relevant articles based on a search strategy involving the reading of titles and abstracts. The full text articles of all the screened references were subsequently evaluated to confirm that they complied with the inclusion criteria. Disagreements were settled by consensus between both reviewers, with the seeking of a third opinion in the event of any persistent doubts.
Following the study search and selection process, we used a data extraction template for each article, compiling information referred to the author and date of the study, the type of humidifiers analyzed, sampling method, culture results, and the conclusions and recommendations of the authors. Data extraction was carried out independently by two investigators, while a third acted as evaluator – contrasting the information obtained by the former and establishing consensus regarding the contents of the final template.
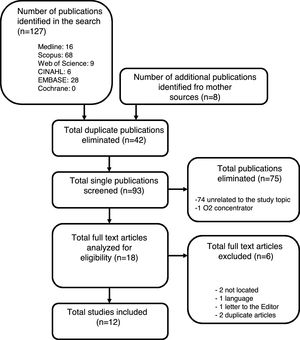
ResultsThe search of the 6 electronic databases yielded a total of 127 references, which were reduced to 85 after eliminating duplicate entries. Expanding the search to the literature references of the articles yielded 8 additional publications that met the inclusion criteria. Of the total selected articles, 75 were discarded after reading of the titles and abstracts: 74 with no relation to the objective of our study, and another referred to humidifiers used with home oxygen devices. Full text access was not possible in two publications (no public access option), and these were therefore excluded from the study. Critical reading of the full text articles of the selected publications excluded another four studies: two considered to be redundant; one written in Korean; and another corresponding to a letter to the Editor with no interest in relation to our study (Fig. 1).
The analysis of evidence was thus finally based on 12 publications,7–18 all comprising descriptive studies based on microbiological agar plate culture of water samples from bubble humidifiers without warming. The main characteristics and results of the included studies are reported in Table 1.
Summary of the evidence contributed by the articles included in the review.
| Author, country, year | Devices studied | Sampling | Culture results | Conclusions |
|---|---|---|---|---|
| Cahill et al. USA, 1990 | 48 reusable humidifiers (without patients) in low flow systems | Daily samplings of the humidifiers for 5 days (24 with sterile water and 24 with non-sterile water). A total of 240 samples were cultured (120 samples of each type of water) | The devices using sterile water yielded more positive results in the bacterial cultures (54/120 versus 39/120). The presence of Legionella or mycobacteria was not studied | The use of non-sterile water in low flow humidification devices is safe and efficient |
| Seigel et al. USA, 1990 | 55 disposable humidifiers (single patient) | Sampling of sterile water of the humidifiers every 72h. In the event of no bacterial growth, the process was repeated until the end of treatment or evaporation of the water. The devices were used by a patient 3–12 days or remained unused for a maximum of 62 days | All the cultures were negative, independently of humidifier use | Disposable devices are safe at least for 12 days. Using humidifiers on an intermittent basis until they are empty is a safe practice |
| Seto et al. China, 1990 | 46 disposable humidifiers (single patient) | Sampling at end of treatment (maximum period of 6 days). After use by the patient, 24 devices were left operating for a maximum of 25 days, with weekly samplings. | All the bacteriological cultures (evaluated after 48 and 72h) were negative | Disposable humidifiers maintain sterility during continuous treatments and periods without use of >10 days |
| Castel et al. France, 1991 | 164 samples of reusable humidifiers and 39 samples of disposable humidifiers (17 multiple patients and 22 single patient) | Sample collection from disposable models once a day or twice a week, depending on the Department, in use for a maximum of 105 days. Sampling of the reusable humidifiers was on a point basis. Samples were also obtained from the connections of the devices | Cultures evaluated after 24, 48h and 7 days. A total of 32.9% of the samples from reusable devices showed bacterial contamination (mainly Pseudomonas), versus none of those from disposable devices | Disposable devices are both safe and efficient |
| Henderson et al. Canada, 1993 | 1311 disposable humidifiers (multiple patients) and 60 reusable (single patient) | Sampling after one month of use or when 10ml of water remained in the disposable models. Sampling after one week of use in the reusable models (filled with sterile water) | Cultures evaluated on days 1, 2, 3, 7 and 10. Only 4/1311 samples from the disposable models presented contamination (epithelial flora). In the case of the reusable devices, 10% (6/60) showed significant contamination. Legionella was not detected | The contamination of disposable humidifiers is unlikely, even when used by various consecutive patients |
| Yamashita et al. Japan, 2005 | 3 disposable humidifiers and 3 reusable (without patients) | Sampling the first day and every 7 days for a maximum of 56 days of use. The disposable models were replaced every 7 days, and the reusable devices were filled with sterile water every 2–3 days | In a total of 108 samples, bacterial contamination was detected (grampositive species) in only one sample from each type of device (after 21 days for disposable and 28 days for reusable). The presence of dust was detected from 35 days in the reusable models | Both systems are safe, with bacterial contamination being unlikely, at least for 56 days |
| Nakipoglu et al. Turkey, 2005 | 50 reusable humidifiers | A point sample was obtained from each humidifier. No data provided on the previous use of the humidifiers | Cultures were made for bacteria (including Legionella), fungal species and amebas. Contamination was detected in 32/50 humidifiers: 45% due to fungal species and 30% due to bacteria. In 20% of the contaminated humidifiers, the causal organisms corresponded to pathogens associated to respiratory infections | A correct protocol is needed for the use of reusable humidifiers, or disposable devices should be made available |
| Kobayashi et al. Japan, 2006 | 15 reusable humidifiers | Samples were obtained on days 0, 1, 3 and 7 from humidifiers filled with non-sterile water. Twelve of the humidifiers were washed before use | All the humidifiers presented bacterial contamination (mainly due to Pseudomonas and grampositive species), especially those without prior cleaning. The presence of Legionella or mycobacteria was not studied | Washing and drying of the devices is necessary in the reusable models; the utilization of disposable humidifiers may be more efficient |
| Kobayashi et al. Japan, 2006 | 8 reusable humidifiers and 12 disposable (multiple patients) | Sampling on days 0, 1, 3 and 7. In the disposable models, sampling was continued on a weekly basis until week 12. The reusable humidifiers were cleaned before use and were filled with sterile water. | Some reusable humidifiers presented bacterial contamination, possibly related to deficient cleaning of the containers. The disposable models exhibited no bacterial growth after 84 days. The presence of Legionella or mycobacteria was not studied | Multiple patient use of disposable humidifiers during long periods of time is safe and efficient |
| Belotti et al. France, 2010 | 10 disposable humidifiers (multiple patients) | Sampling on days 2, 4, 7, 10, 20 and 30 of use in each device | Bacterial and fungal cultures were made. None of the cultures showed contamination | Multiple patient use of disposable humidifiers is safe and efficient |
| Jadhav et al. India, 2013 | 23 reusable humidifiers | Point sampling before and after cleaning and disinfection with ethanol 70° | Bacterial and fungal cultures were made. Numerous contaminant organisms were identified in the samples (42% of the humidifiers presented bacterial contamination. No concrete data referred to fungal contamination). Contamination decreased after cleaning with ethanol | Aseptic handling and protocolized disinfection of the humidifiers is essential |
| Jeong et al. South Korea, 2014 | 60 disposable humidifiers (multiple patients) | The humidifiers were distributed into 3 groups: one was sampled after 7 days, another after 14 days, and the third after 28 days of use | Evaluation of cultures after 24–48h. No bacteria were identified in any of the humidifiers | The use of disposable humidifiers by several consecutive patients is safe |
Four studies analyzed microbiological contamination of reusable humidifiers7,13,14,17 and another four in disposable humidifiers.8,9,16,18 The rest analyzed and compared contamination of both types of humidifiers.10,12,15
Microbiological contamination of reusable humidifiersThe investigators used a heterogeneous series of methods to analyze the presence of microorganisms in humidifiers of this kind. Sample collection for bacterial culture was performed serially over time (for a maximum of 8 weeks) in half of the studies,7,12,14,15 while the other half made use of point sampling.10,11,13,17
Three of the studies failed to specify whether the humidifiers were used with one or more patients,13,15,17 and in two of them the oxygen therapy system was not connected to any patient.7,12 With regard to the rest of the studies, one used a different device for each patient11 while another reused the device, cleaning it before each use.15
One publication used non-sterile water to fill the humidifiers,14 while another contemplated both possibilities, filling half of the devices with sterile water and the other half with non-sterile water.7 Three articles failed to specify the type of water used,10,13,17 and the rest employed sterile water.
All the studies observed bacterial contamination in a variable proportion of sampled humidifiers (between 10% and 100%). In general terms, the isolated organisms were common species of the skin flora, though in some cases potentially pathogenic microorganisms were identified (mainly Pseudomonas and gramnegative bacilli).10,14,17 Two studies specifically evaluated the presence of Legionella pneumophila, with negative culture results.11,13
The presence of microbiological contamination of the reusable humidifiers was probably related to deficient handling during cleaning and preparation, or to the use of non-sterile water for filling – colonies being isolated even before three days after the start of use.
On the other hand, two studies also addressed contamination of humidifiers of this kind by fungal species and amebas.13,17 Both of these studies performed culture of water samples obtained by point sampling on a single day. A notorious proportion of contaminated samples was observed (up to 45%), in some cases due to pathogens associated to respiratory infections (such as fungi belonging to the genus Aspergillus, among others).
Based on these results, emphasis was placed on the need to observe good aseptic practice, with protocolized disinfection of the reusable devices, and underscoring the usefulness of disposable systems as an alternative for guaranteeing patient safety.
Microbiological contamination of disposable humidifiersOf the different studies that used disposable humidifiers, 5 reused them among several patients.10,11,15,16,18
With the exception of a single study11 that performed point samplings, the rest all cultured several samples obtained on a serial basis over a maximum patient utilization time of 105 days – no pathogenic bacterial being isolated in any of them. In addition, three studies8,9,12 also performed cultures of the water from humidifiers not used by patients, for a maximum of 62 days, with similar results.
Only one study16 performed specific cultures for the identification of fungal species, and all proved negative.
Although there was no single criterion regarding the maximum duration of use until the appearance of colonies, all the authors concluded that the utilization of humidifiers of this kind during several weeks is a safe practice, even when consecutively used by different patients. In fact, multiple-patient use of disposable humidifiers was proposed by several authors11,15 as the most cost-effective option – though with discrepancies regarding their efficiency in the Departments of Pneumology.10
DiscussionThe findings of our review reveal the presence of microbiological contamination in the reusable bubble humidifiers, but not in the disposable (prefilled) devices. This applies even when the latter are used consecutively with several patients for prolonged periods of time.
These results may be particularly useful in settings with a high patient turnover rate, such as Emergency Care Departments and pre-hospital emergency care services, short-stay areas or pre- and post-anesthetic units, where one same humidifier system may be used by a number of patients in the course of the day.
From the cost perspective, the use of one same disposable humidifier in consecutive patients for several days appears to be the best strategy. The efficiency of this measure could be further incremented if such devices are employed selectively, since the use of bubble humidifiers with flow-flow oxygen therapy systems appears to be of little use in practice in general. Indeed, there now appears to be some agreement that such humidifiers should not be routinely used with nasal cannulas or masks when low flows are administered with the purpose of achieving a fraction of inspired oxygen of up to 35%, unless the patient suffers nasal dryness or irritation, has been tracheostomized, or presents bronchiectasis or retention of secretions.19–21
Oxygen humidification is a measure of wellbeing that affords beneficial effects in terms of cough, mucociliary clearance and the elimination of secretions, among others. Medicinal oxygen is a cold and dry gas administered at 15°C and with an absolute humidity of 0.3mg/l. In comparison, the humidity of room air at 22°C is about 7mg/ml. As a result, when a bubble humidifier is coupled to an oxygen therapy system, the absolute humidity of the gas is incremented, reaching values of 16mg/l. Nevertheless, the ideal absolute humidity of the mixture of gases reaching the airway is 44mg/l (i.e., a relative humidity of 100%).22
This limitation of the cold bubble humidifiers has caused some Departments to adopt other active and warmed humidification systems based on water nebulization – achieving an effect similar to that of the conventional aerosols and a mixture with room air very similar to that obtained with Venturi masks. However, in these humidification–nebulization systems, since the exhaled condensed water vapor could circulate through the return tube, it does not seem advisable to use them simultaneously with different patients, in view of the plausible risk of contamination or cross-infection.23
On the other hand, despite the presence of biological agents in the oxygen humidifiers, the clinical repercussions of pathogens among patients in which contaminated systems have been used is not known, since few studies to date have evaluated the relationship between respiratory infections and the use of non-sterile or contaminated humidifiers. In this respect, the literature has focused exclusively on infections due to L. pneumophila, a bacterium known to be a sporadic and epidemic cause of community-acquired and nosocomial pneumonia – though these situations generally have been related to domestic environmental humidifiers24,25 and rarely to hospital use oxygen humidifiers.5,26,27 Since Legionella colonizes installations where water accumulates and is transmitted through the inhalation of contaminated water in aerosol form, it would seem pertinent to have conducted specific microbiological investigations of in-hospital humidifiers – though only two of the studies included in our review did so.11,13
Lastly, in addition to the methodological limitations inherent to all systematic reviews (selection and publication bias, etc.), there are some other aspects of our study that deserve comment. The main issue, which affects the external validity of the results, is related to the heterogeneous sampling methods used and the diversity of Departments in which the samples were collected. Likewise, our review did not take into account the use of high-flow systems coupled to active or warmed humidifiers, or the systems employed under conditions of invasive mechanical ventilation. As a result, the results obtained in our study cannot be extrapolated to systems of this kind.
ConclusionsDespite the limitations involved, it can be concluded that microbiological contamination of reusable bubble humidifiers is a frequent situation, even shortly after use of the device has been started, and that this situation is probably related to a lack of aseptic measures during preparation.
On the other hand, there appears to be little risk of contamination in the case of disposable (prefilled) humidifiers, even when these are used for several weeks – such devices being reusable among different patients with no risk of cross-contamination.
Although the precise clinical repercussions of the presence of microbiological contamination in oxygen humidifiers are not clear, it seems reasonable to believe that replacing reusable devices with disposable humidifiers could be the safest option.
Contributions of the authorsItxaro de la Fuente-Sancho and Oscar Romeu-Bordas participated in definition of the search strategy, the screening of titles and abstracts, the retrieval of the full text versions of the relevant articles, the selection of the studies meeting the inclusion criteria, development of the table summarizing the studies, drafting of the first manuscript version, and approval of the final version of the manuscript for publication.
Irrintzi Fernández-Aedo and Gorka Vallejo-De la Hoz contributed to the design of the investigation, review of the selection of studies meeting the inclusion criteria and the table summarizing the evidence, critical review of the manuscript, and approval of the final version of the manuscript for publication.
Sendoa Ballesteros-Peña contributed to the design of the investigation and the systematic review protocol, participated in definition of the search strategy, supervised correct conduction of the study and drafting of the final version of the manuscript, and approved the final version of the manuscript for publication.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: de la Fuente-Sancho I, Romeu-Bordas Ó, Fernández-Aedo I, Vallejo De la Hoz G, Ballesteros-Peña S. Contaminación microbiológica en humidificadores de sistemas de oxigenoterapia de alto y bajo flujo: una revisión sistemática. Med Intensiva. 2019;43:18–25.