Systematic and structured methods must be used to ensure that healthcare risks are effectively managed. Spanish standard UNE 179003:2013 provides healthcare organizations with a framework and a systematic protocol for managing patient safety from a clinical and organizational perspective. Furthermore, it is useful in securing an efficient balance among health risk, health outcomes and costs. The UNE 179003:2013 certifies that a clinical service complies with rules and operating procedures aimed at reducing the incidence of adverse events. It also requires mandatory continuous improvement, given that the standard entails frequent monitoring of the risk management system through periodic audits.
The aims of this paper are to describe the UNE 179003:2013 certification process in an Intensive Care Unit, propose a risk management program for critical patients, and offer some recommendations regarding its implementation.
Para asegurar que el riesgo asistencial se gestiona de manera efectiva es necesario utilizar métodos sistemáticos y estructurados. La Norma española UNE 179003:2013 ofrece a las organizaciones sanitarias un marco y una forma sistemática de abordar la gestión de la seguridad del paciente desde una perspectiva clínica y organizativa, que contribuye a alcanzar un balance eficiente entre riesgo, resultados en salud y costes. Obtener la certificación con UNE 179003:2013 demuestra el cumplimiento de unas normas y unos procedimientos de trabajo dirigidos a disminuir la incidencia de eventos adversos, y obliga a realizar intervenciones de mejora continua, porque la Norma exige realizar un seguimiento periódico del sistema de gestión de riesgos mediante auditorias regulares.
El objetivo de este trabajo es presentar el proceso realizado para obtener la certificación por la Norma UNE 179003:2013 en nuestro Servicio de Medicina Intensiva, proponer un programa de gestión de riesgos del paciente crítico y hacer algunas recomendaciones sobre su implantación.
Healthcare is always associated with a risk of adverse events (AEs) at all care levels,1,2 and particularly in Departments of Intensive Care Medicine (DICMs), due to the seriousness of the condition of the critical patient and the complexity of the provided care.
The incidence of AEs in the SYREC study, carried out in 79 Spanish DICMs, was found to be 29% (interquartile range, IQR: 5–50).3,4 These and other data oblige us to introduce practices designed to improve critical patient safety,5,6 and which at least contemplate adequate risk management; commitment on the part of the healthcare professionals and management staff to adopt a proactive approach to the use of methods for the identification and analysis of safety problems and the detection of underlying causes; the development of preventive strategies; and reactive learning referred to the observed AEs.
Risk management is based on a well defined method derived from the major company business world and which began to be introduced in the healthcare sector in the United States in the 1960s.7,8 Since then, its diffusion has gradually increased worldwide.9 In 2010, the Spanish Normalization and Certification Association, through the Normalization Technical Committee (AEN/CTN 179 Quality and Safety in Healthcare Centers, Subcommittee 5 Patient Safety Risk Management), published standard UNE 179003 to help healthcare organizations to implement a risk management system (RMS), consolidate a safety culture, and secure an efficient balance among risk, health outcomes and costs.
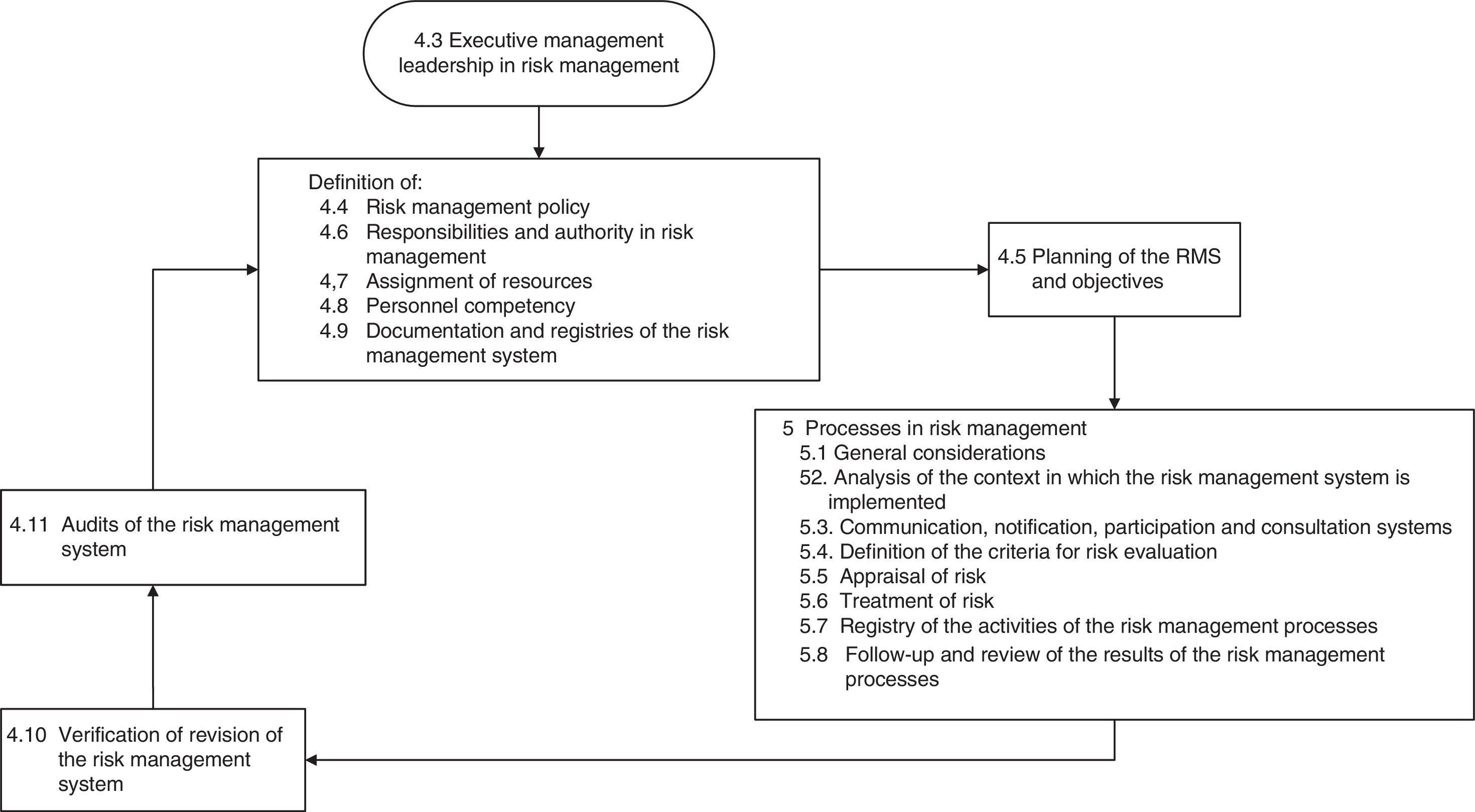
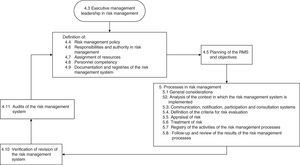
The current version of UNE 179003 is that published in 2013.10 It consists of 6 chapters and three annexes. Sections 4 and 5 are the most relevant parts, and document the requirements for certification (Fig. 1). In this respect, after implementing the RMS, organizations can request their certification, accrediting in writing that they comply with the specific requirements of the mentioned standard, possess a UNE 179003 RMS, and offer safe medical care.
The present study describes the process for obtaining standard UNE 179003:2013 certification in our DICM (the first Department in Spain to do so), proposes a risk management program for the critical patient, presents the first results obtained, and offers some recommendations regarding its implementation.
Implementation of standard UNE 179003:2013The standard was introduced in the DICM of Hospital Can Misses in Ibiza (Spain), belonging to the Servei de Salut de les Illes Balears (Balearic Islands Health Service). Our Department comprises a 9-bed polyvalent Intensive Care Unit (ICU) in the setting of a 220-bed hospital. The staff comprises a Head of Department, 6 intensivists, a pharmacist with part-time dedication, a Nursing Supervisor, 20 nurses, 15 nursing assistants and 5 hospital orderlies. A total of 650 patients were admitted in the course of the year 2012.
The UNE 179003:2013 was implemented by the multidisciplinary quality and safety work group, with representation of all the professional categories, and with prior training and experience in both areas. Three-hour meetings were held every two weeks during one year. The team informed the rest of the personnel of the Unit about the standard and the project to be developed, and obtained collaboration in drafting the documentation and developing the RMS. A diagnosis of the situation was subsequently established to assess the degree to which our routine functioning abides with the requirements of the mentioned standard, with a view to identifying the gap between current risk management in the Department and management as proposed by the standard.
A strong point in our case was the fact that we already had certification referred to standard ISO 9001:2008, which facilitates implementation of standard UNE 179003:2013, since the structure of both standards is similar (continuous improvement, executive management leadership, documental management system, assignation of responsibilities and competencies, training and awareness-enhancing activities, non-conformities, and corrective and preventive actions, follow-up and measurement of systems, management review and internal audits). Furthermore, such prior certification afforded experience in implementing the RMS and in the internal and external audits. We also had the training received in monographic courses and in the Master of the Spanish Ministry of Health, as well as experience (as pupils and lecturers) in the courses of the SEMICYUC.
As weak points we must mention the need for training in standard UNE 179003 and the fact that the RMS was not documented. The possible barriers facing implementation included the fact that ours was the first Spanish DICM to incorporate the standard, and the lack of training in patient safety on the part of executive management–a situation that complicated leadership in the RMS. Nevertheless, the Managing Director supported the certification of our Unit.
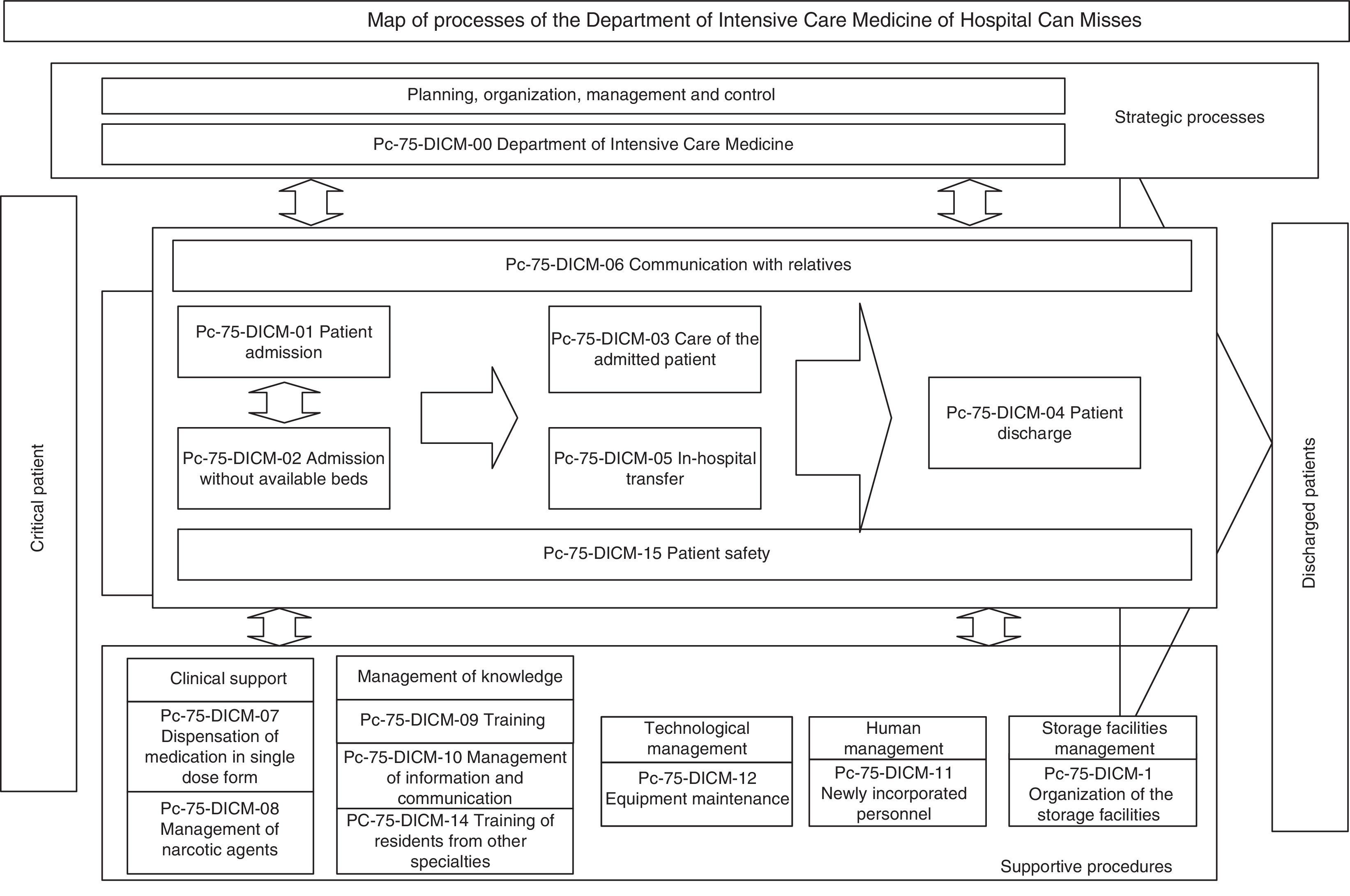
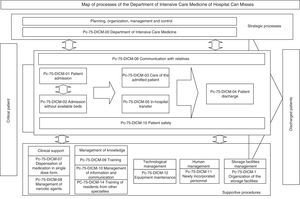
The next step was to address the weaknesses and deal with the barriers detected in two areas: training in standard UNE 179003 and documentation of the RMS. In this respect a semi-physical presence course on patient safety and standard UNE 179003 was designed and imparted among the personnel of the DICM and the hospital management. The policy, objectives, manual and general procedures of the RMS were defined. The process of risk management was included among the processes of the quality management system ISO 9001:2008 (a transverse process affecting the patient from admission to discharge) (Fig. 2). The responsibility and authority, the assignation of resources, follow-up and measurement, review by the management, the obligate documented procedures (control of documents, registries and internal audits), non-conformities, and the corrective and preventive actions are common to those of standard ISO 9001:2008, and are addressed accordingly in the Quality Management System Manual. The specific documents for developing the risk management processes included those referred to the method for the identification, analysis, evaluation and treatment of risks (with definition of the different tools and techniques), the risk prevention and control plans, the working instructions, registries, indicators and the periodic progress reports.
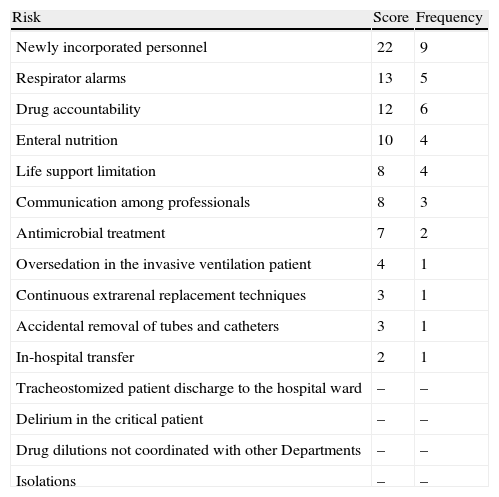
Next, we addressed the risk management process properly speaking, which consisted of identifying, analyzing, evaluating and treating the risks (Fig. 1). There were two complementary ways to address risk: proactive (preventing patient safety incidents before they actually happen) and reactive (intervening after the error has occurred). In the former approach, brain storming was used to identify 15 risks. These were then subjected to pondered selection adopting the nominal group technique, using as criteria the risk severity and occurrence according to the opinion of each of the participants, and designating a risk score from 1 (minimum) to 4 (maximum) (Table 1). An analysis was made of the four risks with the highest scores, and consensus was reached on the actions for improvement, adopting the principle of prevention in order to eliminate the causes of failure from their origin. Alternatively, measures were proposed to lessen the severity of the effect (Table 2).
Risks identified according to assigned priority, by order of score and frequency.
| Risk | Score | Frequency |
| Newly incorporated personnel | 22 | 9 |
| Respirator alarms | 13 | 5 |
| Drug accountability | 12 | 6 |
| Enteral nutrition | 10 | 4 |
| Life support limitation | 8 | 4 |
| Communication among professionals | 8 | 3 |
| Antimicrobial treatment | 7 | 2 |
| Oversedation in the invasive ventilation patient | 4 | 1 |
| Continuous extrarenal replacement techniques | 3 | 1 |
| Accidental removal of tubes and catheters | 3 | 1 |
| In-hospital transfer | 2 | 1 |
| Tracheostomized patient discharge to the hospital ward | – | – |
| Delirium in the critical patient | – | – |
| Drug dilutions not coordinated with other Departments | – | – |
| Isolations | – | – |
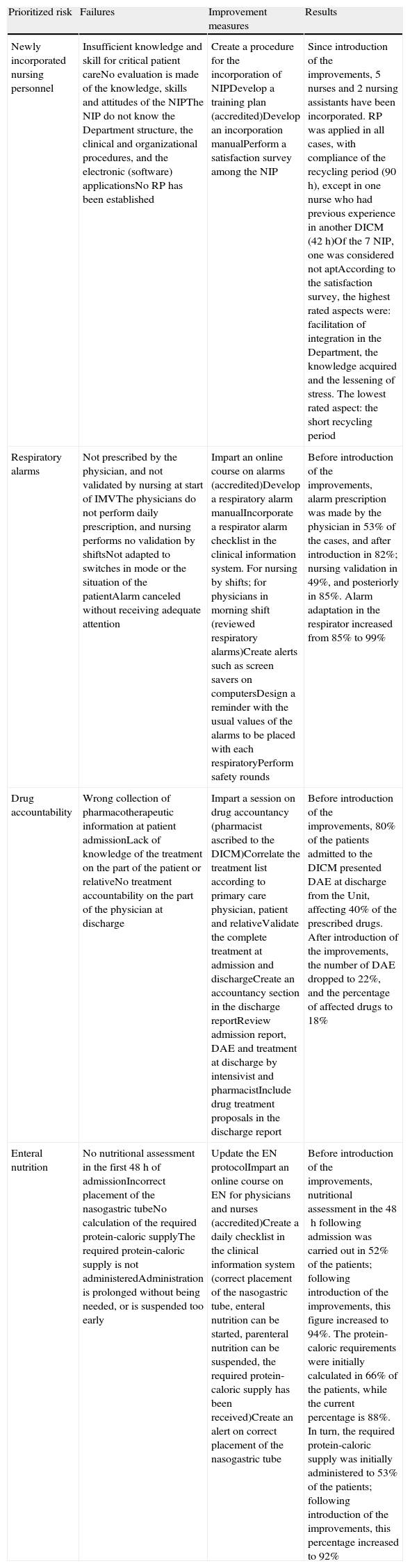
Failure modes, recommended actions for each failure, and results obtained in prioritized risks.
| Prioritized risk | Failures | Improvement measures | Results |
| Newly incorporated nursing personnel | Insufficient knowledge and skill for critical patient careNo evaluation is made of the knowledge, skills and attitudes of the NIPThe NIP do not know the Department structure, the clinical and organizational procedures, and the electronic (software) applicationsNo RP has been established | Create a procedure for the incorporation of NIPDevelop a training plan (accredited)Develop an incorporation manualPerform a satisfaction survey among the NIP | Since introduction of the improvements, 5 nurses and 2 nursing assistants have been incorporated. RP was applied in all cases, with compliance of the recycling period (90h), except in one nurse who had previous experience in another DICM (42h)Of the 7 NIP, one was considered not aptAccording to the satisfaction survey, the highest rated aspects were: facilitation of integration in the Department, the knowledge acquired and the lessening of stress. The lowest rated aspect: the short recycling period |
| Respiratory alarms | Not prescribed by the physician, and not validated by nursing at start of IMVThe physicians do not perform daily prescription, and nursing performs no validation by shiftsNot adapted to switches in mode or the situation of the patientAlarm canceled without receiving adequate attention | Impart an online course on alarms (accredited)Develop a respiratory alarm manualIncorporate a respirator alarm checklist in the clinical information system. For nursing by shifts; for physicians in morning shift (reviewed respiratory alarms)Create alerts such as screen savers on computersDesign a reminder with the usual values of the alarms to be placed with each respiratoryPerform safety rounds | Before introduction of the improvements, alarm prescription was made by the physician in 53% of the cases, and after introduction in 82%; nursing validation in 49%, and posteriorly in 85%. Alarm adaptation in the respirator increased from 85% to 99% |
| Drug accountability | Wrong collection of pharmacotherapeutic information at patient admissionLack of knowledge of the treatment on the part of the patient or relativeNo treatment accountability on the part of the physician at discharge | Impart a session on drug accountancy (pharmacist ascribed to the DICM)Correlate the treatment list according to primary care physician, patient and relativeValidate the complete treatment at admission and dischargeCreate an accountancy section in the discharge reportReview admission report, DAE and treatment at discharge by intensivist and pharmacistInclude drug treatment proposals in the discharge report | Before introduction of the improvements, 80% of the patients admitted to the DICM presented DAE at discharge from the Unit, affecting 40% of the prescribed drugs. After introduction of the improvements, the number of DAE dropped to 22%, and the percentage of affected drugs to 18% |
| Enteral nutrition | No nutritional assessment in the first 48h of admissionIncorrect placement of the nasogastric tubeNo calculation of the required protein-caloric supplyThe required protein-caloric supply is not administeredAdministration is prolonged without being needed, or is suspended too early | Update the EN protocolImpart an online course on EN for physicians and nurses (accredited)Create a daily checklist in the clinical information system (correct placement of the nasogastric tube, enteral nutrition can be started, parenteral nutrition can be suspended, the required protein-caloric supply has been received)Create an alert on correct placement of the nasogastric tube | Before introduction of the improvements, nutritional assessment in the 48h following admission was carried out in 52% of the patients; following introduction of the improvements, this figure increased to 94%. The protein-caloric requirements were initially calculated in 66% of the patients, while the current percentage is 88%. In turn, the required protein-caloric supply was initially administered to 53% of the patients; following introduction of the improvements, this percentage increased to 92% |
DAE: drug accountability error; IMV: invasive mechanical ventilation; EN: enteral nutrition; RP: reception plan; NIP: newly incorporated nursing personnel.
Accredited: accredited by the Continued Training Commission of the Balearic Islands, Conselleria de Salut, Família i Benestar Social.
For each action for improvement, persons in charge of its implementation were selected, with the definition of indicators, timelines, follow-up measures, and review of the controls to ensure effectiveness. Every three months a report was drafted on the degree of implementation of the risk management process, with distribution among the personnel of the Unit through sessions and the Department web facilities. Priority risk analysis was based on failure mode and effect analysis11–the main results and proposed actions for improvement of which are summarized in Table 2.
For the reactive identification of risks, use was made of the information of the hospital reporting and learning system available in web format; the daily clinical sessions in which all the professionals of the Department participate; information from the nursing and assistant personnel changes of shift; and the three-monthly mortality assessment sessions. The study was carried out based on root cause analysis.12 There were two investigated AEs, related to medication and to in-hospital transfer of the patient. After establishing the causes leading to the AE, actions for improvement were defined for each root cause or contributor. In the case of the medication problem, these actions were: prepare a list of high-risk medications, administer such medications with warnings referred to their category, develop a protocol for the standardization of prescription, storage, preparation and administration, and check the similarity of names (phonetics/spelling) and appearance. In the case of in-hospital transfer, the actions included the preparation of a checklist in the clinical information system. Posteriorly, the recommended implementation deadline and corresponding control method were assigned. The results of this reactive analysis were distributed among the personnel of the Unit through sessions and alerts. Since these analyses are confidential, no further information is provided on the AEs analyzed with this tool.
Following compliance with the general requirements and processes corresponding to risk management as specified by the standard, internal and external audits were made in which no non-conformities were detected. UNE 179003 certification for the RMS introduced in our Department was thus finally obtained.
DiscussionSystematic and structured methods are needed in order to ensure that risk is managed effectively, as has been commented on describing standard UNE 179003. However, the use of such methods is still not very widespread in Spain. The application of this standard in our Department has allowed us to produce a list of risks to which the patient may be exposed, and to decide the need for evaluation and treatment.
In addition to the tools used, other very useful simple (flowcharts, cause-effect diagrams) or structured instruments (failure tree analysis, event tree analysis) and qualitative (structured interviews or questionnaires) or quantitative methods (consequence analysis, probability analysis) are available13–the choice of which depends on the characteristics of the organization and the objectives of the RMS.
Assessment and review of the RMS are essential for continuous improvement. Availability of the PICIS clinical information system allows the registry and traceability of healthcare activity and the obtainment of indicators, thereby facilitating compliance with this requirement of the standard.
In order to ensure the effectiveness of the controls and measures adopted, we have carried out periodic monitoring, which has revealed improvement of the markers referred to all the addressed risks. The actions taken have been simple, have required the use of few resources, and have been based on the redesigning of processes, evaluation, training and participation of the personnel.
The lessons learned during implementation of the standard have encouraged us to perform proactive risk management. The teamwork, systematization, documentation, control and improvement of the processes, and the permanent concern to maintain a good team working environment have helped us to improve internal organization by establishing more fluid communication, with clear objectives and responsibilities. Although the internal and external audits have detected no non-conformities, they have been a source of learning–contributing to improve the processes and procedures, alert us to future problems, and produce an appropriate system for improvement.
The implementation of a RMS has contributed to consolidate a patient safety and quality culture, obtain UNE 179003 certification, and offer quality and safety warranted by an independent organism. The professionals also benefit from risk management, for when an AE occurs, the professionals become the second victims.14 Moreover, reductions in AEs increase cost avoidance and lessen their social repercussions, negative external projections (Intensive Care Medicine is a transition service), and the distrust they generate in the healthcare system.15
On the other hand, the interventions are consistent with the evidence-based safety strategies recently recommended in the literature.16
It is not possible to conceive implementation without the active participation of executive management, and which should be reflected in the definition, approval and communication of the policy and objectives of risk management, provision of the necessary resources, training of the personnel in charge of the work, and crucial periodic evaluation and communication (feedback) of the results. The training of executive management in patient safety is another requirement in order to guarantee the success of the RMS, and in our case it undoubtedly facilitated implementation and maintenance of the standard.
It is essential to implicate the personnel in achieving the goals of the EMS. In this respect, the Head of Department and supervisor must act as motivators and ensure that the activities are carried out properly, by adequate personnel, and with the necessary resources. Knowing the degree of occupational wellbeing and its evolution with a view to introducing corrective or preventive measures is crucial in order to keep the personnel committed to the RMS. To this effect, each year we conduct surveys on the levels of burnout and engagement17 of the professionals working in the Unit, and in this sense a low degree of burnout and a high level of engagement was observed before implementation of the standard. However, the changes seen in the health care system are shifting the balance in favor of saturation and discouragement, with a reduction of efficiency and professional commitment. In sum, the changes experienced make it necessary for the leaders to reinforce the principles of professionalism.18,19 Facilitating active participation, allowing all parts interested to be correctly represented and taking their opinions into account in the risk management process, promoting communication and teamwork, and acknowledging the work done and continued training are all aspects that have helped us to implicate the personnel of our Unit in this project.
The RMS must be duly documented. This is an arduous task, though its results make it possible to plan and lessen unjustified variability in healthcare practice, as well as monitor the processes of the organization and set the bases for future improvements.
The UNE 179003 certification is valid for a period of three years, after which another audit for renewal is carried out, along with annual follow-up audits. The procedures must be dynamic, and their effectiveness must be continuously checked. When effectiveness is found to be lacking, the procedures must be modified, with adoption of the opportune corrective and preventive measures. This requires us to continuously think of ways to improve the work setting, policies, RMS and the safety culture and climate–leading us to adopt a system of continuous improvement.
Overall, the intervention consists of performing, supervising, maintaining, evaluating and correcting a number of multifaceted interventions (targeted to different aspects of safety such as those already commented above: individual, systemic and structural, clinical, organizational and referred to management) in a coordinated manner. In this respect, their global effectiveness–defined as proportional improvement in clinical safety attributable to the interventions–is greater than that of the individual actions alone.20
Lastly, our commitment to patient safety is not limited to Intensive Care Medicine. The medical and nursing professionals of the DICM form part of the Functional Risk Management Unit of the hospital, which we also coordinate, and which recently has received ISO 9001:2008 and UNE 179003:2013 certification. Other projects coordinated by the members of the Department are the Sepsis Unit and the Program for the Optimization of Antimicrobial Use (PROA)21–which currently includes the Departments of General Surgery, Traumatology, Urology and Hematology, and which soon will also incorporate the Departments of Maxillofacial Surgery and Gynecology. These are initiatives which undoubtedly will help prevent AEs.
ConclusionsDepartments of Intensive Care Medicine represent a setting in which adverse events is to be expected. We are obliged to deal with our risks as an activity always integrated within the responsibilities of management and in all the processes of the Unit. The adverse consequences of failure to do so (i.e., AEs, morbidity-mortality, and costs not avoided) may be very considerable.
Standard UNE 179003:2013 offers a setting and systematic approach to safety management from a clinical and organizational perspective. Opting for a certification process demonstrates abidance with risk management standards and working procedures destined to lessen the incidence of AEs. We moreover are also obliged to pursue continuous improvement, because the standard demands follow-up of the RMS through periodic audits.
Although ISO 9001:2008 certification is not mandatory to receive UNE 179003:2013 certification, the latter does require the organization to operate under the supervision of a quality management system. Simultaneous implementation of both standards favors integrated management of healthcare safety and quality. Since the two standards share a number of requirements, auditing time and the costs of certification are reduced, because both of them are audited jointly.
Financial supportThis study has received no financial support.
Conflicts of interestBureau Veritas has audited the Department of Intensive Care Medicine of Hospital Can Misses. The authors declare that they have no conflicts of interest.
The authors of the study thank all the personnel members of the of the Department of Intensive Care Medicine for their contribution in obtaining UNE 179003:2013 certification, and the Managing Director of the center, Ignasi Casas, for the support received.
Please cite this article as: Merino P, Bustamante E, Campillo-Artero C, Bartual E, Tuero G, Marí J. Certificación en seguridad del paciente en un Servicio de Medicina Intensiva: nuestra experiencia con la norma UNE 179003:2013. Med Intensiva. 2014;38:297–304.