During the recent COVID-19 pandemic, non-invasive respiratory support has played a crucial role1 in the management of patients with acute hypoxemic respiratory failure.
The best therapeutic option for these patients has always been a matter of discussion.2 Compared to traditional CPAP based non-invasive mechanical ventilation or the use of 2 different levels of pressure, oxygen therapy administered through high-flow nasal cannula (HFNC) has been gaining popularity probably due to how easy it is to use, its high tolerability, and the possibility of applying it outside the ICU setting.3 All these qualities made it an attractive therapeutic option within the first difficult days of the pandemic. However, scientific evidence that backed its use was insufficient.4 This triggered our former letter where we claimed, at least temporarily, a tie in this match between both non-invasive respiratory supports.
In our own opinion, the evidence generated by the RECOVERY-RS5 trial has turned the tide in favor of non-invasive mechanical ventilation (NIMV). In sports terminology «tie has been undone at the overtime».
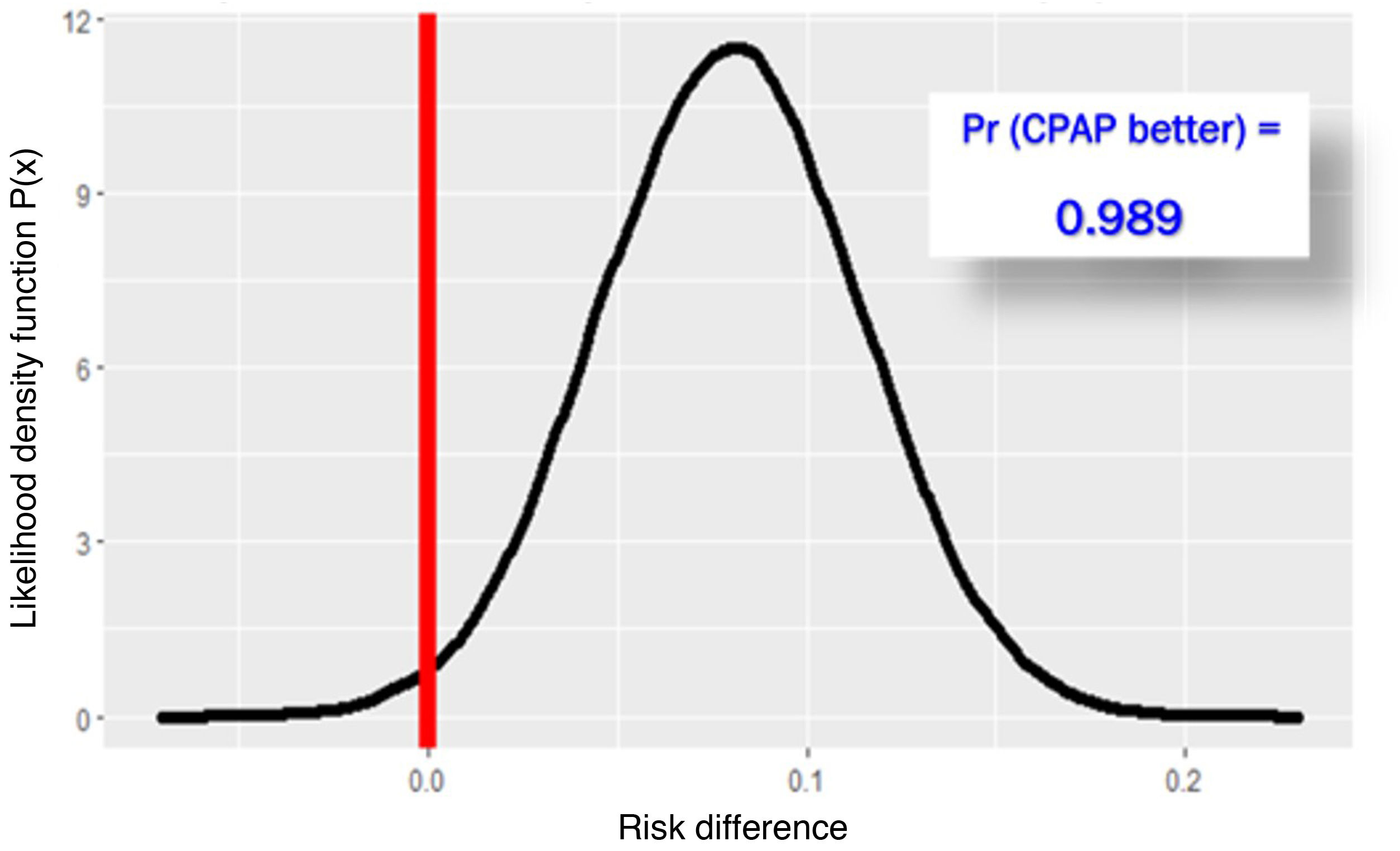
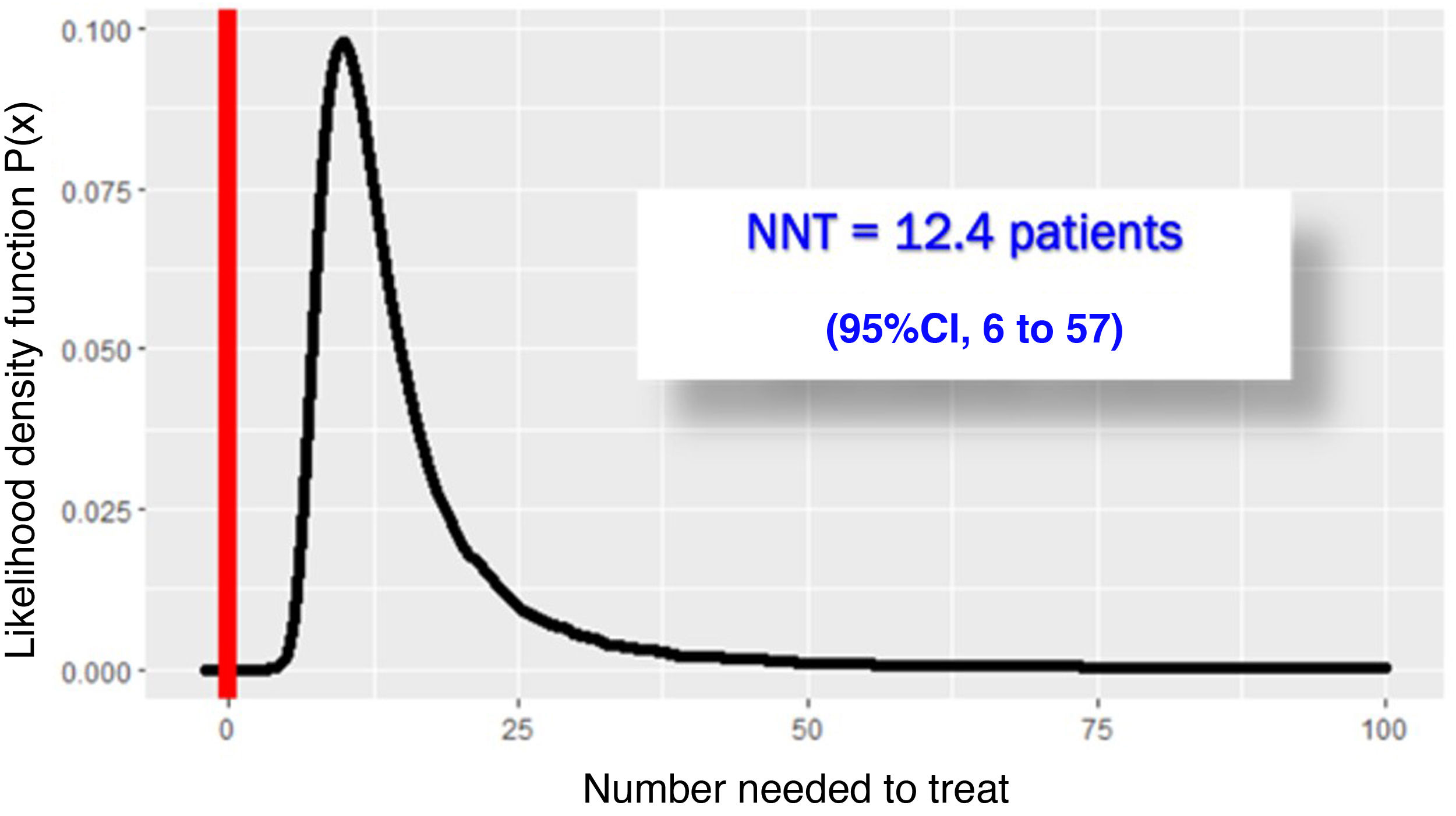
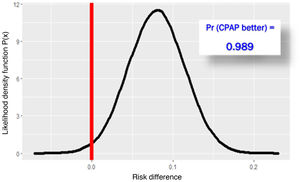
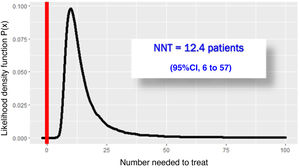
The RECOVERY-RS trial was conducted from April 6, 2020 through May 3, 2021 in 48 hospitals from the United Kingdom and Jersey. Patients were randomized to receive CPAP (N = 380), high-flow nasal oxygen (N = 418) or conventional oxygen therapy (N = 475). To maximize performance given the harsh conditions of the pandemic, the study design is a little special. The study consists of 2 parallel randomized clinical trials that share the same control group: in NIMV only capable hospitals, patients were randomized to receive CPAP or conventional oxygen therapy. In HFNC only capable hospitals, however, patients were randomized to receive HFNC or conventional oxygen therapy. In hospitals with the 3 ventilation systems available, randomization occurred among the 3 groups. Primary endpoint was a composite of orotracheal intubation or 30-day mortality. In the 1273 patients studied, the need for orotracheal intubation or 30-day mortality was significantly lower with CPAP (137/377 = 36.3%) compared to conventional oxygen therapy (158/356 = 44.4%), an absolute difference of −8% (95%CI, − from 15% down to –1%); P = .03. However, the difference between HFNC (184/415 = 44.3%) and conventional oxygen therapy (166/368 = 45.1%) was not statistically significant, an absolute difference of −1% (95%CI, from 8% to 6%); P = .83. Due to the randomization-generated comparison and assuming interchangeability between both control groups, Bayesian analysis with the beta-binomial model using a non-informative prior distribution confirmed that the chances of CPAC exceeding HFNC are 0.988 (Fig. 1) with a number needed to treat of 12.4 patients (95%CI = 6–52) (Fig. 2).
To improve the quality of our healthcare we can assess the meaning of these results by applying these findings to our own data. To perform this easy analysis, we conducted consecutive sampling of patients included in the registry of patients with COVID-19 from our ICU—after approval by the local research ethics committee and after obtaining the patients or their legal representatives’ informed consent—from March 2020 through March 2022 with the diagnosis of moderate or severe hypoxemic respiratory failure. In 788 (88.14%) out of a total of 894 patients with SARS-CoV-2 infection confirmed the reason for ICU admission was acute hypoxemic respiratory failure. We decided to use HFNC on 477 of these patients (53.4%) in our ICU as a first-line respiratory support therapy. The 30-day mortality rate of these patients was 7.33%, and the composite endpoint of orotracheal intubation or 30-day mortality appeared in 263 patients (55.13%). Ceteris paribus, if we had used CPAP instead of HFNC in these 477 patients, we would have had 40 fewer patients (95%CI = 8–79) in whom we would have had to use intubation or who would have died otherwise.
With the knowledge generated by the RECOVERY-RS trial, tie has been definitively undone. The abuse of HFNC and the lack of rigorous management—based on the scientific evidence available—of non-invasive ventilatory support devices will lead HFNC to dying from its own success without any benefits for the patients whatsoever.
FundingNone whatsoever.
Conflicts of interestNone reported.