We present some of the most important developments in advanced life support incorporating the new international recommendations for resuscitation 2010. The study highlights aspects related to prevention and early detection of in-hospital cardiac arrest, resuscitation in the hospital, the new advanced life support algorithm, the techniques and devices for cardiopulmonary resuscitation, post-resuscitation care, assessment of the prognosis of patients who survive initially, and specific aspects of non-beating heart organ donation and the creation of cardiac arrest referral centers.
Se presentan a continuación algunas de las novedades más importantes en soporte vital avanzado que incorporan las nuevas recomendaciones internacionales en resucitación de 2010. Se destacan los aspectos relacionados con la prevención y detección precoz de la parada cardiaca intrahospitalaria, la resucitación en el hospital, el nuevo algoritmo de soporte vital avanzado, las técnicas y dispositivos de resucitación cardiopulmonar, los cuidados posresucitación, la valoración del pronóstico de los pacientes que sobreviven inicialmente a la parada y aspectos específicos relativos a la donación de órganos a corazón parado y la creación de centros de referencia de parada cardiaca.
In October 2010, the journals Resuscitation1 and Circulation2 simultaneously published the guides of the ERC (European Resuscitation Council) and of the AHA (American Heart Association) relating to cardiopulmonary resuscitation (CPR). These guides offer an update to the previous guidelines, published in the year 2005, and are based on the more recent International Consensus on Resuscitation Science and Treatment Recommendations (CoSTR). In this context, the new guides incorporate the results of systematic reviews, involving strict methodological criteria, corresponding to over 270 topics related to CPR and prepared by over 300 international experts.
These reviews of the literature, carried out to offer answers to the questions raised by each of the working groups of the International Liaison Committee on Resuscitation, were prepared using a standardized worksheet that included a grading system designed to define the level of evidence of each study. The International Consensus Conference, held in Dallas in February 2010, and its published conclusions and recommendations, constitute the basis of the ERC guides of 2010. Although the guides are derived from the CoSTR document of 2010, they represent consensus among the members of the Executive Committee of the ERC. The Committee considers that these new recommendations are the most effective and easiest to learn interventions supported by the current state of knowledge, research and experience.
The current guides on advanced life support (ALS) recommend some changes with respect to the previous guidelines.3 These changes are described in the present article, together with the reasons for such changes. However, many of the recommendations in the ERC guides of 2005 remain without change in the year 2010, either because no new studies have been published, or because the new evidence generated since 2005 simply reinforces the already existing evidence. In addition, the current universal algorithm for advanced life support (ALS) is presented.
The changes in the recommendations are presented divided into the following sections:
- 1.
Prevention of in-hospital cardiac arrest (CA)
- 2.
Resuscitation in the hospital
- 3.
Simplified ALS algorithm
- 4.
CPR techniques and devices
- 5.
Post-resuscitation care
- 6.
Prognosis
- 7.
“Non-heart beating” donors and reference centers in CA
The current recommendations stress the importance of the early identification of those hospitalized patients who are experiencing a worsening of their condition, and of the possibility of avoiding progression towards cardiac arrest (CA)-thus defining prevention of the latter as a first link in the chain of survival.
In order to prevent in-hospital CA, hospital centers should incorporate a care system including the following elements: (a) training of the healthcare personnel to recognize the signs of patient worsening and the reasons for offering a rapid response to the disease; (b) adequate and regular monitorization of the vital signs of hospitalized patients; (c) clear guidelines (e.g., based on call criteria or warning or alarm sign scores) to help the personnel in the early detection of patient worsening; (d) a uniform and clear system for requesting help; and (e) a clinical response to calls for help that is both appropriate and on time.4
The following strategies are proposed for preventing avoidable in-hospital CA:
- 1.
The provision of care for critical patients or patients at risk of clinical deterioration in the appropriate hospital areas.5 In other words, care should be provided adapted to the seriousness of the condition of each individual patient.
- 2.
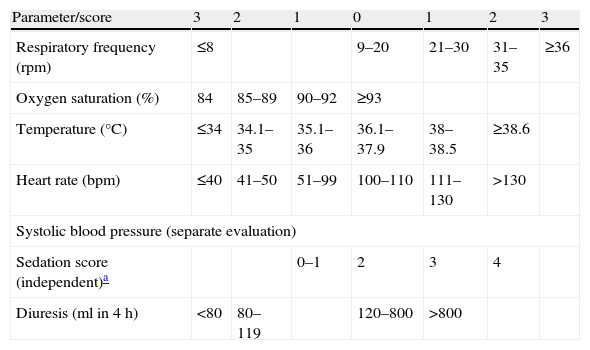
Each patient should have a documented plan for monitoring of the opportune vital signs, identifying the variables to be measured and the frequency of measurement, in accordance to the severity of the disease or the probability of clinical worsening. The guides suggest the monitorization of simple physiological variables (heart rate, blood pressure, respiratory frequency, level of consciousness, temperature and SpO2).6–8 A data collection sheet should be used, allowing regular measurement and registry of the vital signs, and of the early warning scores, when used.
- 3.
An alarm and tracing system (“call criteria” or early warning system) should be used to identify patients in critical condition and/or at risk of clinical worsening and CA.9Table 1 shows an example of early warning score for the identification of critical patients, based on the evaluation of multiple vital signs.10
- 4.
An adequate clinical response to alterations in physiological parameters should be provided, based on the alarm and tracing system used.
- 5.
The hospital should have a clearly identified response to critical disease. This may include the designation of a resuscitation team capable of responding in an adequate period of time to the acute clinical situations identified by the alarm and tracing system or other indicators. This service should be available 24h a day. The team should include professionals capable of resolving situations requiring acute or critical patient care.
- 6.
All the clinical personnel should be trained to identify, monitor and manage critical patients, and must know their role in the rapid response system.
- 7.
Identification is required of those patients in which CA represents a foreseeable terminal event, of those subjects in which CPR is inappropriate, and of those patients who do not wish to receive CPR.11 Accordingly, hospitals should have an “orders not to start CPR” policy, based on national guides that are understood by all clinical personnel members.
- 8.
Auditing should be ensured of CA, “false arrests”, unexpected deaths and non-expected admissions to the ICU, making use of common databases. The antecedents and clinical responses of such events must also be audited.
Modified early warning score.10 Vital signs to evaluate.
| Parameter/score | 3 | 2 | 1 | 0 | 1 | 2 | 3 |
| Respiratory frequency (rpm) | ≤8 | 9–20 | 21–30 | 31–35 | ≥36 | ||
| Oxygen saturation (%) | 84 | 85–89 | 90–92 | ≥93 | |||
| Temperature (°C) | ≤34 | 34.1–35 | 35.1–36 | 36.1–37.9 | 38–38.5 | ≥38.6 | |
| Heart rate (bpm) | ≤40 | 41–50 | 51–99 | 100–110 | 111–130 | >130 | |
| Systolic blood pressure (separate evaluation) | |||||||
| Sedation score (independent)a | 0–1 | 2 | 3 | 4 | |||
| Diuresis (ml in 4h) | <80 | 80–119 | 120–800 | >800 | |||
Report if score≥4.
Sedation score:
0: Awake/alert
1: Asleep, responds to stimuli
2: Mild: occasionally drowsy, easily awakened
3: Moderate: often drowsy, easy to awaken but cannot keep awake
4: Severe: difficult to awaken
When CA occurs in the hospital, the division between basic life support (BLS) and advanced life support (ALS) is arbitrary; in practice, the resuscitation process is a continuum and is based on common sense applied to each concrete situation.
Cardiac arrest must be identified immediately, and help must be requested using a pre-established standard telephone number. CPR is to start immediately, using airway accessories (e.g., pocket masks) and, where indicated, defibrillation should be performed as soon as possible (in all cases within 3min after CA).
The precise sequence of actions after in-hospital CA depends on many factors, including:
- -
The location where arrest occurs (clinical/non-clinical area, monitored/non-monitored area). Patients with monitored arrest are generally diagnosed quickly. In contrast, patients in wards may have suffered a period of worsening and non-witnessed arrest. Ideally, all patients at high risk of suffering CA should be attended in a monitorized area with the availability of immediate resuscitation measures.
- -
Training of the first responders, and the number of responders. In principle, it is advisable for all healthcare professionals to be able to recognize CA, call for help and start the CPR maneuvers. Each hospital healthcare professional should do what he or she has been trained to do, since there may be different levels of training and skill in dealing with the airway, breathing and circulation. Thus, the resuscitators should undertake only those activities in which they have been trained and are competent. When there is only one responder, he or she must ensure that help has been called for and is underway. If several professionals are available, different actions can be taken simultaneously.
- -
Available equipment and hospital response system for CA and medical emergencies. All clinical areas should have immediate access to the resuscitation equipment and to drugs allowing rapid patient resuscitation. The equipment to be used in CPR (including defibrillators), its distribution, and the medication should be standardized throughout the hospital.12 The resuscitation team may take the form of a conventional CA team, which is notified only when a case of CA has been identified. Alternatively, however, hospitals may have strategies for identifying patients at risk of CA and for calling or alerting a team, e.g., the medical emergencies team, before CA actually occurs. In-hospital CA is rarely sudden or unexpected; a strategy that includes the identification of patients at risk of CA may be able to prevent some of these arrests, or may contribute to avoid futile resuscitation attempts in patients who are unlikely to benefit from CPR.
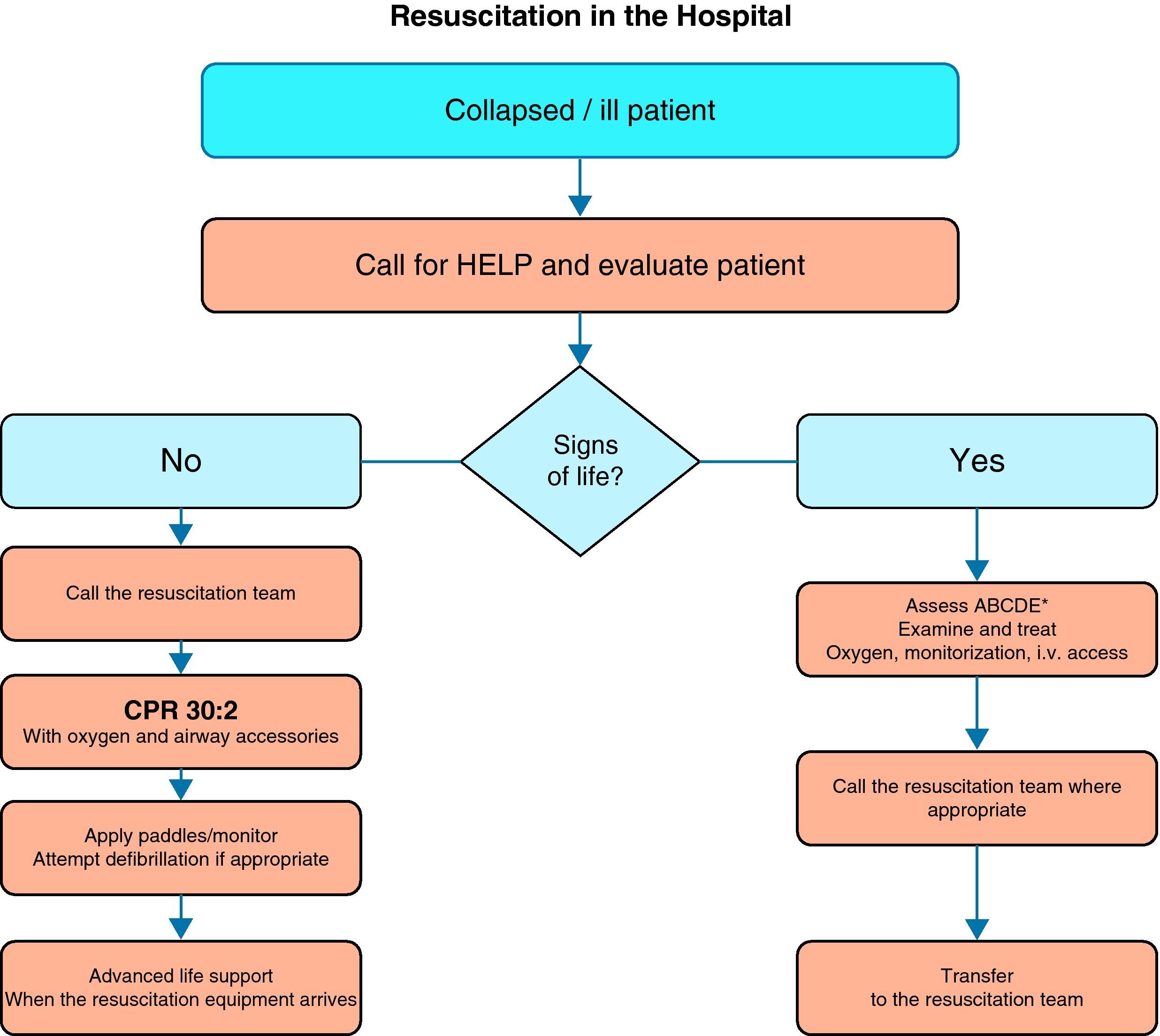
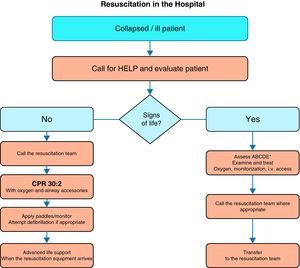
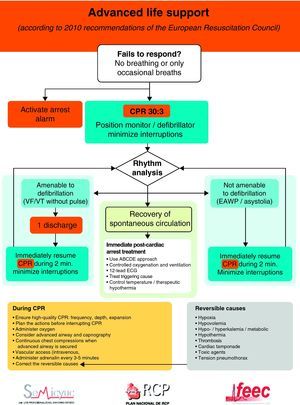
Fig. 1 shows the general algorithm for the initial management of in-hospital CA:
- -
Ensure the safety of the personnel dealing with the arrest.
- -
Check whether the patient responds.
- -
When the healthcare personnel witness patient collapse or find an apparently unconscious patient in a clinical area, the first thing to do is call for help, and then determine whether the patient responds. The patient should be shaken at the shoulders and asked out loud: “Are you OK?”
- -
If there are other healthcare personnel members nearby, simultaneous actions can be carried out.
If the patient responds, urgent medical evaluation is required, in accordance with the acute critical patient care protocol applied in each hospital center. While the arrival of help is being awaited, oxygen should be administered, with monitorization and the insertion of a venous catheter.
If the patient fails to respond, the precise sequence of actions will depend on the training of the personnel attending the patient, and on their experience in evaluating breathing and circulation. It must be taken into account that healthcare personnel, even when trained, may not assess breathing and pulse reliably enough to confirm CA. Agonal breathing (occasional breaths, slow, laborious or noisy breathing) is a sign of CA that should not be confused with life/circulation signs. The following sequence of actions is indicated in such cases:
- 1.
Call out for help (if this has not been done yet).
- 2.
Turn the patient on to his or her back and open the airway.
- 3.
On opening the airway, check for breathing:
- -
Open the airway using the head tilt/chin lift maneuver.
- -
Examine inside the mouth. Attempt to remove any foreign body or elements.
- -
If neck injury is suspected, attempt to open the airway applying mandibular traction. If there are sufficient personnel members at hand, aligned manual stabilization is indicated in order to minimize head movements. The efforts to protect the cervical spine should not place oxygenation and ventilation at risk.
- -
Keeping the airway open, check whether the chest moves, listen at the mouth of the patient for breathing sounds, feeling the breath on the cheek, in order to assess normal respiration. It should take no more than 10s to determine whether the patient is breathing normally or not.
- -
- 4.
Check the circulation signs:
- -
If the patient shows no signs of life (consciousness, purposeful movements, normal breathing or cough), start CPR maneuvering until more experienced help arrives, or until the patient shows signs of life.
- -
Those with experience in clinical assessment should evaluate the carotid pulse while simultaneously seeking signs of life, during no more than 10s.
- -
If the patient seems to show no signs of life, or in case of doubt, CPR maneuvering should be started immediately. Chest compression in a patient with a scantly beating heart is unlikely to cause injury. However, delays in diagnosing CA and in starting CPR have a negative impact upon patient survival, and therefore should be avoided.
- -
In the presence of a pulse or signs of life, urgent medical assessment is required.
If there is no breathing but a pulse is present (i.e., respiratory arrest), the patient should be ventilated, checking circulation every 10 respirations.
Start of CPR in the hospital- -
A person starts CPR while others alert the resuscitation team and retrieve the resuscitation equipment and defibrillator. If only one personnel member is present, this would mean having to momentarily leave the patient.
- -
Apply 30 chest compressions, followed by two ventilations.
- -
Minimize the interruptions and ensure quality compression.
- -
Maintain the airway and ventilate the lungs with the most appropriate equipment immediately available: pocket masks, supraglottic devices and an auto-inflatable balloon or balloon-mask, according to the locally applied protocol. Tracheal intubation should only be attempted by trained persons who are competent and experienced in the technique.
- -
Administer sufficient volume to allow normal chest elevation. Add supplementary oxygen as soon as possible.
- -
Following tracheal intubation of the patient or the insertion of a supraglottic device, chest compression should be continued without interruption (except for defibrillation or checking of the pulse where indicated), at a rate of at least 100min−1, with lung ventilation at approximately 10 respirations min−1.
- -
Once the defibrillator has arrived, the rhythm in arrest must be analyzed. If self-adhering defibrillation patches are available, these should be placed without interrupting the chest compressions. The use of self-adhering patches or of the “quick look” technique will allow rapid assessment of the cardiac rhythm. With a manual defibrillator, if the rhythm corresponds to ventricular fibrillation/ventricular tachycardia without a pulse (VF/VT), the defibrillator should be charged while another resuscitator continues with the chest compressions. Once the defibrillator is charged, a discharge should be applied. If an automated external defibrillator (AED) is used, follow the audiovisual indications of the AED.
- -
Resume the chest compressions immediately after the defibrillation attempt. Minimize interruption of the chest compressions.
- -
Continue resuscitation until the resuscitation team arrives, or until the patient shows signs of life. Follow the voice instructions if an AED is used. If a manual defibrillator is being used, follow the universal advanced life support algorithm (Fig. 2).
- -
Once resuscitation is underway, and if sufficient personnel are available, prepare an intravenous catheter and the drugs that will probably be used (e.g., adrenalin).
- -
Even brief interruptions in chest compression worsen the prognosis. Every effort therefore must be made to maintain effective chest compression throughout the resuscitation attempt. The team leader should monitor the quality of CPR and alternate the participants in CPR if quality proves poor.
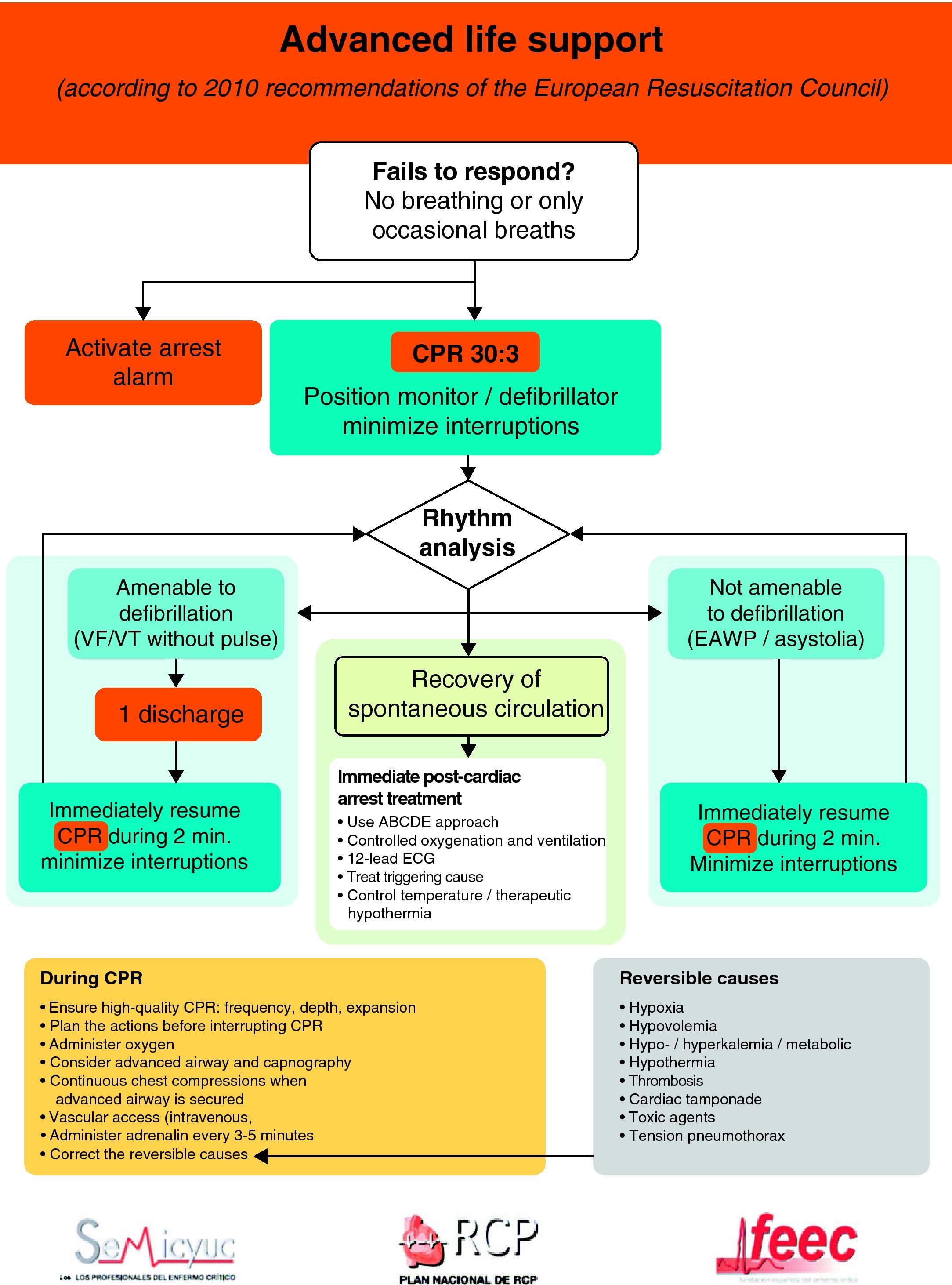
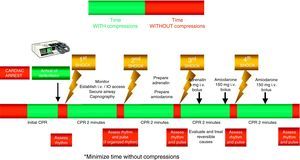
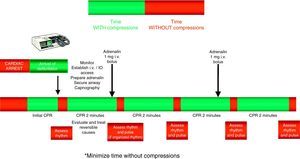
The universal ALS algorithm of the ERC 2010 recommendations (Fig. 2) is similar to the previous algorithm corresponding to the year 2005, though the recommendations show some relevant changes, and especially a different emphasis on some of them. In general, priority centers on simplification and rationalization to facilitate application of the algorithm. The following should be underscored:
- 1.
Interventions, which undoubtedly contribute to improve survival in CA, are effective basic life support (BLS), with uninterrupted high-quality chest compressions and an early defibrillation in VF/VT. Accordingly, special emphasis is placed on the need for quality CPR.13 This would include the following:
- -
The performance of high-quality chest compressions, of adequate depth (approximately 5cm), and allowing complete expansion of the chest. If the airway has not been isolated, a compression: ventilation ratio of 30:2 should be maintained; if the airway has been isolated, the frequency should be 100bpm.
- -
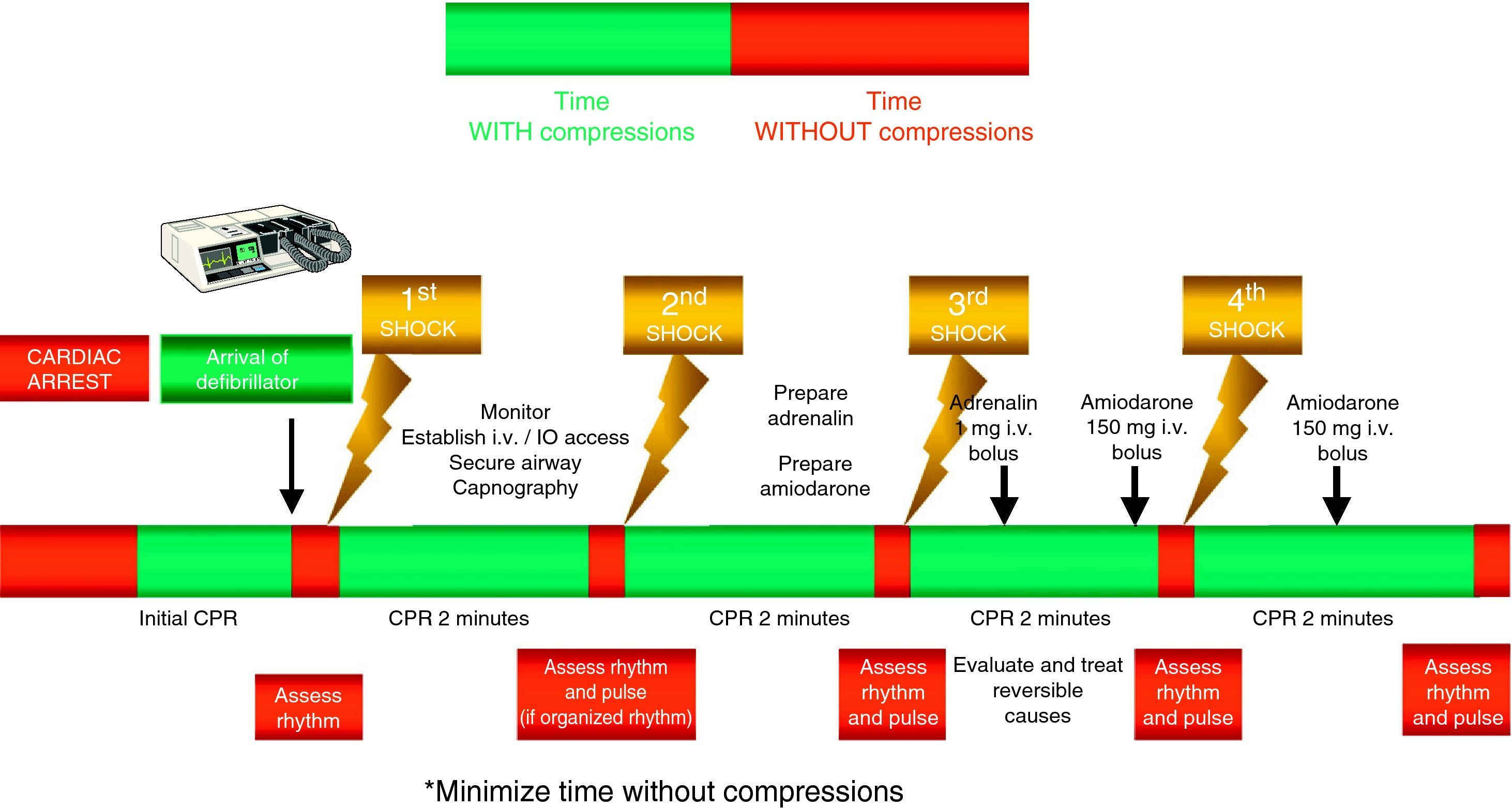
The compressions should be interrupted as little as possible through the resuscitation period.14,15 Brief interruptions should only be made to allow specific interventions such as defibrillation or tracheal intubation. A delay of only 5–10s is enough to reduce the chances for success in dealing with CA. In order to shorten the pre-discharge pause, the chest compressions should be continued while the defibrillator is being charged.16,17 Without assessing cardiac rhythm or pulse, the chest compressions should be resumed immediately after discharge. Even in cases where discharge proves successful and restores a perfusion rhythm, it takes some time for post-discharge circulation to become established.
- -
Avoid excessive ventilation.
- -
- 2.
Special emphasis continues to be placed on early defibrillation in patients with CA who present rhythms amenable to defibrillation.18–20 In the same way as in the previous recommendations, the new guidelines maintain the protocol of one discharge versus the sequence of three discharges for rhythms amenable to defibrillation, with the same energy ratings in both mono- and biphasic waves, and an increase in voltage for the second and subsequent discharges, instead of maintaining a fixed voltage (in defibrillators with biphasic waves).
- 3.
In contrast to other clinical situations, the recommendation is to deliver up to three rapid and consecutive (grouped) discharges in ventricular fibrillation/ventricular tachycardia without a pulse (VF/VT) occurring in the cardiac catheterization room or in the immediate postoperative period of heart surgery.21 This same strategy can also be considered in the case of witnessed CA with VF/VT, when the patient has already been connected to a manual defibrillator.
- 4.
In the case of out-hospital CPR, the new guidelines eliminate the recommendation to apply a predetermined period of CPR before defibrillation following CA not witnessed by the medical emergency services (MES).16,22–24
- 5.
For the administration of drugs, the recommendation to establish a peripheral venous access remains, due to the rapidity, efficacy and safety of the technique. If a venous access cannot be established in the first 2min of resuscitation maneuvering, an intraosseous (IO) route should be attempted for the administration of drugs. The increasing availability of these devices has made it easier to apply this technique.25,26 The IO drug doses are the same as with the intravenous route. This form of administration has been found to be safe and effective.27,28
- 6.
Drug treatment. Despite a lack of data from human clinical studies reporting improvements in survival, the current 2010 guides continue to indicate adrenalin as the only vasopressor drug in the treatment of CA.
- -
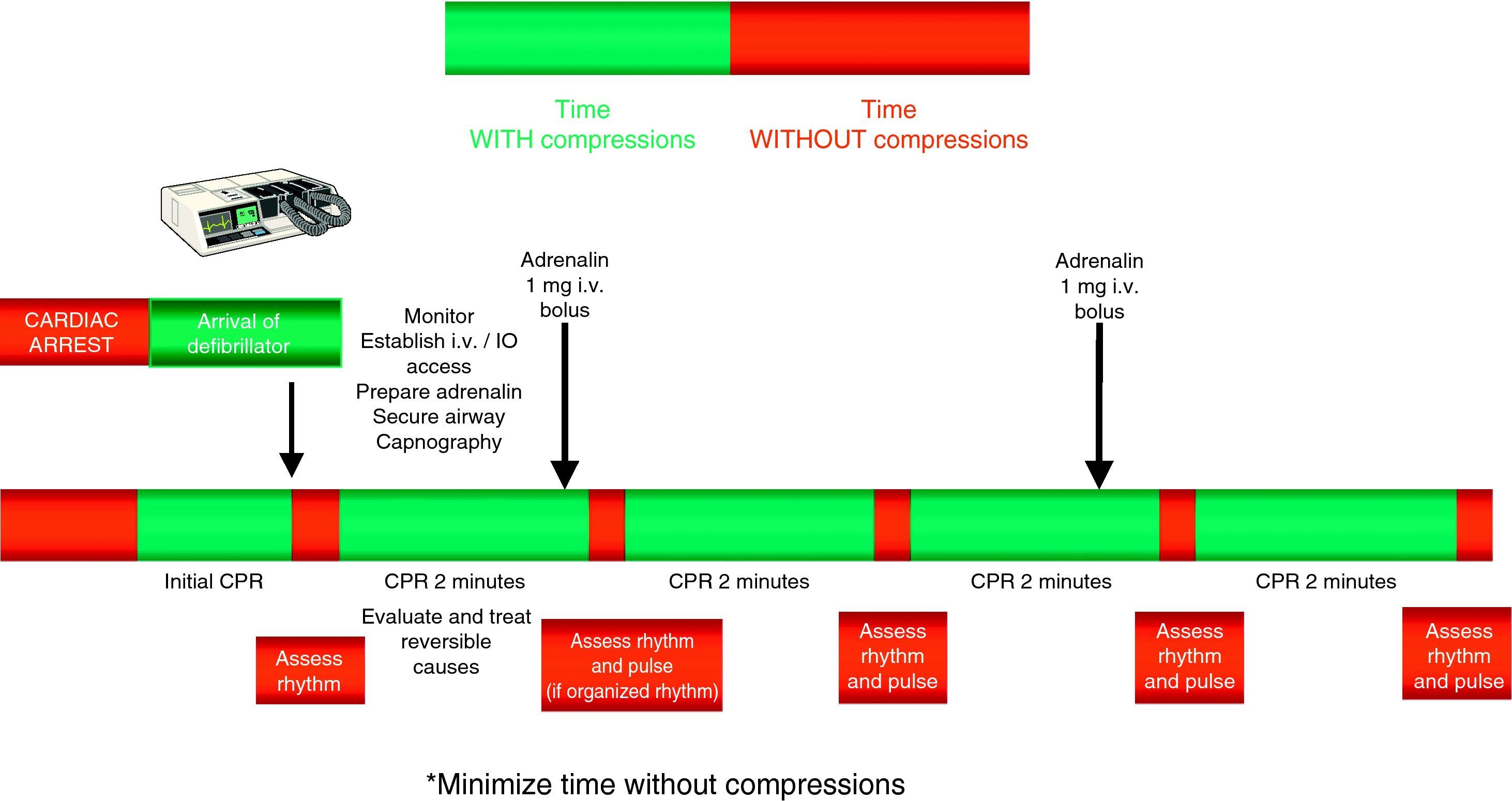
The administration of drugs should only be considered once the first discharges have been applied (where indicated), and following the start of chest compressions and ventilation. Therefore, during the treatment of CA secondary to VF/VT, we should administer 1mg of adrenalin after the third discharge, once the chest compressions have been resumed, and then every 3–5min (during alternate CPR cycles). CPR should not be interrupted for the administration of drugs. There are no alternative vasopressors capable of improving survival versus adrenalin.
- -
Although there is no evidence that the administration of any antiarrhythmic drug, on a routine basis, is able to improve survival at hospital discharge, the new guides continue to recommend the administration of amiodarone in refractory ventricular fibrillation,29,30 after the third discharge. The dose likewise remains the same: 300mg for the first dose, and the perfusion of 900mg in 24h. A posterior 150mg bolus dose can be administered.
- -
It is acknowledged that asystolia is fundamentally due to the primary myocardial disease rather than to an excessive vagal tone, and that there is no clear evidence that atropine improves the results in CA.31,32 Therefore, and in contrast to the previous guidelines of 2005, the routine use of atropine in asystolia or electrical activity without a pulse (EAWP) is no longer recommended, and has been eliminated from the ALS algorithm. Such a medication would only be used in the context of bradyarrhythmias.
- -
In recent years, a number of studies have examined the role of fibrinolytic treatment in the context of CA, with a view to eliminating the coronary and/or pulmonary thrombus. The conclusions are that such treatment should not be used on a routine basis in CA, but that should be considered when CA is caused by acute pulmonary embolism–either diagnosed or suspected.33–36 CPR in course is not a contraindication to fibrinolysis.
- -
- 7.
Less emphasis is placed on early tracheal intubation. This technique should only be performed by highly skilled resuscitators, with minimum interruption of the chest compressions. Only a small pause in the compressions should be allowed in order to advance the tube beyond the vocal cords (no more than 10s). Alternatively, and in order to avoid the interruptions, attempted tracheal intubation can be postponed until spontaneous circulation has been recovered.37 There is no conclusive clinical evidence that early intubation improves survival without sequelae at hospital discharge. When the personnel members dealing with CA are not trained in tracheal intubation, the use of supraglottic devices (e.g., a laryngeal mask) is regarded as an acceptable alternative for airway isolation.
- 8.
Since plasma drug concentration is unpredictable when medication is administered via the tracheal route, the optimum drug dosage is not known, and on the other hand there is now an increased availability of intraosseous devices; as a result, the administration of drugs through the tracheal tube is no longer recommended. The administration of drugs through a supraglottic device is even less reliable.
- 9.
In line with the previous recommendations, the new guides do not advise the routine use of any circulatory device as a substitute for manual chest compression. However, in certain patients requiring prolonged CPR maneuvering, as in the case of transfers, hypothermia, pulmonary embolism subjected to fibrinolysis, or patients undergoing computed tomography or percutaneous coronary interventions, mechanical devices are effectively being used.
- 10.
The same importance as before is placed on correction of the potential reversible causes, maintaining the rule of the 4 “Hs” and 4 “Ts”.
Figs. 3 and 4 provide a schematic summary of the sequence of interventions in CA, in both rhythms amenable to defibrillation and in those not amenable to defibrillation, respectively.
Cardiopulmonary resuscitation techniques and devicesAs regards the different techniques and devices used during CPR maneuvering, mention should be made of the following:
- 1.
Less emphasis is placed on the role of precordial percussion.38,39 This technique should not be used in non-witnessed out-hospital CA. Precordial percussion should be considered in patients presenting witnessed, monitored and unstable VT (including VT without a pulse), if a defibrillator cannot be immediately used. However, the technique should not delay either CPR maneuvering of defibrillator discharges.
- 2.
The 2005 guides recommended the use of an exhaled CO2 detector to confirm placement of the tracheal tube. In addition, it was indicated that end-tidal CO2 (PetCO2) monitorization could be useful as a noninvasive indicator of cardiac output during CPR maneuvering. The current 2010 guidelines place greater emphasis on the use of capnography, recommending quantitative registry of the capnographic wave to confirm and continuously monitor the position of the tracheal tube; monitor the quality of CPR; and afford an early indication of the recovery of spontaneous circulation. Although other methods are available for confirming the position of the tracheal tube, continuous capnographic wave registry is the most reliable option.40,41 The monitorization of this wave is particularly important in moments when the tracheal tube may become displaced from its correct position, as during patient transfer. In order for the capnographic system to measure exhaled CO2, there must be blood flow through the lungs. In this context, ineffective compressions, a drop in cardiac output, or a new situation of CA (in a patient who had already recovered spontaneous circulation), are associated with decreased PetCO2. In contrast, the restoration of spontaneous circulation increases PetCO2.
- 3.
The potential role of ultrasound in ALS is acknowledged. Although no study has shown the use of ultrasound to improve the prognosis of CA, it is clear that echocardiography is able to detect a number of the potentially reversible causes of CA (e.g., pericardial tamponade, pulmonary embolism, hypovolemia, pneumothorax).42–44 However, the incorporation of ultrasound to ALS requires important training in order to be used in only certain situations, and with minimum interruption of the chest compressions (attempting to obtain “useful” images in under 10s). The sub-xiphoid window is advised.45
In contrast to the guides of 2005, the current guidelines of 2010 attach special attention and importance to post-CA syndrome and post-resuscitation care. Post-CA syndrome comprises post-CA brain damage, post-CA myocardial dysfunction, the systemic response to ischemia/reperfusion, and persistence of the triggering or causal disorder.46 The severity of this syndrome varies according to the duration and cause of CA. Post-CA brain damage can be exacerbated by failure of the microcirculation, impaired auto-regulation, hypercapnia, hyperoxia, fever, hyperglycemia and seizures.
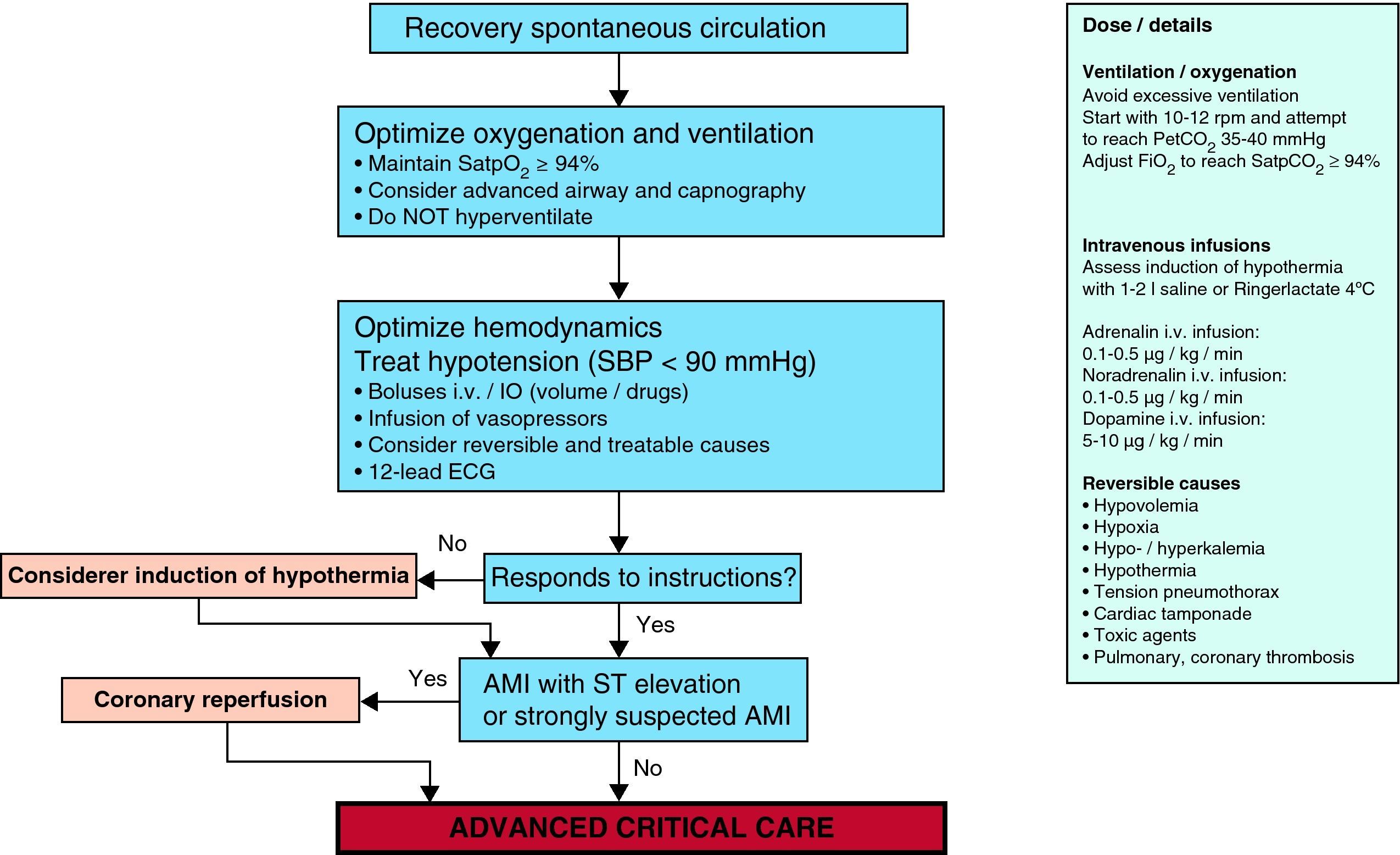
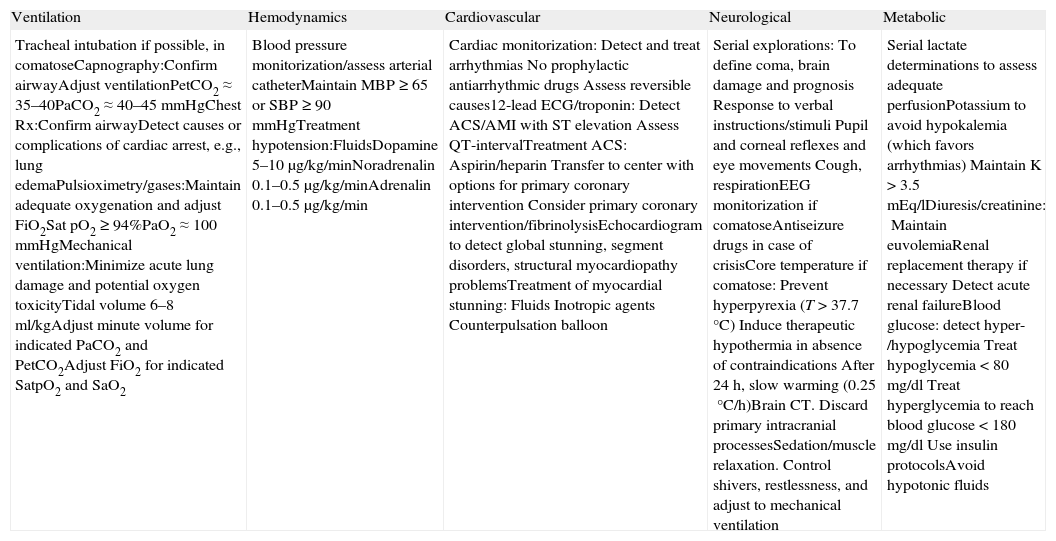
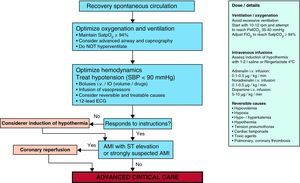
It is clearly accepted that success in the recovery of spontaneous circulation is only the first step towards full recovery in patients with CA. It is important to acknowledge that the treatment received in this post-resuscitation period exerts a significant influence upon the ultimate neurological prognosis.47–49 The post-resuscitation phase begins in the place where the recovery of spontaneous circulation is achieved, though once stabilized, the patient must be moved to intensive care for continuous monitorization and treatment. Fig. 5 and Table 2 summarize the actions and multisystemic approach required in the post-resuscitation care of the adult patient.
Multisystemic approach to post-cardiac arrest management.
| Ventilation | Hemodynamics | Cardiovascular | Neurological | Metabolic |
| Tracheal intubation if possible, in comatoseCapnography:Confirm airwayAdjust ventilationPetCO2≈35–40PaCO2≈40–45mmHgChest Rx:Confirm airwayDetect causes or complications of cardiac arrest, e.g., lung edemaPulsioximetry/gases:Maintain adequate oxygenation and adjust FiO2Sat pO2≥94%PaO2≈100mmHgMechanical ventilation:Minimize acute lung damage and potential oxygen toxicityTidal volume 6–8ml/kgAdjust minute volume for indicated PaCO2 and PetCO2Adjust FiO2 for indicated SatpO2 and SaO2 | Blood pressure monitorization/assess arterial catheterMaintain MBP≥65 or SBP≥90mmHgTreatment hypotension:FluidsDopamine 5–10μg/kg/minNoradrenalin 0.1–0.5μg/kg/minAdrenalin 0.1–0.5μg/kg/min | Cardiac monitorization:Detect and treat arrhythmiasNo prophylactic antiarrhythmic drugsAssess reversible causes12-lead ECG/troponin:Detect ACS/AMI with ST elevationAssess QT-intervalTreatment ACS:Aspirin/heparinTransfer to center with options for primary coronary interventionConsider primary coronary intervention/fibrinolysisEchocardiogram to detect global stunning, segment disorders, structural myocardiopathy problemsTreatment of myocardial stunning:FluidsInotropic agentsCounterpulsation balloon | Serial explorations:To define coma, brain damage and prognosisResponse to verbal instructions/stimuliPupil and corneal reflexes and eye movementsCough, respirationEEG monitorization if comatoseAntiseizure drugs in case of crisisCore temperature if comatose:Prevent hyperpyrexia (T>37.7°C)Induce therapeutic hypothermia in absence of contraindicationsAfter 24h, slow warming (0.25°C/h)Brain CT. Discard primary intracranial processesSedation/muscle relaxation. Control shivers, restlessness, and adjust to mechanical ventilation | Serial lactate determinations to assess adequate perfusionPotassium to avoid hypokalemia (which favors arrhythmias)Maintain K>3.5 mEq/lDiuresis/creatinine:Maintain euvolemiaRenal replacement therapy if necessaryDetect acute renal failureBlood glucose: detect hyper-/hypoglycemiaTreat hypoglycemia<80mg/dlTreat hyperglycemia to reach blood glucose<180mg/dlUse insulin protocolsAvoid hypotonic fluids |
The most important changes in the current guides can be summarized as follows:
- 1.
The introduction of a detailed and structured post-resuscitation treatment protocol can improve survival among patients with CA following the recovery of spontaneous circulation.50
- 2.
Airway and ventilation: As in the previous guides, consideration is required of tracheal intubation, sedation and mechanical ventilation in any patient with altered brain function. Emphasis is placed on the fact that both hypoxemia and hypercapnia increase the probability of ulterior CA, and can contribute to secondary brain damage. Different animal studies have found that hypoxemia induces oxidative stress and causes post-ischemic neuron damage.51 In an experimental study and in a clinical registry it has been found that post-resuscitation hyperoxemia is associated to a poorer prognosis compared with normo- or hypoxemia.52 In clinical practice, the recommendation is to adjust the inhaled oxygen fraction in order to keep arterial oxygen saturation (as determined by blood gases and/or pulsioximetry) in the range of 94–98%, which could be referred to as “controlled reoxygenation”.
Following CA, hypocapnia induced by hyperventilation produces brain ischemia (secondary to cerebral vasoconstriction and a decrease in cerebral blood flow). There are no data in support of a specific target arterial PCO2 following resuscitation, though it seems reasonable to adjust ventilation to secure normocapnia, with monitorization based on capnography and the arterial blood gas values.
- 3.
Circulation: Increased emphasis is placed on the usefulness of primary coronary intervention in appropriate patients, including comatose individuals, with the sustained recovery of spontaneous circulation after CA. Given the high percentage of patients with CA who suffer coronary disease, and the well established indication of coronariography and early primary coronary intervention in post-CA patients with ST-segment elevation acute myocardial infarction (AMI), it is recommended that this intervention should be considered in all post-CA patients in which the existence of coronary disease is suspected.50,53–56 In addition, several studies have indicated that the combination of therapeutic hypothermia and primary coronary intervention is both feasible and safe after CA secondary to AMI.50,57,58
The treatment of post-CA hemodynamic instability with fluids, inotropic agents and vasopressors can be guided by physiological and laboratory test parameters such as blood pressure, heart rate, diuresis, plasma lactate and central venous oxygen levels. Although early target-guided therapy is well established in the management of sepsis and has been proposed as a therapeutic strategy after CA, no controlled and randomized studies have warranted its routine use. As targets, we should use mean blood pressure for ensuring adequate diuresis (1ml/kg/h) and normal or decreasing plasma lactate levels, taking into consideration the normal blood pressure of the patient, the cause of CA, and the severity of any myocardial dysfunction.
- 4.
Control of seizures: The appearance of seizures increases brain metabolism and can cause brain damage. In a way similar to the specifications of the previous guidelines, the new guides stress the immediate and effective management of seizures with benzodiazepines, phenytoin, sodium valproate, propofol or a barbiturate. Myoclonus may prove difficult to treat; clonazepam is the most effective drug, but sodium valproate, levetiracetam and propofol can be effective alternatives. There are no studies definitively warranting the use of prophylactic drug treatment against seizures after CA in adult patients.
- 5.
Blood glucose control: There is a strong correlation between elevated blood glucose after CA resuscitation and a poor neurological prognosis. Control of blood glucose is therefore recommended. The resuscitation guidelines of 2010 have revised this recommendation. Based on the data available at the time of preparation of the guides, the recommendation is to keep blood glucose after the recovery of spontaneous circulation at levels of ≤10mmol/l (180mg/dl).59 Severe hypoglycemia is associated to increased mortality in critical patients,60 and comatose individuals are at special risk of suffering undetected hypoglycemia. It is therefore agreed that hypoglycemia should be avoided. Strict blood glucose control (72–108mg/dl, 4–6mmol/l) should not be applied in adults with recovery of spontaneous circulation after CA, due to the increased risk of hypoglycemia.
- 6.
Therapeutic hypothermia: In a clearer manner and in the context of more global post-resuscitation care, the new 2010 guidelines underscore the key role of therapeutic hypothermia. This technique would be applicable to comatose CA survivors initially associated to both rhythms not amenable to fibrillation and to rhythms amenable to defibrillation. There is admittedly less evidence in favor of its use after CA due to rhythms not amenable to defibrillation.
In the recommendations, careful revision is made of the following:
- -
The physiological bases explaining why moderate hypothermia has been shown to be neuroprotective and improves the prognosis after a period of global hypoxia-brain ischemia.61
- -
What post-CA patients should receive therapeutic hypothermia? There is good evidence supporting the use of induced hypothermia in comatose survivors of out-hospital CA caused by rhythms amenable to defibrillation. It seems reasonable to extrapolate these data to other types of CA involving other initial rhythms or in-hospital arrest, though the supporting evidence in these cases is of lower level.
- -
How should cooling be carried out? The guides revise the possible different techniques for the induction and maintenance of hypothermia, and the ways to posteriorly induce warming. Internal and/or external techniques can be used to start cooling. The infusion of 30ml/kg of saline solution at 4°C reduces the core temperature approximately 1.5°C,62–65 and has been shown to be safe and effective. It can be used to start cooling from the pre-hospital setting.66,67 In the maintenance phase it is preferable to use a cooling technique with effective temperature monitorization, in order to avoid temperature fluctuations. Temperature is normally monitored in the bladder and/or esophagus. There are no data suggesting that any specific cooling method improves survival when compared with any other cooling technique; however, internal devices allow more precise control of temperature compared with the external techniques. Warming in turn is to be carried out slowly: although the optimum rate has not been established, the current consensus is that warming should be carried out at a rate of 0.25–0.5°C/h.68
- -
When should hypothermia be carried out? Data from experimental studies indicate that earlier cooling after the recovery of spontaneous circulation results in a better prognosis.69 Although a number of studies have shown that hypothermia can be started early, to date in the pre-hospital setting there are no data in humans proving that a given temperature–time target offers improved prognosis.
- -
The guides also revise the physiological effects, complications and contraindications of hypothermia, thus facilitating understanding of the technique and its application in clinical practice.
- -
- 7.
Other treatments. The current guides review the literature on neuroprotective drugs (coenzyme Q10, thiopental, glucocorticoids, nimodipine, lidoflazine or diazepam) used either alone or added to therapeutic hypothermia. It is emphasized that these agents have not been shown to increase survival with neurologically intact situations when included in post-CA management. There is also insufficient evidence to support the routine use of high-volume hemofiltration to improve the neurological prognosis in patients with recovery of spontaneous circulation after CA.
The resuscitation guides of the year 2010 point out that many of the accepted predictors of poor survival in comatose CA survivors are not reliable, particularly if the patient has been subjected to therapeutic hypothermia. In general, the potential predictors have been examined:
- -
Clinical exploration. There is no neurological clinical sign capable of reliably defining a poor prognosis (Cerebral Performance Category (CPC) 3 or 4, or death) in under 24h after CA. The absence of pupil reaction to light and of corneal reflexes for over 72h can reliably indicate a poor prognosis70 in adult comatose patients who have not been subjected to hypothermia and do not present confounding factors such as hypotension, sedatives or muscle relaxants.
- -
Biochemical markers. Although many markers have been studied, the current evidence does not warrant the use of serum or cerebrospinal fluid biomarkers isolatedly as indicators of a poor prognosis in comatose patients after CA, regardless of whether they are subjected to therapeutic hypothermia or not. This is due to the limitations of the studies made to date, with the inclusion of only a small number of patients and/or to inconsistencies in the cutoff values used to predict a poor prognosis.
- -
Electrophysiological markers: No electrophysiological study is able to offer a fully reliable prognosis of the comatose patient in the first 24h after CA. In the absence of confounding factors or circumstances (sedation, hypotension, hypothermia or hypoxemia), it may be reasonable to use EEG (identifying generalized suppression at under 20μV, burst-suppression pattern with generalized epileptic activity, or diffuse periodic complexes over flattened basal activity), performed between 24 and 72h after the recovery of spontaneous circulation, as a help in predicting a poor prognosis among comatose CA survivors not subjected to hypothermia.71 If somatosensory evoked potentials (SSEPs) are recorded after 24h in comatose CA survivors not subjected to therapeutic hypothermia, the bilateral absence of N20 cortical response to stimulation of the median nerve is indicative of a poor prognosis (death or CPC 3 or 4).69
- -
Imaging studies: Likewise, no studies offering level one or two evidence have been found supporting the use of any imaging technique to reliably establish the prognosis of comatose CA survivors. Overall, the imaging studies that have been carried out are limited to small sample sizes, with variability in the timing of imaging, the lack of a standardized prognostic method for comparison purposes, and the early withdrawal of care measures. Despite its enormous potential, neuroimaging is not recommended for routine decision taking in this context.
As has been commented above, defining a prognosis is even more complicated in patients who have been subjected to therapeutic hypothermia after cardiac arrest. No neurological clinical signs, electrophysiological studies, biomarkers or imaging techniques have been found to offer a reliable neurological prognosis in the first 24h after CA. Based on the limited available evidence, the potentially most reliable predictors of poor prognosis in patients subjected to therapeutic hypothermia are the bilateral absence of peak N20 in the SSEPs ≥24h after CA (false-positive rate 0%, 95% CI: 0–69%) and the absence of corneal and pupil reflexes three or more days after CA (false-positive rate 0%, 95% CI: 0–48%).72,73 In a study carried out in patients subjected to post-CA therapeutic hypothermia, an algorithm was developed based on a series of data (clinical and electrophysiological), demonstrating that the presence of two independent predictors of poor neurological prognosis (incomplete recovery of trunk reflexes, early myoclonus, a non-reactive EEG tracing and bilateral cortical absence of SSEPs) confirms a poor prognosis with a false-positive rate of 0% (95% CI: 0–14%). In general, and given the limited available evidence, decisions to limit care should not be taken on the basis of the results obtained with a single prognostic tool.
Non-heart beating donors and reference centers in cardiac arrestLastly, these recent resuscitation guides of 2010 raise two concepts that should be taken into account in relation to resuscitation. Firstly, since successful solid organ transplants have been performed after cardiac death, the possibility has been suggested of recruiting some post-CA patients to expand the pool of organ donors (“non-heart beating” donors), which remains so scarce in comparison with the potential number of organ recipients. Graft function after transplantation is conditioned by the duration of warm ischemia from the cessation of cardiac output to the time of organ preservation. When a delay is expected in the time to starting organ preservation, mechanical chest compression devices may be useful for maintaining adequate organ perfusion while the steps needed to allow organ donation are taken.74
There is enormous variability in survival among different hospitals that deal with patients resuscitated from cardiac arrest. There is some low-level evidence that ICUs which treat more post-CA patients a year show improved survival figures compared with those Units which receive fewer such cases. Several studies have reported improvements in survival after the implementation of a series of post-resuscitation care measures including therapeutic hypothermia and primary coronary intervention. On the other hand, several studies of out-hospital CA in adults have reported no effects upon survival at hospital discharge attributable to the transfer interval from the scene of CA to arrival in hospital, provided recovery of spontaneous circulation is achieved on the scene and transfer is brief (3–11min).75,76 This means that it may be safe not to transfer post-CA patients to local hospital centers but to ensure transfer to a regional CA center. There is indirect evidence that regional cardiac resuscitation systems improve the prognosis of myocardial infarction with ST-segment elevation. The consequence of these data is that the centers and healthcare systems specialized in CA may be effective, though there is still no direct evidence in support of this hypothesis.77,78
Summary of the changes since the guides of 2005Finally, it should be commented that many of the recommendations of the ERC guides of 2005 remain without changes, either because no new studies have been published, or because the new evidence generated since 2005 simply reinforces the already existing evidence. However, the evidence published since 2005 does point to the need for changes in some parts of the 2010 guidelines. The changes of 2010 in relation to the guides of 2005, referred to advanced life support, can be summarized as follows3:
- -
Increased emphasis on the importance of high-quality chest compressions with minimum interruption throughout any ALS attempt: chest compressions are only briefly interrupted to allow specific interventions.
- -
Increased emphasis on the use of “alarm and tracing systems” to detect patient worsening and allow treatment for the prevention of in-hospital CA.
- -
Increased attention to the alarm signs associated to the potential risk of sudden cardiac death outside the hospital.
- -
Elimination of the recommendation of a predetermined period of CPR before out-hospital defibrillation after CA not witnessed by the medical emergency services (MES).
- -
Maintenance of the chest compressions while the defibrillator is being charged (this serving to minimize the pre-discharge pause).
- -
Less emphasis on the role of precordial percussion.
- -
The delivery of up to three rapid and consecutive (grouped) discharges in ventricular fibrillation/ventricular tachycardia without a pulse (VF/VT) occurring in the cardiac catheterization room or in the immediate postoperative period of heart surgery.
- -
Drug administration through the tracheal tube is no longer advised (if an intravenous access cannot be established, the drugs should be administered via the intraosseous (IO) route).
- -
During the treatment of CA secondary to VF/VT, we should administer 1mg of adrenalin after the third discharge, once the chest compressions have been resumed, and then every 3–5min (during alternate CPR cycles). After the third discharge, 300mg of amiodarone are also administered.
- -
The routine use of atropine in asystolia or in electrical activity without a pulse (EAWP) is no longer recommended.
- -
Less emphasis is placed on early tracheal intubation, unless carried out by trained persons who are very experienced in the technique, and ensuring minimal interruption of the chest compressions.
- -
Increased emphasis on the use of capnography to confirm and continuously monitor the position of the tracheal tube, the quality of CPR, and to afford an early indication of the recovery of spontaneous circulation.
- -
The potential role of ultrasound in ALS is acknowledged.
- -
Recognition of the potential damage caused by hyperoxemia after achieving the recovery of spontaneous circulation: once spontaneous circulation and arterial blood oxygen saturation (SaO2) have been restored, reliable monitorization can be carried out using pulsioximetry and/or arterial blood gas measurements-adjusting the inhaled oxygen concentration to obtain SaO2 94–98%.
- -
Much greater attention and emphasis is placed on treatment of post-CA syndrome.
- -
The implementation of a detailed and structured post-resuscitation treatment protocol is recognized as being able to improve survival among CA victims after the recovery of spontaneous circulation.
- -
Increased emphasis is placed on the use of primary coronary intervention in appropriate cases (including comatose patients) with sustained recovery of spontaneous circulation after CA.
- -
Revision is made of the recommendation referred to blood glucose control: in adults with sustained recovery of spontaneous circulation after CA, blood glucose values of >10mmol/l (>180mg/dl) should be treated, though avoiding hypoglycemia.
- -
Utilization of therapeutic hypothermia also in comatose CA survivors initially associated to rhythms both amenable and not amenable to defibrillation. It is recognized that there is less evidence in favor of such use after CA involving rhythms not amenable to defibrillation.
- -
Recognition that many of the accepted predictors of poor prognosis in comatose CA survivors are not reliable, particularly if the patient has been subjected to therapeutic hypothermia.
The authors declare no conflicts of interest.
Please cite this article as: Pérez-Vela JL, et al. Novedades en soporte vital avanzado. Med Intensiva. 2011;35:373–87.