Pulmonary thromboembolism (PTE) is still one of the leading causes of morbimortality in the world.1 The European clinical guidelines stratify this entity into various categories based on the risk of death during the first 30 days.2 High risk PTEs start with shock or maintained blood hypotension (defined as systolic blood pressure<90mmHg for more than 15min not explained by other causes), and in these cases, mortality rates are 35–58 per cent of the cases.3 Even though the treatment with systemic fibrinolysis may reduce mortality, it does not come without serious complications. Registries from daily clinical practices estimate that a low percentage of patients with high risk PTE receive fibrinolytic therapy due to various contraindications to receive such therapy.4 In these situations, both the surgical thrombectomy and the percutaneous pulmonary thrombectomy (PPT) have been reported.5
Now we will describe our own experience in the therapeutical management of patients with high risk PTE and contraindications for fibrinolysis from January 2015 through January 2016. During this time 15 patients with high risk PTE were admitted in our unit, of which 4 patients (26.7 per cent) underwent one PPT due to contraindications for fibrinolytic therapy.
Case #1 is that of an eighty-year-old female with reported syncope due to cranioencephalic traumatism (CET) that initiated with subdural hematoma. Bilateral PTE is diagnosed and she develops cardiogenic shock that needs precise vasoactive support. PPT is performed and an immediate hemodynamic improvement is confirmed.
Case #2 is that of a fifty-year-old female with one active cerebral oligodendroglioma. She is admitted to the hospital with acute respiratory failure and cardiogenic shock; the CT scan confirms presence of bilateral PTE. PTT was performed but yet despite the extraction of a great amount of thrombus, the hemodynamic improvement was insufficient, so we decided to combine local fibrinolysis with a single bolus of 10mg of rtPA administered directly with the catheter with a good response.
Case #3 is that of an eighty-one-year-old female admitted to the hospital due to an episode of out-of-hospital recovered cardiac arrest (RCA). Upon arrival and while studying the etiology of the RCA, one cerebral CT scan is performed that confirms presence of subdural hematoma due to CET. One emergent echocardiogram is performed in the emergency room that confirms presence of dilation and severe right ventricular dysfunction with high probability of PTE.
Due to the situation of shock refractory to high doses of vasoactive drugs, the patient is referred to the unit of hemodynamics where support using venoarterial extracorporeal membrane oxygenation (VA-ECMO) is implanted, one pulmonary angiography is performed that confirms the diagnostic suspicion, and ultimately one PTT was performed with excellent clinical and hemodynamic response that allows the withdrawal of the VA-ECMO after 3 days. Unfortunately, the patient died due to anoxic encephalopathy secondary to the time of the RCA.
The last case is that of a forty-four year-old female with a history of breast neoplasm who was admitted to the hospital for breast reconstruction surgery with abdominal flap. Six hours after the surgery the patient developed sudden shock and acute respiratory failure, and the echocardiogram showed data suggestive of PTE. PTT was performed with excellent results and clinical progression.
All our cases were women of an average age of 63.75 years old. Four women were in shock due to severe right ventricular dysfunction and needed vasoactive support with dobutamine and noradrenaline, plus one of the patients needed support with VA-ECMO. The characteristics of patients are shown in Table 1. Affectation in all cases was proximal and bilateral in both pulmonary arteries.
Clinical data, hemodynamic and echocardiographic characteristics, and clinical progression.
| Patient | Age | Cause of PTE | Diagnostic test | Contraindicated fibrinolysis | Pre mPASP (mmHg) | Post mPASP (mmHg) | 6-Month follow-up |
|---|---|---|---|---|---|---|---|
| 1 | 80 | DVT | CT | CET (subdural hematoma) | 58 | 24 | Asymptomatic |
| 2 | 50 | DVT | CT | Cerebral neoplasm (oligodendroglioma) | 45 | 38 | Asymptomatic |
| 3 | 81 | Not established | ECG | CET (subdural hematoma) | – | – | Exitus |
| 4 | 44 | Immobilization | ECG | Major surgery | 55 | 45 | Asymptomatic |
| Patient | Pre mPASP | Post mPASP | Pre B RVTD | Pre M RVTD | Pre L RVTD | Post B RVTD | Post M RVTD | Post L RVTD | Pre TAPSE | Post TAPSE |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | 63 | 59 | 56 | 68 | 40 | 41 | 65 | 9 | 15 |
| 2 | 43 | 40 | 53 | 45 | 70 | 42 | 40 | 68 | 8 | 23 |
| 3 | 65 | 50 | 63 | 54 | 67 | 44 | 42 | 64 | 8 | 15 |
| 4 | 50 | 24 | 56 | 49 | 71 | 39 | 40 | 69 | 8 | 21 |
B RVTD: basal right ventricular telediastolic diameter; L RVTD: longitudinal right ventricular telediastolic diameter; M RVTD: medial right ventricular telediastolic diameter; ECG: echocardiogram; mPASP: mean pulmonary artery systolic pressure; pre: pre-interventional; post: post-interventional; prox: proximal; PASP: pulmonary artery systolic pressure through echocardiogram; TAPSE: tricuspid annular plane systolic excursion (mm); CT: computed tomography; CET: cranioencephalic traumatism; DVT: deep venous thrombosis.
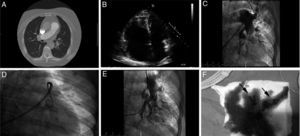
Once the diagnosis of PTE was confirmed, the indication of immediate perfusion due to its high risk was established and the patients were referred to the unit of hemodynamics to perform one PTT since the systemic fibrinolytic treatment was contraindicated. The percutaneous technique used was the same one in all the cases and consisted of obtaining right femoral venous access, make one pigtail catheter advance through both pulmonary arteries and inject contrast in order to identify the occluded branch. Once the branch to be treated was identified an interchange was tried from extra support guide-wire to catheter-guide-wire or 8F sheath (e.g., Torq Vue®, St Jude Medical) that was placed proximal to the thrombus. Fragmentation was accomplished using one pigtail catheter and introducing it through the 8F sheath; then aspiration was attempted through such sheath with support from multipurpose catheters (Fig. 1). During the PTT all patients received treatment with IV sodium heparin with one dose adjusted to activated coagulation time values of 200s, being the infusion continued when completing the proceeding adjusted to APPT-ratio values of 1.5–2. In all the cases, one variable amount of thrombus was extracted that would lead to significant clinical and hemodynamic improvement.
(A) CT-angiography of pulmonary arteries showing thrombotic content in the 2 main pulmonary arteries. (B) Transthoracic echocardiogram, apical four-chamber plane, severe dilation of right ventricle. (C) Basal left pulmonary angiography. (D) Fragmentation using pigtail catheter. (E) Left pulmonary angiography after mechanical thrombectomy. (F) Aspirated thrombotic content (arrows).
Before and after the proceeding, pulmonary pressures were measured invasively and an average reduction of the mean pulmonary pressure of 17.0±14.8mmHg was observed. In the patient with VA-ECMO support no measurements of the pulmonary pressure were taken since such measurements cannot be assessed with this kind of support. Improvement of the right ventricular function in the echocardiogram performed 72h after the proceeding was confirmed. The telediastolic diameters of the basal, medial, and longitudinal right ventricle were reduced an average of 16.5±3.8mm, 10.3±4.3mm, and 2.5±0.5mm, respectively. Also, the right ventricular function measured using the tricuspid annular plane systolic excursion (TAPSE) was increased an average of 10.3±4.4mm. After 6 months, the subjects from cases #1, #2 and #4 are still alive with no signs of developing pulmonary hypertension in the control echocardiogram.
The PPT technique includes the mechanical fragmentation of the thrombus and may be completed with the different protocols of local fibrinolysis. Although today there is no unanimous consensus on what the indications and optimal technique are, it is widely accepted that the PPT technique may be assessed in patients with contraindications for fibrinolysis or fibrinolytic failure. Based on our initial experience, we should avoid the combined treatment with local fibrinolysis in patients with contraindications for systemic fibrinolysis, yet despite the fact that the dose of the fibrinolytic agent is lower. In this sense, there are many studies that show that isolated PTT is a feasible and safe technique.6–9 Also, an immediate improvement of pulmonary pressures has been reported – something that may be useful in patients with shocks and severe right ventricular failure.6 During the last years, there were also several studies on the use of this technique in patients with sub-massive PTEs with good results.7 The advances of technology with the development of new devices that facilitate the extraction of thrombi together with the growing experience with this type of techniques may, in the coming years, generalize the use of the PPT technique for the management of patients with PTEs.
The goal of this paper is to show our own initial experience in the implementation of an urgent PTT program while benefiting from the presence of one on-call interventional cardiologist 24h/day and 365 days/year, within the recommendations established for the management of ST-segment elevation acute coronary syndrome (STACS). In Spain this type of program would be easier and cheaper to generalize to the large majority of centers with programs of primary angioplasty. To that end, the creation of multidisciplinary teams (ER, ICU, cardiology, radiology, etc.) may improve the management and prognosis of this type of patients.
FundingThe authors declare that they have received no funding while conducting this study.
Please cite this article as: Castro-Garay JC, Uribarri A, Cruz-González I, Martín-Moreiras J, Sánchez PL. Tratamiento percutáneo del tromboembolismo pulmonar masivo. Med Intensiva. 2017;41:437–439.