To compare rSO2 (muscle oxygen saturation index) static and dynamic variables obtained by NIRS (Near Infrared Spectroscopy) in brachioradialis muscle of septic shock patients and its prognostic implications.
DesignProspective and observational study.
SettingIntensive care unit.
SubjectsSeptic shock patients and healthy volunteers.
InterventionsThe probe of a NIRS device (INVOS 5100) was placed on the brachioradialis muscle during a vascular occlusion test (VOT).
VariablesBaseline, minimum and maximum rSO2 values, deoxygenation rate (DeOx), reoxygenation slope (ReOx) and delta value.
ResultsSeptic shock patients (n=35) had lower baseline rSO2 (63.8±12.2 vs. 69.3±3.3%, p<0.05), slower DeOx (−0.54±0.31 vs. −0.91±0.35%/s, p=0.001), slower ReOx (2.67±2.17 vs. 9.46±3.5%/s, p<0.001) and lower delta (3.25±5.71 vs. 15.1±3.9%, p<0.001) when compared to healthy subjects (n=20). Among septic shock patients, non-survivors showed lower baseline rSO2 (57.0±9.6 vs. 69.8±11.3%, p=0.001), lower minimum rSO2 (36.0±12.8 vs. 51.3±14.8%, p<0.01) and lower maximum rSO2 values (60.6±10.6 vs. 73.3±11.2%, p<0.01). Baseline rSO2 was a good mortality predictor (AUC 0.79; 95%CI: 0.63–0.94, p<0.01). Dynamic parameters obtained with VOT did not improve the results.
ConclusionSeptic shock patients present an important alteration of microcirculation that can be evaluated by NIRS with prognostic implications. Monitoring microvascular reactivity in the brachioradialis muscle using VOT with our device does not seem to improve the prognostic value of baseline rSO2.
Comparar las variables microcirculatorias estáticas y dinámicas obtenidas mediante espectroscopia cercana al infrarojo en el músculo braquiorradial de pacientes con shock séptico y sus implicaciones pronósticas.
DiseñoEstudio prospectivo y observacional.
ÁmbitoUnidad de Cuidados Intensivos.
PacientesPacientes con shock séptico y voluntarios sanos.
IntervencionesEn el músculo braquioradial de todos los sujetos se realizaron mediciones NIRS (del inglés Near Infrared Spectroscopy) durante un test de oclusión vascular.
VariablesValores rSO2 basal, mínimo y máximo, pendiente de desoxigenación, pendiente de reoxigenación y valor delta.
ResultadosLos pacientes con shock séptico (n=35) presentaron unos valores basales de rSO2 más bajos (63,8±12,2 frente a 69,3±3,3%; p<0,05), una pendiente de desoxigenación y reoxigenación más lenta (−0,54±0,31 frente a −0,91±0,35%/s, p=0,001; 2,67±2,17 frente a 9,46±3,5%/s, p<0,001) y un delta menor (3,25±5,71 frente a 15,1±3,9%; p<0,001) en comparación con los sujetos sanos (n=20). De los pacientes con shock séptico, los no supervivientes presentaron unos valores basales de rSO2 más bajos (57,0±9,6 frente a 69,8±11,3%; p=0,001), de rSO2 mínimo y máximo igualmente inferiores (36,0±12,8 frente a 51,3±14,8%; p<0,01; 60,6±10,6 frente a 73,3±11,2%; p<0,01). El rSO2 basal fue un buen factor predictivo de mortalidad (AUC 0,79; IC del 95%: 0,63-0,94; p<0,01). Los parámetros dinámicos obtenidos mediante la prueba de oclusión vascular no mejoraron los resultados.
ConclusiónLos pacientes con shock séptico presentan una alteración importante de la microcirculación que se puede evaluar mediante la espectroscopia cercana al infrarrojo con implicaciones pronósticas. La monitorización de la reactividad microvascular en el músculo braquiorradial mediante el test de oclusión vascular con nuestro dispositivo no parece mejorar el valor pronóstico de la rSO2 basal.
Septic shock remains the most frequent cause of death in non-coronary intensive care units.1,2 It is characterized by hemodynamic alterations associated with organ dysfunction. Although systemic oxygen transport is usually preserved after the resuscitation of septic shock, multiorgan failure frequently develops as a probable consequence of an imbalance between oxygen delivery and consumption (DO2 and VO2) at tissue level. Microcirculatory dysfunction could be involved in this phenomenon.
The microcirculatory derangements caused by sepsis consist in decreased functional vascular density and heterogeneity of capillary transit-time.3–7 They may persist despite the normalization of systemic hemodynamics and oxygen transport in the large blood vessels, having relevant prognostic implications. It is well known that recovery of macrohemodynamic stability does not necessarily involve a parallel enhancement in microcirculation, organ function restoration, and a subsequent better prognosis.8–10 Therefore, some macrohaemodynamical markers such as blood pressure, cardiac output, and other systemic cardiovascular variables are not reliable predictors of the evolution of septic shock.11 In contrast, the assessment of oxygen release to the peripheral tissues would better reflect the microcirculation status.
However, peripheral oxygenation depends on many different factors including the ensemble of small vessels, local blood flow, hemoglobin content and oxygen partial pressure, among others.6 This complexity has meant that clinicians have not been able to assess the status of microcirculation and peripheral tissue oxygenation at the bedside until last years. Nevertheless, both can be easily approximated with Near Infrared Spectroscopy (NIRS)12 through determination of skeletal muscle oxygen tissue saturation (StO2) or skeletal muscle oxygen saturation index (rSO2) depending on the device used. StO2 is a measure of oxygen saturation in the microcirculation where oxygen is exchanged with tissue and it is usually measured in the thenar eminence. On the other hand, rSO2 (“regional”) value is an index of the OxyHb present within a volume of tissue (expressed as the percentage of oxygenated hemoglobin relative to total) and it can be easily assessed in other locations.
In this regard, both have demonstrated its prognostic value in septic shock.13–18 In addition to simple determination of StO2, several studies have evaluated the behavior of this variable during an ischemic challenge (vascular occlusion test, VOT) in septic shock patients.19–26 This test assesses the reactivity of small vessels and appears to improve the predictive ability of simple StO2 determination.19
We have previously showed the relevance of measuring rSO2 at the brachioradialis muscle as an outcome predictor in septic shock.17,18 The behavior of skeletal brachioradialis rSO2 in response to VOT and its relationship to mortality in such patients has not been assessed yet. We hypothesized that this test can further improve the predictive value of simple rSO2 measurement in the brachioradialis muscle of septic shock patients as StO2 in the thenar eminence. Accordingly, the aim of the present study was to compare the prognostic implications relate to mortality of rSO2 static and dynamic variables in the brachioradialis muscle of septic shock patients.
Materials and methodsThe investigation was conducted according to the principles outlined in the Declaration of Helsinki for studies in humans. The study protocol was approved by the Ethics Committee at our institution, and informed consent was given by each patient or their next of kin.
Study populationA prospective and observational study was conducted in a 30-bed Department of a medical-surgical Intensive Care Unit (ICU) at our university hospital. Consecutive patients admitted in the ICU with the diagnosis of septic shock and healthy volunteers (controls) were included in the study from June 2012 through December 2013. Severe sepsis and septic shock were defined according to these criteria established27: “a sepsis complicated by organ dysfunction” and “a sepsis-induced hypotension which persists despite adequate fluid resuscitation, and requires the administration of vasopressor agents”, respectively. The treatment for septic shock was administered by an independent clinical team and following locally adapted standards recommended by the Surviving Sepsis Campaign.1 Exclusion criteria were tissue edema, upper extremity fractures, amputations, hematomas over forearms, morbid obesity (body mass index>35kg/m2), pregnancy, age<18 years, diabetes requiring insulin therapy, cachexia, alcoholism, severe peripheral vascular disease, chronic renal insufficiency requiring hemodialysis, evidence of cirrhosis or immunodeficiencies.
Study protocolPatients were always enrolled within the first 24h after the onset of severe sepsis, during an episode of septic shock. Information collected comprised: demographic characteristics including age and sex, the Acute Physiology and Chronic Health Evaluation (APACHE) II score,28 the Sequential Organ Failure Assessment (SOFA) score,29 the primary site and type of infection, as well as hemodynamic and respiratory variables including heart rate (HR), mean arterial pressure (MAP), temperature, arterial blood gases and acid–base balance, hemoglobin concentration, norepinephrine use and dose, mechanical ventilation and PaO2/FIO2.
All patients were treated with fluid resuscitation and norepinephrine to maintain MAP above 65mm Hg (maximum dose, 2μg/kg/min). Fluid resuscitation was administered by a fluid bolus challenge with crystalloids to increase stroke volume and/or allow the vasopressor doses to be decreased when required. In accordance with our local guidelines, each subject was monitored through the entire process. All the NIRS parameters were studied related to ICU mortality.
MeasurementsValues of rSO2 were obtained with updates every 6s using a NIRS spectrometer (INVOS 5100C Oximeter and adult SomaSensor model SAFB-SM, Somanetics Corporation, Troy, MI, USA), coupled to a skin surface probe. The probe was placed on the medial forearm, 5cm distal to the elbow, in the brachioradialis muscle. To ensure the validity of measurements in septic shock patients, rSO2 measurements were only performed when MAP>60mm Hg and the vasopressor infusion rate remained unchanged for at least the preceding 2h.
Vascular occlusion test (VOT)The VOT consisted in stopping the blood flow of the brachial artery by wrapping a sphygmomanometer cuff around the arm and inflating it until 200mm Hg. Briefly, after the stabilization of the baseline NIRS signal (defined as a rSO2 variation<2% over 30s), baseline rSO2 was recorded (defined as the average of the three firsts values of rSO2). Afterwards, arterial inflow was stopped. After 3min of ischemia the cuff pressure was released and arterial blood flow returned. The rSO2 signal was continuously recorded through the entire procedure and until the final signal stabilization. Only one vascular occlusion test was made in every subject and all the parameters were included without known the final patient outcome.
The parameters obtained during VOT were: (1) the deoxygenation rate (DeOx), which indicates the velocity of the rSO2 decrease during the ischemia and is believed to reflect the status of the local aerobic metabolism including oxygen consumption in the NIRS-assessed area30–32; (2) the reoxygenation rate, which is the velocity of the rSO2 recovery following VOT, which would reflect the time required to wash out stagnant blood and it is thought to be determined by the reserve capacity and functionality of the microvascular system. In this regard we calculated the reperfusion rate in the first 12s (slope 12s) and in the entire reperfusion time (ReOx); (3) the difference (delta) between the baseline rSO2 and its maximum value during the hyperemic phase. This variable would represent the tissue's ability to regulate local blood flow and oxygenation; (4) the lowest rSO2 value at the end of the ischemic period; and (5) the highest rSO2 value during the reperfusion phase.
Statistical analysisSample size was calculated based on previous study.17 It was estimated that the rSO2 standard deviation between the survivors and non-survivors septic shock patients would be 8.5%, and a 50% of mortality was assumed. With a statistical power of 80% and an alpha error of 0.05, at least 16 patients would be needed in both survivors and non-survivors groups, to detect a difference of 1 standard deviation, considered as the lowest clinical relevance. A descriptive analysis was performed. Normal distribution of the studied variables was confirmed using the Kolmogorov–Smirnov test. Discrete variables were expressed as counts (percentage) and continuous variables as mean±standard deviation (SD) or median (25th–75th percentiles) as appropriate. Differences between groups were assessed using a Fisher's exact test and Student's t-test, Mann–Whitney U-test and Chi-square test, as appropriate. The signal slopes were determined by linear regression analysis. Pearson's correlation coefficient was used to assess the association between rSO2 and VOT with others variables.
The predictive value of the baseline rSO2 and slopes were calculated using a receiver operator characteristic curve (ROC), and the area under the curve (AUC) was also computed. A p<0.05 was considered significant. Data were analyzed using the SPSS Statistics package 20 version (IBM, Armonk, NY, USA).
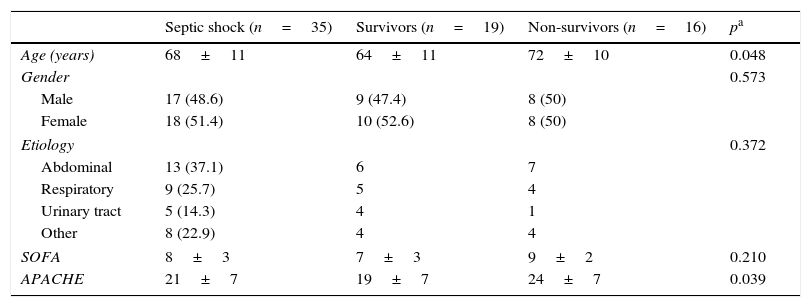
ResultsThirty-five patients with septic shock (68±11 years of age, 48.6% males) and twenty healthy volunteers (55±8 years of age, 45% males) were finally included. Only three patients were not included because exclusion criteria: two patients with diabetes requiring insulin therapy and one patient with chronic renal insufficiency requiring hemodialysis. The main characteristics of patients are shown in Tables 1 and 2. Abdomen was the most common source of infection (37.1%), followed by community-acquired pneumonia (25.7%), urinary tract infections (14.3%) and others. Even though the differences did not reach statistical significance, non-survivors had more tachycardia, hyperlactatemia, acidosis and norepinephrine dosing compared with survivors.
Demographics characteristics of the study groups. SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation.
| Septic shock (n=35) | Survivors (n=19) | Non-survivors (n=16) | pa | |
|---|---|---|---|---|
| Age (years) | 68±11 | 64±11 | 72±10 | 0.048 |
| Gender | 0.573 | |||
| Male | 17 (48.6) | 9 (47.4) | 8 (50) | |
| Female | 18 (51.4) | 10 (52.6) | 8 (50) | |
| Etiology | 0.372 | |||
| Abdominal | 13 (37.1) | 6 | 7 | |
| Respiratory | 9 (25.7) | 5 | 4 | |
| Urinary tract | 5 (14.3) | 4 | 1 | |
| Other | 8 (22.9) | 4 | 4 | |
| SOFA | 8±3 | 7±3 | 9±2 | 0.210 |
| APACHE | 21±7 | 19±7 | 24±7 | 0.039 |
Data are shown as mean±SD or n (%).
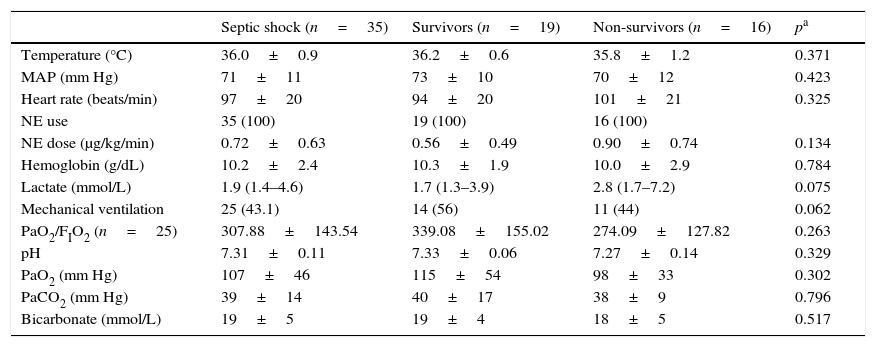
Clinical characteristics of the study groups. MAP, mean arterial pressure; NE, norepinephrine; PaO2, partial pressure of oxygen in arterial blood; PaCO2, partial pressure of carbon dioxide in arterial blood; BD, base deficit.
| Septic shock (n=35) | Survivors (n=19) | Non-survivors (n=16) | pa | |
|---|---|---|---|---|
| Temperature (°C) | 36.0±0.9 | 36.2±0.6 | 35.8±1.2 | 0.371 |
| MAP (mm Hg) | 71±11 | 73±10 | 70±12 | 0.423 |
| Heart rate (beats/min) | 97±20 | 94±20 | 101±21 | 0.325 |
| NE use | 35 (100) | 19 (100) | 16 (100) | |
| NE dose (μg/kg/min) | 0.72±0.63 | 0.56±0.49 | 0.90±0.74 | 0.134 |
| Hemoglobin (g/dL) | 10.2±2.4 | 10.3±1.9 | 10.0±2.9 | 0.784 |
| Lactate (mmol/L) | 1.9 (1.4–4.6) | 1.7 (1.3–3.9) | 2.8 (1.7–7.2) | 0.075 |
| Mechanical ventilation | 25 (43.1) | 14 (56) | 11 (44) | 0.062 |
| PaO2/FIO2 (n=25) | 307.88±143.54 | 339.08±155.02 | 274.09±127.82 | 0.263 |
| pH | 7.31±0.11 | 7.33±0.06 | 7.27±0.14 | 0.329 |
| PaO2 (mm Hg) | 107±46 | 115±54 | 98±33 | 0.302 |
| PaCO2 (mm Hg) | 39±14 | 40±17 | 38±9 | 0.796 |
| Bicarbonate (mmol/L) | 19±5 | 19±4 | 18±5 | 0.517 |
Data are shown as mean±SD, median (IR) or n (%).
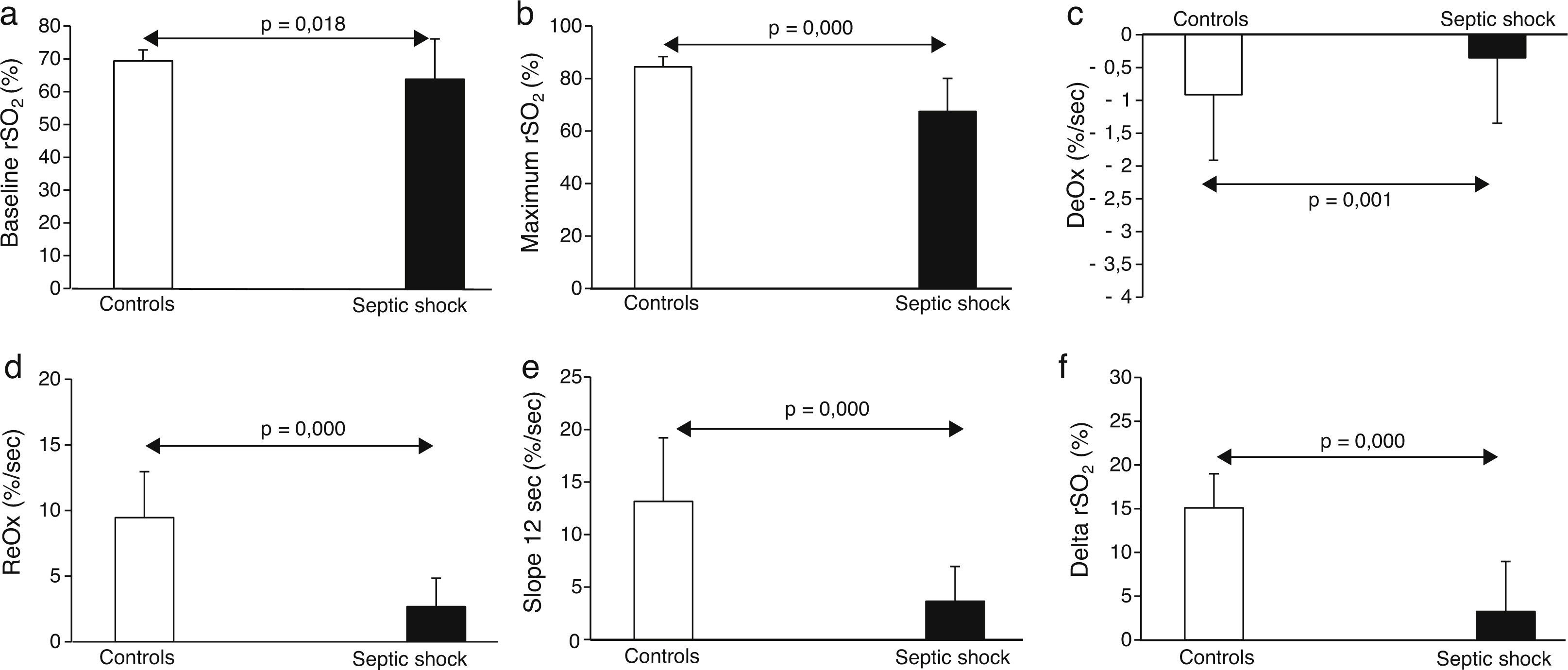
The main results obtained with NIRS are shown in Fig. 1. Briefly, septic shock patients showed lower baseline rSO2 compared with healthy controls (63.8±12.2 vs. 69.3±3.3%, p<0.05). Moreover, the DeOx obtained in the ischemic phase and the ReOx slopes obtained in the reperfusion phase (total ReOx and at 12s) were slower in septic shock patients (−0.54±0.31 vs. −0.91±0.35%/s, p=0.001; 2.67±2.17 vs. 9.46±3.5%/s, p<0.001; and 3.64±3.32 vs. 13.15±6.07%/s, p<0.001). The same accounted for the delta value (3.25±5.71 vs. 15.1±3.9%, p<0.001) being the maximum rSO2 post-reperfusion greater in the control group (67.48±12.55 vs. 84.45±3.92, p<0.001). No differences were found in the minimum rSO2 between groups.
We just observed significant correlation between the SOFA score at ICU admission and DeOx slope at 12s (−0.369, p=0.034). No other correlations with significant values were observed.
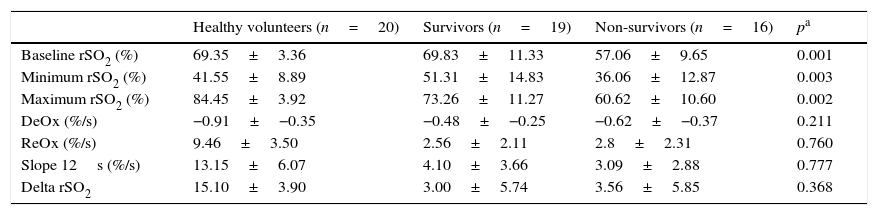
Survivors vs. non-survivorsThe ICU mortality in septic shock patients was 46%, and the comparison between values obtained with NIRS in survivors and non-survivors is shown in Table 3. It is worth noting that baseline rSO2 was significantly greater in the former group. Minimum rSO2 and maximum rSO2 post-reperfusion were lower in non-survivors. There were no differences in any of the slopes neither in the delta value between two groups.
rSO2 curve variables in survivors (n=19) and non-survivors (n=16). Minimum rSO2, minimum rSO2 post-ischemia; Maximum rSO2, maximum rSO2 during the reperfusion; DeOx, deoxygenation rate; ReOx, reoxygenation rate; Slope 12s, slope in the firsts 12s; Delta rSO2, difference between baseline rSO2 and its maximum value.
| Healthy volunteers (n=20) | Survivors (n=19) | Non-survivors (n=16) | pa | |
|---|---|---|---|---|
| Baseline rSO2 (%) | 69.35±3.36 | 69.83±11.33 | 57.06±9.65 | 0.001 |
| Minimum rSO2 (%) | 41.55±8.89 | 51.31±14.83 | 36.06±12.87 | 0.003 |
| Maximum rSO2 (%) | 84.45±3.92 | 73.26±11.27 | 60.62±10.60 | 0.002 |
| DeOx (%/s) | −0.91±−0.35 | −0.48±−0.25 | −0.62±−0.37 | 0.211 |
| ReOx (%/s) | 9.46±3.50 | 2.56±2.11 | 2.8±2.31 | 0.760 |
| Slope 12s (%/s) | 13.15±6.07 | 4.10±3.66 | 3.09±2.88 | 0.777 |
| Delta rSO2 | 15.10±3.90 | 3.00±5.74 | 3.56±5.85 | 0.368 |
Data are shown as mean±SD.
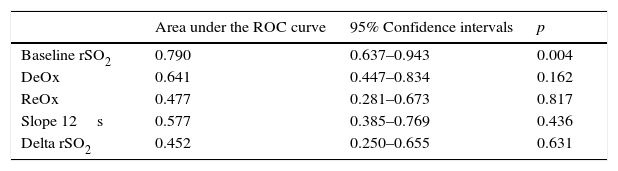
Finally, the baseline rSO2 ROC curve confirmed a good discriminatory power for mortality (Table 4). In contrast, none of the dynamic parameters obtained with the VOT showed a significant AUC value (Table 3). The baseline rSO2 cutoff value of 60% showed a sensitivity of 83%.
Discriminative power of rSO2 and other VOT-derived variables relate to mortality in septic shock patients.
| Area under the ROC curve | 95% Confidence intervals | p | |
|---|---|---|---|
| Baseline rSO2 | 0.790 | 0.637–0.943 | 0.004 |
| DeOx | 0.641 | 0.447–0.834 | 0.162 |
| ReOx | 0.477 | 0.281–0.673 | 0.817 |
| Slope 12s | 0.577 | 0.385–0.769 | 0.436 |
| Delta rSO2 | 0.452 | 0.250–0.655 | 0.631 |
The study suggests that among septic shock patients, baseline rSO2 differed between survivors and non-survivors and was able to predict mortality. On the contrary and against our hypothesis, dynamic variables obtained with our device did not differ between survivors and non-survivors. To our knowledge this prospective study is the first to assess the microcirculatory dysfunction in this muscle after an ischemic challenge and to study its association with mortality.
Although NIRS rSO2 is a measure of muscle tissue oxygenation and not a direct marker of microcirculatory perfusion, it permits the quantification of the microcirculatory dysfunction.32 NIRS during a VOT might give additional information about local VO2, reactive hyperemia, and microcirculation recruitment and it seems to be sensitive to the progress and outcome of sepsis in clinical illness.19,20,25,26
Several studies have assessed the microvascular reactivity in the thenar eminence in sepsis with other NIRS devices.13,15,16,19–26 Most of them found differences in the baseline StO2 between septic patients and healthy volunteers.13,15,16,19–21,24,26 In this sense, Leone et al.13 found that StO2 static values were significantly lower in the non-survivors than in survivors septic shock patients. In contrast, some of them22,23,25 did not show differences between both groups. However, in these studies dynamic NIRS improved the discrimination power between normal and abnormal states of microcirculation. Furthermore, a recent meta-analysis that included twenty articles dealing with the prognostic implications of StO2, showed that the static value is lower in septic patients than in control subjects.14
Our group have recently demonstrated that in septic shock throughout the first 24h after ICU admission, the patients with brachioradialis skeletal muscle rSO2<60% had significantly greater mortality than patients with rSO2>60% in the same location.17 Accordingly to these data, in the present study we confirm that brachioradialis baseline rSO2 is a good prognostic predictor in this group of patients and that the cut-off value of 60% presents a good sensitivity for mortality prediction. Furthermore, in this study we improved the methodology of our previous work using a dynamic test.
Most of the studies that analyze the association between static and dynamic NIRS variables and mortality in septic shock patients were conducted in the thenar eminence with other NIRS devices. In one hand, in these studies static thenar StO2 was variably reported as associated13,14 or not associated19,20,25 with mortality. On the other hand, some of these studies demonstrated that the recovery slope outperformed the other NIRS-derived variables because it was more strongly associated with organ dysfunction and mortality.19,20,25
In this sense, Creteur et al.19 showed a significant association between a reduced reperfusion slope after VOT and mortality in septic shock patients while there were no differences in the static parameters between patients and control subjects neither in mortality. Payen et al.20 also showed a depressed reperfusion slope in non-survivors septic shock patients compared with survivors with a significant ROC curve for the prediction of mortality. They also showed a lower static StO2 in patients than in healthy subjects but without any difference between survivors and non-survivors. In the same way, Shapiro et al.25 observed that baseline StO2 did not discriminate between septic shock patients and control subjects, while the deoxygenation and recovery slope distinguish them. Non-survivors presented slower slopes than survivors with a relevant ROC curve.
Mesquida et al.26 also reported that in a population of septic shock patients impaired DeOx was associated with no improvement in organ failures after 24h. They did not found differences in baseline StO2 between SOFA improvers and SOFA non-improvers patients.
Finally, Neto et al.14 in the meta-analysis above mentioned, concluded that static StO2 distinguish between survivors and non-survivors septic shock patients, just like reoxygenation slope.
More recently, different groups have evaluated the microcirculation by NIRS in other locations in order to find better anatomic sites with different results.15–18
Ait-Oufella et al.15 evaluated the prognostic value of StO2 measured around the knee in septic shock patients and compared it with thenar eminence StO2. They concluded that after initial septic shock resuscitation, StO2 measured around the knee is a strong predictive factor of 14-day mortality. In the same way, Colin et al.16 demonstrated that masseter and deltoid StO2, but not thenar StO2 were strong predictors of 28-day mortality.
We recently have assessed and compared rSO2 obtained simultaneously in two different muscles, brachioradialis and deltoid.18 Our results showed that both measurements present a strong correlation and adequate consistency and both muscles also showed consistent discriminatory power for mortality.
Our present results are in accordance with these data, showing that static rSO2 measurements in the brachioradialis muscle are good predictors of mortality. Although some articles showed that dynamic tests improve the prognostic value of the static parameters in the thenar eminence, our results show that VOT test in brachioradialis muscle of septic shock patients is not better to assess the microcirculatory alterations. Static NIRS data in this location is easier to obtain and better mortality predictor than dynamic parameters. However, it has to be taken into account that the studies without differences in dynamic tests between survivors and non-survivors included a small sample size. Future studies with a large number of patients will be necessaries to elucidate this debate.
Although skeletal muscle StO2 may be equivalent to rSO2, they are obtained with different devices and the values are calculated with different algorithms. For this reason it is difficult to compare both parameters between them. However, further studies will be necessary to compare the prognostic value of the static NIRS measurements in the thenar eminence and in the other muscles. Muscle myofiber composition, oxygen consumption and metabolism may differ between these muscles and it may contribute to these differences. In addition, thenar muscle may be more influenced by abnormal peripheral perfusion than the other territories,33 a major confounder in assessing StO2.34
In other way and in contrast with the reported in the literature, we did not found differences in SOFA Score at admission between survivors and non-survivors due to the small sample size. Even though some differences did not reach statistical significance, non-survivors patients had more tachycardia, hyperlactatemia and norepinephrine dosing. These results are in accordance with Edul et al.35 who recently demonstrated the association of microcirculatory alterations with these three variables in septic shock patients.
This study and the NIRS technology have some limitations that should be taken into account. First of all, the methodologies for assessing the microcirculation by NIRS are very inconsistent in the literature, and consequently, results vary from study to study, making data comparison difficult and potentially inadequate. Two major aspects concerning the inconsistent methodology are measurement site and probe spacing. The measurement site is important because differences may exist in the sensitivity of muscle groups and/or other anatomical structures to the VOT during health and/or pathophysiological conditions. Probe spacing, on the other hand, will determine the depth of measurement within the respective muscle group. To address these issues, Bezemer et al.36 demonstrated in healthy subjects that although not apparent at baseline, the probe spacing and measurement site significantly influenced VOT-derived StO2 variables. In addition, the optimal way of performing VOT is a matter of debate.37,38 Second, we have assessed microvascular dysfunction within the firsts 24h of the septic shock diagnosis, but the time of measurement also varies between the different studies and it could be a confounding factor. Further studies will be necessary to check the utility of NIRS parameters along the time and their changes with the treatment. Third, to our knowledge, this is the first study that evaluates every NIRS-derived variable in the braquioradialis muscle. Although increased thickness of the subcutaneous layer due to edema may reduce the muscle layer assessed, this was not a major problem in our study, since the tissue edema was an exclusion criteria. In any case, we evaluated the NIRS variables in the early phase of septic shock before the development of significant edema.
We conclude that baseline rSO2 obtained at the braquioradialis muscle is a good predictor of mortality in septic shock. In our population, VOT-derived variables in this muscle does not seem to improve the prognostic value of the static parameters. Future studies will be necessaries to confirm our results and to evaluate the usefulness of these parameters to evaluate the microcirculatory response to treatment.
Conflicts of interestNone.
We are thankful to Sergi Mojal from Institut Hospital del Mar d’Investigacions Mèdiques (IMIM) for his statistical support.
This study has been supported by Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación [Fondo de Investigaciones Sanitarias (FIS)PI10/01538 and PI13/02011], by Sociedad Española de Neumología y Cirugía Torácica [SEPAR 264/2012] and Agència de Gestió d’Ajuts Universitaris i de Recerca [AGAUR 2014-SGR926].