Cardiogenic shock is associated to high mortality. Early detection and adequate risk stratification are essential in order to avoid delays in treatment.1 The CardShock risk score,2 composed of 7 easily obtainable basal variables (patient age, previous heart surgery or infarction, neurological dysfunction upon admission, lactate, glomerular filtration rate, cause of shock and left ventricular ejection fraction), has recently demonstrated adequate performance in predicting mortality among patients with cardiogenic shock.
Extracorporeal membrane oxygenation (ECMO) has shown promising results3,4 in patients with refractory cardiogenic shock in the absence of other therapeutic alternatives. There is no information on the predictive performance of the CardShock score in patients with profound shock treated with venoarterial ECMO (VA-ECMO). The present study describes the capacity of this score to predict in-hospital mortality in a series of consecutive patients with cardiogenic shock treated with VA-ECMO.
We consecutively included patients with cardiogenic shock subjected to VA-ECMO in a Cardiological Intensive Care Unit (CICU) between January 2010 and September 2018. A prospective registry was made of the basal clinical characteristics, hemodynamic data, laboratory test results, echocardiographic and angiographic data, and information referred to patient evolution in hospital. Although the CardShock score had not yet been published at the start of the recruitment period, its items formed part of the variables collected; the score therefore could be calculated on a retrospective basis. In view of the observational and retrospective design of the study, the obtainment of patient informed consent was not considered necessary. The patient data were processed in compliance with the specifications of Spanish Organic Act 3/2018, referred to personal data protection and digital rights, and the manuscript was approved for publication by the Research Ethics Committee of Hospital Universitari de Bellvitge (Barcelona, Spain).
The clinical characteristics of the patients were compared according to outcome at discharge. Quantitative variables were contrasted with the Student t-test, while categorical variables were compared using the chi-squared test with continuity correction where required. The capacity of the CardShock score to predict in-hospital mortality was assessed through binary logistic regression analysis, maintaining the original coding of the variables. In addition, we examined the predictive capacity of a model with the same variables, but coding age, lactate and glomerular filtration rate as continuous variables. The discriminative capacity of the model was assessed by means of the receiver operating characteristic (ROC) curve and the corresponding area under the curve (AUC).
A total of 83 patients were included (84.3% males), with a mean age of 53.4 years (standard deviation [SD] 13). The most common cause of cardiogenic shock was acute coronary syndrome (27 cases, 32.5%), followed by decompensated chronic cardiomyopathy (14 cases, 16.9%). In turn, 95.2% of the patients were in INTERMACS5 (Interagency Registry for Mechanically Assisted Circulatory Support) class 1/2 upon admission. The mean CardShock score was 4.25. The mean duration of ECMO support was 6.7 days.
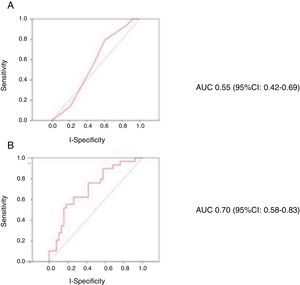
A total of 40 patients (48.2%) suffered bleeding complications during admission, 50 patients (60.2%) developed infectious complications requiring antibiotherapy, 21 (25.3%) suffered vascular complications, and 24 (28.9%) developed pulmonary congestion during circulatory support. The mean hospital stay was 43.8 days, with a mean CICU stay of 28.2 days. The in-hospital mortality rate was 47% (39/83 cases). The deceased patents were older, had a greater prevalence of chronic obstructive pulmonary disease (COPD), and presented significantly lower systolic blood pressure upon admission (Table 1). The CardShock score presented an AUC for predicting mortality of 0.55 (95% confidence interval [95%CI]: 0.42–0.69; p=0.459). The predictive model with age, lactate and creatinine clearance coded as continuous variables presented an AUC of 0.70 (95%CI: 0.58–0.83; p=0.001). Fig. 1 shows the ROC curves for the prediction of in-hospital mortality of the CardShock score (A) and of the model with age, lactate and glomerular filtration rate coded as continuous variables (B).
Clinical characteristics of the patients according to outcome at discharge.
| Deceased (n=39) | Alive (n=44) | p-value | |
|---|---|---|---|
| Age (years) | 56.1 (12) | 52.6 (13) | 0.223 |
| Male gender | 31 (79.5) | 39 (88.6) | 0.252 |
| Diabetes mellitus | 9 (23.1) | 9 (20.5) | 0.772 |
| Active smoker | 18 (47.4) | 20 (45.5) | 0.226 |
| Arterial hypertension | 17 (43.6) | 15 (34.1) | 0.375 |
| Previous stroke | 3 (7.7) | 0 | 0.120 |
| Peripheral arterial disease | 3 (7.7) | 1 (2.3) | 0.250 |
| COPD | 7 (17.9) | 1 (2.3) | 0.019 |
| Previous AMI | 7 (17.9) | 5 (11.4) | 0.395 |
| Previous heart surgery | 1 (2.6) | 1 (2.3) | 0.931 |
| Cause of shock | 0.634 | ||
| ACS | 13 (33.3) | 14 (31.8) | |
| Myocarditis | 3 (7.7) | 7 (15.9) | |
| Decompensated chronic cardiomyopathy | 9 (23.1) | 5 (11.4) | |
| Ventricular arrhythmias | 5 (12.8) | 6 (13.6) | |
| Others | 9 (23.1) | 12 (27.2) | |
| INTERMACS class upon admission | 0.566 | ||
| 1 | 30 (76.9) | 30 (69.8) | |
| 2 | 7 (17.9) | 11 (25.6) | |
| 3 | 2 (5.2) | 2 (4.7) | |
| Systolic BP upon admission | 74 (24) | 86 (17) | 0.021 |
| Differential BP upon admission | 29 (13) | 34 (13) | 0.138 |
| Management strategy | 0.580 | ||
| Referral to decision | 13 (33.3) | 9 (20.5) | |
| Referral to recovery | 15 (38.5) | 21 (47.7) | |
| Referral to transplantation | 4 (10.3) | 4 (9.1) | |
| Referral to surgery | 7 (17.9) | 10 (22.7) | |
| Cardiorespiratory arrest | 17 (43.6) | 18 (40.9) | 0.805 |
| Neurological dysfunction upon admission | 13 (33.3) | 10 (22.7) | 0.196 |
| LVEF | 27 (18) | 26 (13) | 0.791 |
| Basal creatinine clearance | 38 (21) | 46 (22) | 0.126 |
| Basal serum pH | 7.26 (0.2) | 7.33 (0.2) | 0.255 |
| Basal serum bicarbonate | 19.5 (4) | 19.5 (4) | 0.988 |
| Basal lactate | 8.6 (7) | 6.1 (5) | 0.081 |
| CardShock score | 4.4 (1) | 4.1 (2) | 0.324 |
| Multivariate analysis for the prediction of in-hospital mortality | ||
|---|---|---|
| Predictor | Odds ratio (OR) (95%CI) | p-value |
| Age | 1.03 (0.97–1.10) | 0.314 |
| Neurological dysfunction upon admission | 0.56 (0.08–4.07) | 0.566 |
| Previous AMI or coronary surgery | 3.30 (0.45–24.02) | 0.239 |
| Lactate upon admission | 1.10 (0.97–1.25) | 0.150 |
| Glomerular filtration rate | 0.97 (0.93–1.01) | 0.106 |
| Shock due to ACS | 2.34 (0.50–10.89) | 0.279 |
| LVEF <40% | 2.55 (0.36–17.9) | 0.346 |
COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; AMI: acute myocardial infarction; ACS: acute coronary syndrome; BP: blood pressure.
The results of our study evidence suboptimal performance of the CardShock score in patients of this kind. Undoubtedly, one of the most probable reasons for this is the difference in clinical profile of the patients. While the CardShock2 registry used the classical shock criteria (i.e., hypotension with signs of peripheral hypoperfusion, altered mental state, oliguria, peripheral coldness or lactate >2mmol/l) in the first 6h after the diagnosis, the patients in our series mostly presented a profile corresponding to profound cardiogenic shock, since over 95% were in INTERMACS class 1/2 upon admission. Profound cardiogenic shock,6 which is characterized by lower blood pressure values and more consolidated signs of hypoperfusion, is the scenario in which ECMO has shown the best outcomes.7
Furthermore, the patients in our series were significantly younger, with significantly higher lactate concentrations than in the CardShock registry.2 It should be noted that the cut-off age in the CardShock is 75 years, which was exceeded by 25% of the cases in the mentioned registry, versus by only one of the 83 patients in our study. On the other hand, the lactate thresholds in the CardShock score are 2 and 4 points. In this regard, over 80% of the patients in our series presented >2. The scant variability around these components of the score explains the loss of its predictive capacity in our patients. The observed improvement in performance of the scale on coding age, lactate and glomerular filtration rate as continuous variables clearly supports this idea.
The present study has some limitations, such as its limited sample size or the retrospective nature of calculation of the CardShock score. Since this was an observational registry, we are unable to rule out selection bias and the effect of non-analyzed confounding factors. Lastly, as this was a single-center registry, the findings need to be replicated in other series involving different clinical management practices.
Nevertheless, our results reasonably illustrate the performance of the CardShock score in patients with profound shock. We were unable to obtain adequate predictive capacity on applying the score to patients of this kind; the instrument probably needs to be adapted with different cut-off points in this concrete scenario. Optimization of risk stratification and the prognosis of patients as complex as these could have important clinical, economic and social consequences.
Please cite this article as: Sánchez-Salado JC, Lorente V, Alegre O, Llaó I, Blázquez L, Ariza-Solé A. Rendimiento de la escala CardShock en pacientes con shock cardiogénico profundo tratados con membrana de oxigenación extracorpórea venoarterial. Med Intensiva. 2020;44:312–315.