We sought to delineate the mortality outcome time frames reported in septic shock randomized control trials (RCTs).
DesignSystematic review of PubMed, EMBASE, and the Cochrane Database of Systematic Reviews.
SettingIntensive care units.
ParticipantsStudies that included adult patients with septic shock.
InterventionsAny type of intervention.
Main variables of interestInformation about the study, specific patient population, type of study intervention, specific intervention, and number of patients. Mortality time frames were analyzed for geographical differences and changes over time.
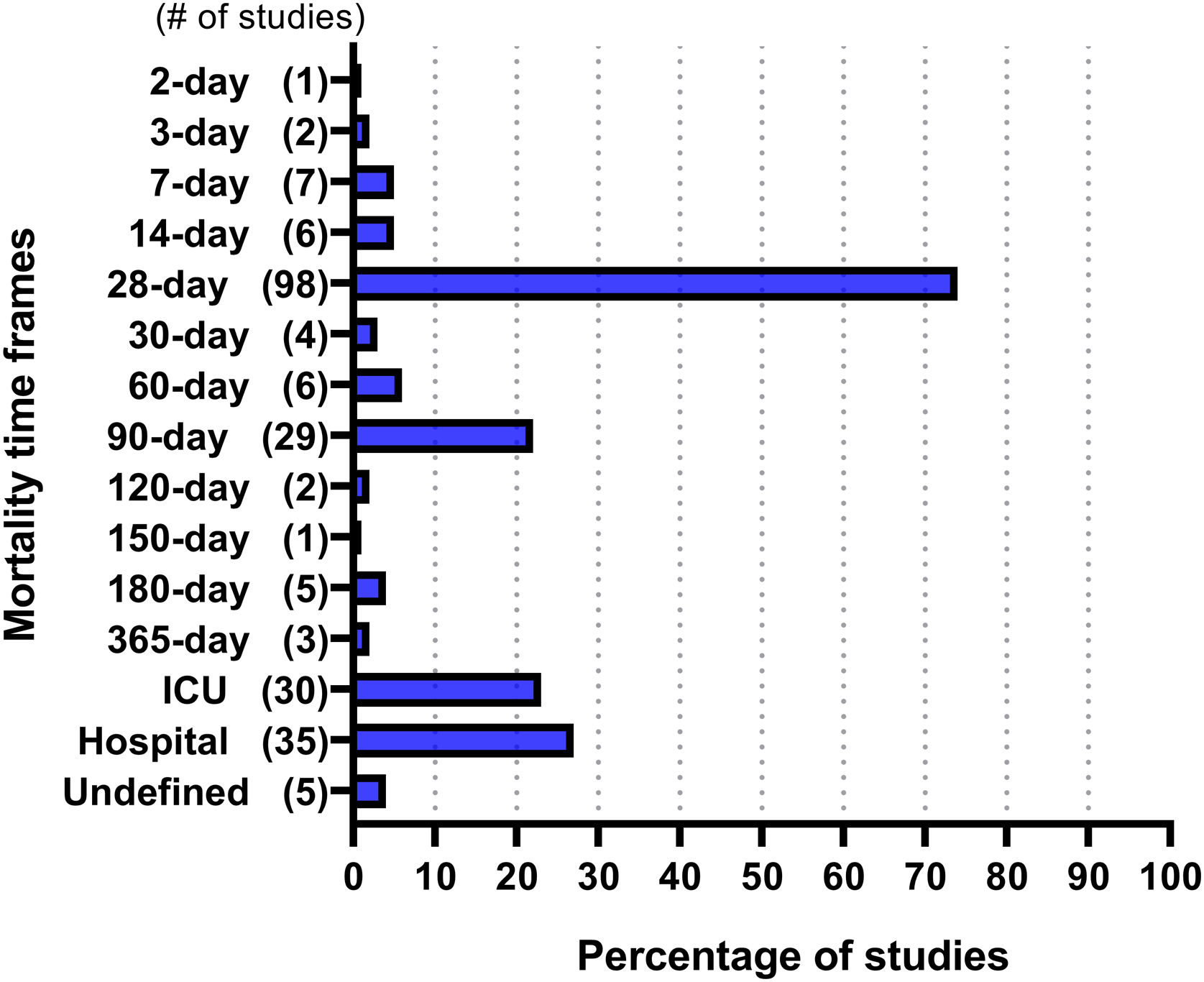
ResultsThe search yielded 2660 unique citations. After screening, 132 eligible studies were identified. A total of 234 mortality time frames were collected from the included studies, of which 15 timeframes were unique. The most frequently reported time frame was 28-day mortality (n = 98, 74% of trials), followed by hospital mortality (n = 35, 27%), ICU mortality (n = 30, 23%), and 90-day mortality (n = 29, 22%). The most reported mortality time frame was 28 days in studies from every continent except Africa. The studies published between 2008 and 2013 (25%) more frequently reported hospital and ICU mortality combination than studies published between 2014 and 2019 (11.4%) (P = 0.043).
ConclusionsThere was considerable variability in the mortality time frames reported in ICU-based septic shock trials. This variability may lead to under or overestimation of the problem, overlooking the effectiveness of the interventions studied, and further limiting the application of trials and their pooling in meta-analyses. A consensus regarding time frame reporting in septic shock trials is long overdue.
Delinear los lapsos de tasas de mortalidad reportados en ensayos clínicos aleatorizados (ECA) sobre choque séptico.
DiseñoRevisión sistemática de PubMed, EMBASE y la Base de Datos Cochrane de Revisiones Sistemáticas.
ÁmbitoUnidades de cuidados intensivos (UCI).
Pacientes o participantesEstudios de adultos con choque séptico.
IntervencionesCualquier intervención.
Variables de interés principalesPoblación, tipo de intervención y número de pacientes. Se analizaron los lapsos de mortalidad en busca de diferencias geográficas y cambios a lo largo del tiempo.
ResultadosSe encontraron 2660 citas únicas. Después de la selección, se identificaron 132 estudios elegibles. Se recopilaron 234 lapsos de mortalidad de los estudios incluidos, 15 fueron únicos. El lapso de mortalidad reportado con mayor frecuencia fue la mortalidad a 28 días (n = 98, 74% de los ensayos), seguida de la mortalidad hospitalaria (n = 35, 27%), la mortalidad UCI (n = 30, 23%) y la mortalidad a 90 días (n = 29, 22%). El lapso de mortalidad más reportado fue el de 28 días en los estudios de todos los continentes, excepto África. Los estudios publicados entre 2008 y 2013 (25%) informaron con mayor frecuencia la combinación de mortalidad hospitalaria y en la UCI que los publicados entre 2014 y 2019 (11%) (P = 0,043).
ConclusionesSe halló una variabilidad considerable en los lapsos de mortalidad reportados en los ensayos de choque séptico. Esta variabilidad puede llevar a una subestimación o sobrestimación del problema, pasando por alto la efectividad de las intervenciones estudiadas y limitando aún más la aplicación de los ensayos y su agrupación en metanálisis.
Septic shock is sepsis resulting in organ dysfunction through metabolic derangement and hypotension refractory to fluid therapy.1 It represents 20% of all deaths globally and is associated with a 60% mortality rate at 6 months.2,3 Considerable efforts have been made to lessen the impact of sepsis and septic shock; however, there is no approved sepsis-specific medication to date.4,5 Several randomized controlled trials (RCTs) have assessed different treatments for septic shock without clear success in reducing mortality.6 Recent systematic reviews have investigated mortality in patients with severe sepsis and septic shock,7–9 but numerous studies were excluded from these analyses because they did not report a 28-day mortality rate endpoint. Reporting inconsistency of mortality endpoints, and in some cases questionable time frames, can create selection biases that affect external validity of these analyses, clinical practice, or research.6
A preliminary exploration of the literature performed by our group showed heterogeneity in the mortality time frames reported in RCTs,10 with 28-day mortality being the most frequently reported endpoint in RCTs studying critically ill patients with sepsis. The present systematic review was designed to further delineate the mortality time frames used by septic shock RCTs, to identify differences between geographical regions, publication types, and changes over the past decade.
MethodsProtocol and registrationThis systematic review followed the Cochrane methodology.11 The protocol was registered in the International Prospective Register of Systematic Reviews, #CRD42018107855. This report is based on the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.12
Eligibility criteriaRCTs enrolling adult patients with septic shock and published between January 2008 and August 2019 were included. Only articles that had at least an abstract in English were included in the final analytical cohort. We excluded studies published before 2008 to reflect current practices in the design of clinical trials. Studies of “severe sepsis” interventions that included patients with “septic shock” as a subgroup and reported mortality outcomes for this subgroup were included in the review. Since many relevant studies that are relevant to our systematic review were already designed or published using the Sepsis-2 definitions, we decided to retain the term severe sepsis in our inclusion criteria. In order to get a complete picture of the literature, both full articles and conference abstracts were included. Basic science studies, protocols, commentaries and opinions, observational studies, secondary analyses, and studies that included pediatric patients were excluded. If mortality was listed as an outcome but the mortality rate was not included in the text, figures, or tables, the study was excluded. Studies with the same authors, affiliations, and enrollment periods were excluded from the analysis to avoid double counting potentially duplicate data.
Information sources and search strategyA comprehensive literature search was conducted by a professional librarian (Cl F). Electronic databases, including PubMed, EMBASE, and the Cochrane Database of Systematic Reviews were searched on August 2019. The terms used were “septic shock” and its synonyms, combined with the term “mortality” plus the term “randomized controlled trial” and their synonyms. The full search strategy and included terms can be found in the Appendix. Duplicates and overlapping results were identified and removed in the title screening phase. The time frames of studies with authors in common were checked against one another to confirm that they were not the same study. Conferences proceedings were also assessed as part of the grey literature evaluation.
Study selection processTwo independent investigators (PM and JAC) individually screened the titles and abstracts. Relevant studies were moved to full-text screening, only articles that had at least an abstract in English were included in the final analytical cohort. The platform Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) was used to manage the screening process. Any disagreements were resolved by consensus between the investigators.
Data collection processTwo investigators (PM and JAC) independently abstracted information from the included studies using a standard table. Afterwards, the results were compared, and when disagreements arose, a consensus was reached between the two abstractors and a third investigator (AL).
Data itemsTitle, first author, year of publication, country of study, publication type, specific patient population, type of study intervention, specific intervention, and number of patients in each arm were extracted. The primary outcome was the mortality time frame reported in RCTs enrolling critically ill patients with septic shock. Secondary outcomes included the frequency of each reported mortality time frame across continents and changes in the reported time frames across the study period (2008–2019). Mortality rates not specified by time frame in the article were categorized as “undefined.” Time points from survival curves derived from Kaplan–Meier analysis were not estimated or extracted.
Risk of bias assessmentIncluded studies were assessed by two authors independently (PM, JAC, AL) for potential risk of bias using the Cochrane Risk of Bias tool version 2. All studies were ranked as low risk, high risk, or unclear in seven items: random sequence generation, allocation concealment, selective reporting, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and other bias. Conflicts in grading risk of bias were resolved through discussion. Most conference abstracts were classified as “unclear” because character limits prevented full disclosure of the methods used to reduce bias.
Synthesis methodsWe reported the number and percentage of studies that assessed mortality time frames by continent and study publication type. Studies were grouped by publication types in: abstracts or full-text articles, and high or low-impact factor (IF) studies. For studies that had an IF and that were published as full-text the IF of the journal on the year of publication was recorded. To classify the studies into high or low IF we calculated the IF quartiles. RCTs with an IF within the first or second quartile were considered of high IF. Studies published between 2008–2013 and 2014–2019 were compared to assess changes over time. Descriptive statistics were used to describe the frequencies of the reporting of the different time frames and the most frequent combinations. Differences among continents and 5-year publication periods were assessed using the Fisher exact test, chi-squared test, or Mann-Whitney U test as appropriate. P values <0.05 were considered significant. Statistical analyses were performed using GraphPad Prism v8.0 (San Diego, USA) and IBM SPPS Statistics, version 25 (SPSS Inc., Chicago, USA).
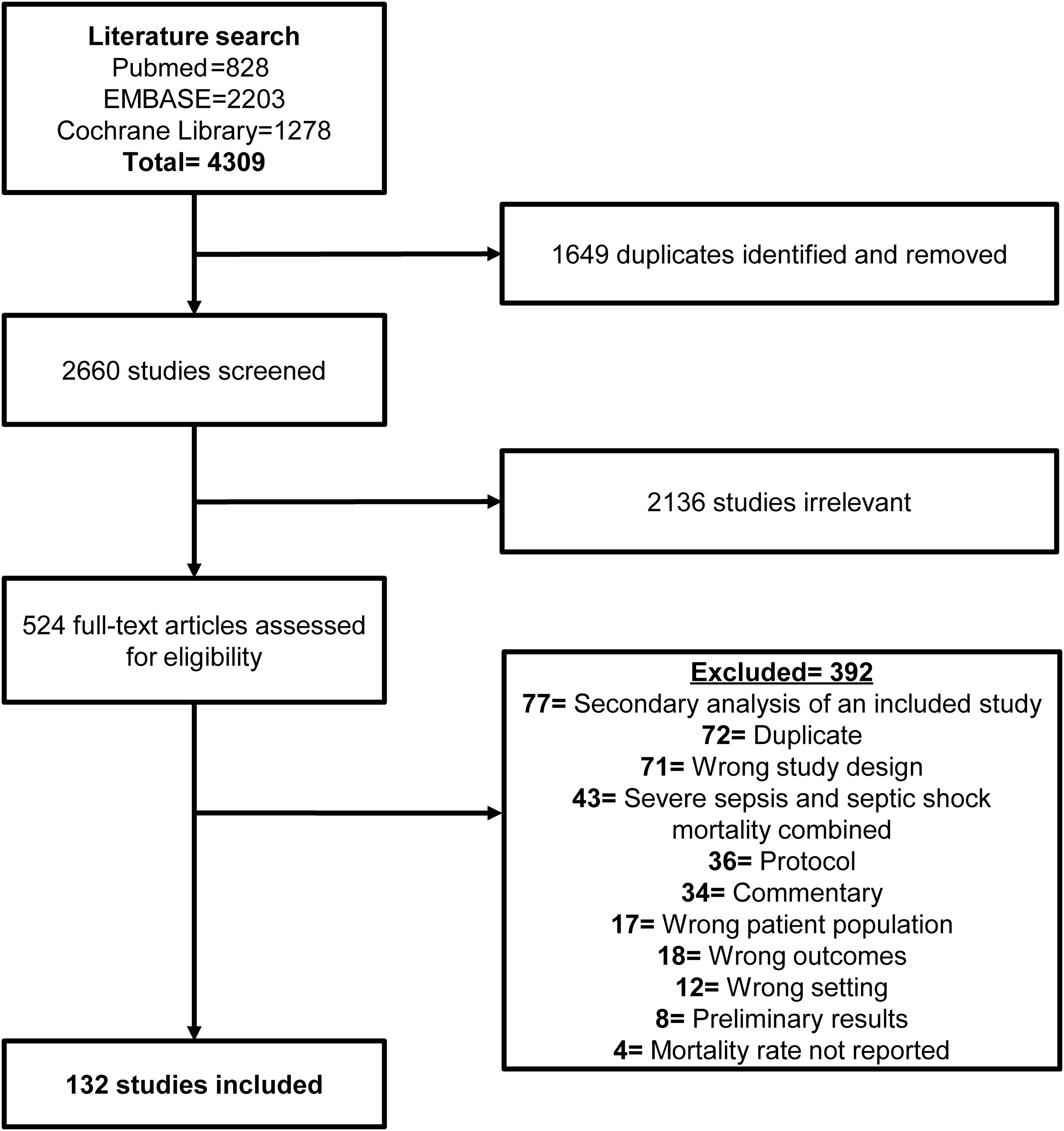
ResultsStudy selectionWe found 4309 citations (Fig. 1), of which 524 were reviewed in full text. and 132 included for data extraction.13–144
Study characteristicsThe characteristics of the included RCTs are presented in the Supplemental Table S1. A total of 34,465 patients were included in the studies. Most of the studies included were published in English as full articles (88, 67%); the remaining 44 (33%) were published in English as abstracts. Only 61 (46%) RCTs focused on a specific subpopulation of septic shock patients. In the 132 studies, the most common subpopulations were patients receiving invasive mechanical ventilation (10%), patients with early septic shock (6%), and patients with refractory septic shock (6%).
Risk of bias assessmentThe quality assessments are presented in Supplementary Fig. S1. Although blinding of participants and personnel was not performed in 33% of the included studies, only 25% were considered to have a high risk of detection bias. The lack of blinding to outcome was not deemed a detection bias since the reported mortality time frames were not likely to be influenced by blinding.
Frequency of mortality time framesAmong the 132 included RCTs, 234 mortality time frames were identified. Fig. 2 provides the frequency at which the included studies reported each mortality time frame. Overall, of the 234 identified time frames, the 28-day, hospital, intensive care unit (ICU), and 90-day mortality rates were the most commonly used, accounting for 74%, 27%, 23%, and 22% of the reported mortality time frames, respectively. Most studies reported one time frame (57%), followed by two (23%), and three (12%).
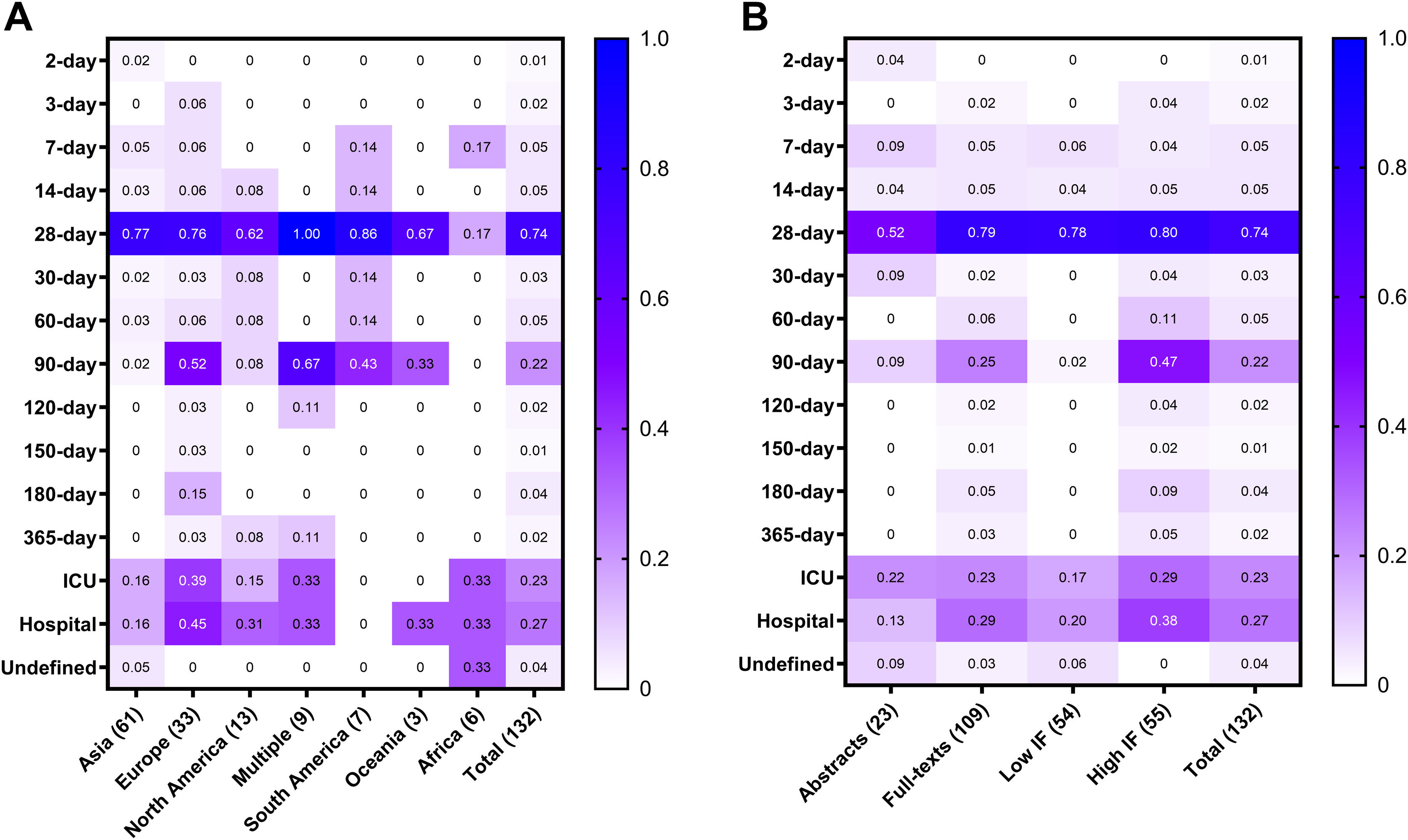
Mortality time frames by continent, study type and yearJust under half of the studies were carried out in Asia (n = 61, 46%), followed by Europe (n = 33, 25%), North America (n = 13, 10%), South America (n = 7, 5%), Africa (n = 6, 5%), Oceania (n = 3, 2%), and across multiple continents (n = 9, 7%). Supplementary Table S2 summarizes the characteristics of the included studies by continent.
The most commonly reported mortality time frame was 28 days for every continent except for Africa, where ICU mortality, hospital mortality, and an ‘undefined’ time frame were more frequently reported (Fig. 3A). Reporting of 28-day mortality ranged from 17% in African studies to 100% in transcontinental studies. Similarly, there was reporting heterogeneity by publication type (Fig. 3B). For instance, more full-text publications reported 28-day time frame as compared to only-abstract publication (79% vs. 52%), and less than 50% of the studies published in high IF journals reported the Hospital (38%) and 90-day (47%) mortality rates, which were the second and third most common timeframes overall.
Frequencies of reported mortality time frames by continent (A) and by study type (B). Frequencies are measured from 0% (0) to 100% (1.0). Overall, 74% of the studies reported 28-day mortality; however, heterogeneity between continents was observed (A). For instance, 17% of the 6 studies conducted in Africa reported 28-day time frame, while 100% of the 9 multi-continent studies reported 28-day mortality. The mortality heterogeneity was also present regardless of the study type (B) such as abstract, full-text, published in a high or low impact factor (IF) journals. For example, even though 90-day was the second most commonly reported time frame in high IF studies, it was reported in less than 50% of those trials.
Because multiple mortality time frames were reported by several studies, we evaluated the frequency in which studies reported more than one of the most common time frames (28-day, hospital, ICU, and 90-day mortality) (Supplementary Tables S3–S6). Overall, 22 studies (17%) reported both 28-day and hospital mortality, whereas 25 studies (19%) reported both 28-day and 90-day mortality. Compared to other regions, studies from Europe and those spanning multiple continents more often reported more than one time frame.
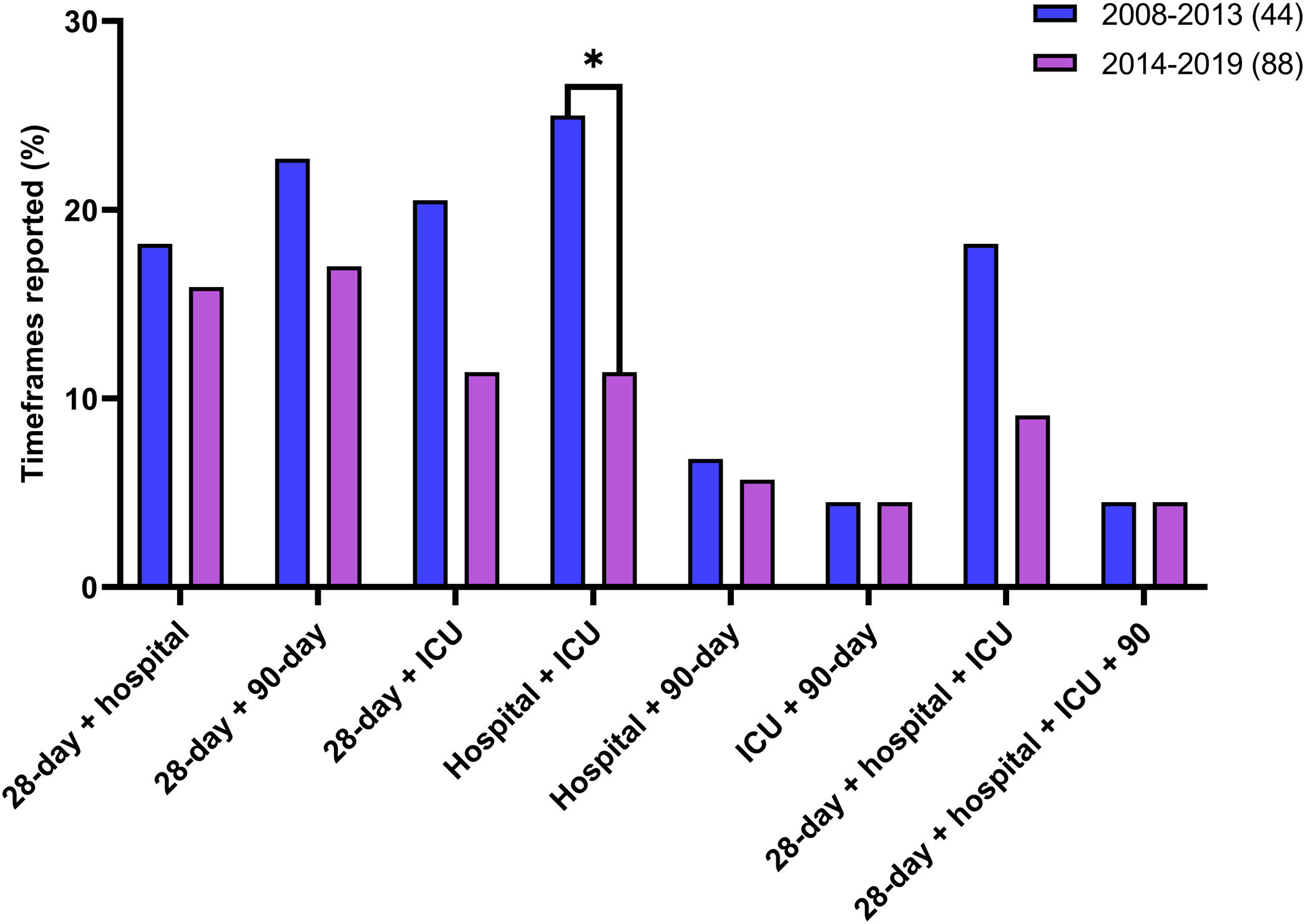
There were 44 studies published between 2008 and 2013 and 88 studies published between 2014 and 2019. No difference was observed between these two periods in the median number of time frames reported per study (1.5 [IQR, 1–2.75] versus 1 [IQR, 1–2], P = 0.21). The studies published between 2008 and 2013 more frequently reported two or more of these common time frames (Fig. 4); in particular, the hospital and ICU mortality combination was reported significantly more than in studies published between 2014 and 2019 (P = 0.043).
DiscussionIn this systematic review of mortality outcome data extracted from septic shock RCTs published between 2008 and 2019, 28-day mortality was the most commonly reported mortality outcome overall, followed by hospital mortality, ICU mortality, and 90-day mortality. There was, however, substantial interregional variability in the time frames selected for reporting. Few studies from North America, Africa, and Asia reported 90-day mortality as an outcome, whereas 40%–60% of the studies from Europe, South America, and those from multiple countries included this time frame. The variability was also present regardless the study publication type (full-text vs. abstract) and IF.
These results highlight considerable discrepancies in outcome reporting exposing the lack of consensus between clinicians and investigators despite decades of sepsis research. The importance of homogeneity among reported RCTs outcomes and the clinical implications cannot be overstated. Short-term mortality time frames can lead to underestimation of the real problem; at the same time, reports such as 90-day mortality can lead to misallocation of the cause of death or failing to recognize effective therapies due to confounding.145 This reporting variability also reduces the accuracy of meta-analyses limiting our ability to extract meaningful conclusions from the pooled data of septic shock patients.
Although sepsis mortality has been decreasing,6,146 approximately 25% of patients with sepsis are still expected to die within 28 days.6 Because a predefined outcome measure is required to establish whether an intervention is effective, mortality rate is a commonly used endpoint in RCTs of therapeutic interventions for sepsis. Nonetheless, as Vincent suggested in 2004,147 the ideal time at which mortality should be recorded remains uncertain. Many licensing authorities, including the US Food and Drug Administration, consider 28-day all-cause mortality to be a germane time frame for mortality assessments, as this endpoint counterbalances the time needed to assess a given intervention’s impact on mortality against the time in which unrelated events could cloud and confound this impact.147,148 The original sepsis consensus paper published 30 years ago also acknowledged the lack of well-defined endpoints and advocated for the reporting of 28-day and in-hospital mortality.149 Nonetheless, the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis 3) study developed its diagnostic criteria using only in-hospital mortality as a primary outcome.150,151 Furthermore, the current Surviving Sepsis Campaign guidelines do not suggest any specific mortality time frame for explorations of the efficacy of novel treatments for sepsis and septic shock.4 These discrepancies highlight the lack of widely accepted consensus on mortality time frame reporting and sheds light on the need for more standardized reporting.
Different approaches may be considered to establish an optimal time frame for mortality reporting in septic shock RCTs. Early mortality time frames (14 days or less) may be the most suitable endpoints to reflect mortality directly related to septic shock and assess the impact on survival of a studied therapeutic intervention. However, because patients with sepsis and septic shock have a reduced mean life span and a higher risk for long-term mortality,152 a shorter time span for evaluation would leave unrecognized a given intervention’s potential effect on longer-term survival. That said, only 16 (12%) of the included studies reported at least one early mortality endpoint.
Arguments exist for the presentation of alternative mortality endpoints, such as 90-day, 180-day, 6-month, and 12-month mortality rates. While these longer time spans may render the links between interventions and mortality rates more tenuous,148 some authors have questioned the justification for interventions that significantly impact 28-day mortality but do not affect mortality by at least the 3-month mark.147 At longer time points, when the primary insult of septic shock has been overcome, mortality rates may reflect non-sepsis-related causes of death (for instance, cardiovascular disease, stroke, or heart failure) or may reflect the effects of subsequent septic events, such as nosocomial infections, during the ICU or hospital stay.153 This consideration is of paramount importance, as temporality is a basic principle of causal inference and is lost when a late outcome is measured as the effect of an intervention.154 Nevertheless, an intervention that may lead to early recovery or rehabilitation may still impact long-term mortality outcomes. If these outcomes are not measured in clinical trials, a relevant amount of disease information may be ignored. In addition, differential mortality rates may be present among different populations of ICU patients. Assessment of long-term mortality outcomes may thus elucidate the impacts of an intervention in patients with different baseline status (e.g., medical, surgical, oncological).155–159
There are additional key points worthy of discussion. First, it is crucial to consistently define the beginning of the mortality time frame (i.e., when counting begins) when assessing the impact of septic shock on mortality or evaluating the impact of a treatment in an RCT. As stated in the 1992 sepsis consensus paper,149 “the interval between fulfillment of entry criteria and administration of experimental intervention…should be noted” because a lack of clear information on this subject may introduce lead-time bias. Machine learning models for the early detection of sepsis have at times identified a condition consistent with sepsis hours prior to its recognition in the emergency department and ICU, suggesting that, both in patients arriving with clinical sepsis and those developing it while in the hospital, providers may already be working against a significant delay.160–162 Second, the reporting of the causes of death in clinical trials is of paramount importance, as it allows researchers to understand the real effect of an intervention and may shed light on potential long-term events that influence survival and merit further research. A step in that direction was offered by Moskowitz and colleagues,163 who proposed five cause-of-death categories and reported their frequency among patients with sepsis and septic shock. Nonetheless, classifying the cause of death in these patients is challenging, as illustrated by the moderate concordance between the three independent experts in that study.163
With the increasing research emphasis on precision medicine, new sepsis therapies are likely to be developed in the coming years. In actual practice and under research conditions, subpopulations of patients are typically heterogeneous. For this reason, mortality analyses should also consider patient-level differences, not only with regard to clinical and study-related factors, such as patients’ underlying conditions, shock severity, sepsis origins, country of study, or causative pathogen, among others, but also with regard to molecular-level differences. Such studies would illustrate the inherent clinical variability among septic shock patients and septic shock outcomes, which would further justify the use of consistent outcome durations.164,165 For instance, a recent study in Spain with more than 2000 ICU patients identified 3 different types of sepsis secondary to COVID-19 with distinct mortality rates.166 These differences will likely impact the design of clinical trials in the near future.167
This study has limitations. First, we did not divide mortality outcomes into primary or secondary outcomes; thus, we were unable to determine the priority authors gave to their studied outcomes. Second, while we extracted specific data from each study, including the type of intervention and patient characteristics, we were not able to collect information addressing why authors elected to measure one outcome over another. For this reason, factors driving the included trials’ endpoint selections could not be analyzed, representing a gap that requires further attention. Third, our study did not explore the mortality time frames based on study phase or number of patients which could play a role in the variability observed. Fourth, the inclusion of abstracts could also have skewed our findings. Finally, we did not include evidence that emerged during the COVID-19 pandemic because many COVID-19-related studies used an expedited approach that might not reflect the evidence-generating practices used in the sepsis literature over the last decade.
While 28 days was the most widely used time frame for mortality reporting among RCTs involving ICU patients with septic shock, this systematic review found considerable variability in outcome reporting of mortality outcomes. This variability may lead to under or overestimating the problem, failure to recognize the effectiveness of the interventions studied, and further limiting the applicability of RCTs findings and their pooling in meta-analyses. Given the burden of sepsis worldwide, reaching a consensus regarding the mortality time frames in septic shock trials is of paramount importance and long overdue. This consensus should also consider the relevant characteristics of each studied population.
CRediT authorship contribution statementPM, JAC, AL, and JLN conceived the study. ClF developed the search strategy and performed the literature search. PM, JAC, AL, CoF, and NM did the study selection and data extraction for the systematic review. JAC performed the formal analysis. PM, JAC, AL, JB-C, and JLN wrote the first draft of the manuscript. Both AL and JAC contributed equally and have the right to list their name first in their CV. All authors contributed to the interpretation of data and critical revision of the manuscript and approved the final manuscript. All authors confirm the accuracy and integrity of the work.
Consent to participateNot applicable.
Ethical approvalNot applicable.
Code availabilityAvailable upon reasonable request.
Meeting presentationPreliminary data used in this study were presented virtually at the Society of Critical Care Medicine’s 51th Critical Care Congress; April 2022.
Declaration of Generative AI and AI-assisted technologies in the writing processNo generative AI or AI-assisted technologies were used in the writing process.
FundingThis work is supported by The University of Texas MD Anderson Cancer Center Grant Resources, and the National Institutes of Health/National Cancer Institute under award number P30CA016672. Dr. Lopez-Olivo’s work is supported by the National Cancer Institute (CA237619). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materialData will be available upon reasonable request.
We appreciate the editorial contributions made by Amy Ninetto of the Research Medical Library at The University of Texas MD Anderson Cancer Center.