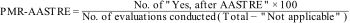From a safety perspective, the pandemic imposed atypical work dynamics that led to noticeable gaps in clinical safety across all levels of health care.
ObjectivesTo verify that Real-Time Random Safety Analyses (AASTRE) are feasible and useful in a high-pressure care setting.
DesignProspective study (January–September 2022).
SettingUniversity Hospital with 350 beds. Two mixed ICUs (12 and 14 beds).
Interventions: Two safety audits per week were planned to determine the feasibility and usefulness of the 32 safety measures (grouped into 8 blocks).
Main variables of interest1) Feasibility: Proportion of completed audits compared to scheduled audits and time spent. 2) Utility: Changes in the care process made as a result of implementing AASTRE.
ResultsA total of 390 patient-days were analyzed (179 were Non-COVID patients and 49 were COVID patients). In the COVID patient subgroup, age, ICU stay, SAPS 3, and ICU mortality were significantly higher compared to the Non-COVID patient subgroup. Regarding feasibility, 93.8% of planned rounds were carried out with an average audit time of 25 ± 8 min. Overall, changes in the care process were made in 11.8% of the measures analyzed.
ConclusionsIn a high-complexity care environment, AASTRE proved to be a feasible and useful tool with only two interventions per week lasting less than 30 min. Overall, AASTRE allowed unsafe situations to be turned safe in more than 10% of the evaluations.
Desde el punto de vista de la seguridad la pandemia impuso dinámicas de trabajo atípicas que provocaron visibles brechas en la seguridad clínica en todos los niveles de la atención sanitaria.
ObjetivosComprobar que los Análisis Aleatorios de Seguridad en Tiempo Real (AASTRE) son factibles y útiles en un escenario de elevada presión asistencial.
DiseñoEstudio prospectivo (enero y septiembre de 2022).
ÁmbitoHospital Universitario con 350 camas. Dos UCIs polivalentes (12 y 14 camas).
IntervencionesSe planificaron 2 auditorías de seguridad a la semana para determinar la factibilidad y la utilidad de las 32 medidas de seguridad.
Variables de Interés principales1) Factibilidad: proporción de auditorías completadas respecto a las programadas y el tiempo empleado; 2) Utilidad: cambios en el proceso de atención realizados como resultado de la aplicación de AASTRE.
ResultadosSe analizaron un total de 390 pacientes día (179 fueron pacientes No-COVID y 49 COVID). En los pacientes COVID la edad, el SAPS 3, la estancia y la mortalidad en UCI fueron significativamente mayores respecto a los pacientes No-COVID. En cuanto a la factibilidad, el 93.8% de las rondas planificadas fueron realizas con un tiempo promedio empleado por auditoría de 25 ± 8 minutos. Globalmente se produjeron cambios en el proceso de atención en el 11.8% de las medidas analizadas.
ConclusionesAASTRE, en un ambiente de elevada complejidad asistencial, resultó ser una herramienta factible y útil con sólo dos intervenciones semanales de menos de 30 minutos. Globalmente, AASTRE permitió revertir situaciones inseguras a seguras en más del 10% de las evaluaciones.
Still recent, it is easy to recall the strain exerted by the pandemic triggered by SARS-CoV-2 virus on the health care systems everywhere.1 Long before, in Intensive Care Medicine, there was already a strong association between high care pressure and a lack of adherence to clinical practice guidelines, which secondarily led to a worsening prognosis, including increased mortality.2–4
From a safety perspective, the pandemic imposed atypical work dynamics that caused gaps in clinical safety at all levels of health care.5 Specifically, significant changes in the perception of safety culture were described, associated with structural, leadership, and communication deficits.6 Other authors highlighted inefficiencies in the system due to a lack of process standardization.7
In the ICU setting, incidents related to patient safety (IRSP) during the pandemic led to an increase in primary bacteremia,8 central venous catheter-associated bacteremia,9 ventilator-associated pneumonia, catheter-associated urinary tract infections, renal failure, and thromboembolic and vascular events, all of which were associated with increased mortality.10,11 In response to these IRSPs, health care professionals were further strained as they were forced to adapt to new safety standards.12 Simultaneously, during this period, IRSP reporting systems were underutilized.13–15
Our group has developed a proactive safety tool called Real-Time Random Safety Audits (AASTRE), which has been associated with improvements in structure, process, and outcome quality indicators. AASTRE has proven particularly useful in situations of high care pressure and for the more severe critically ill patients.16,17 This tool is based on a set of evidence-based measures that are considered mandatory during ICU care activities. Randomization refers to the fact that neither the measures nor the patients audited can be known in advance of the safety rounds, as both are randomized on the day of the audit.
Emphasizing the importance of clinical safety and considering the paradox of its neglect during the pandemic, the objectives of this study are: 1) To describe the adaptation of AASTRE to the pandemic work dynamic; 2) To demonstrate that AASTRE is feasible and useful in a real pandemic scenario; 3) To build a web platform that makes the results visible in a simple, continuous, and intuitive way for clinicians.
Materials and methodsDesignWe conducted a prospective study in a teaching hospital with 350 beds and 2 multipurpose ICUs (12 and 14 beds). During the study period, both units treated COVID and non-COVID patients interchangeably. The ICU has a Clinical Information System (CIS) where data from patient bedside devices, information generated in other departments, and information generated/recorded by professionals during patient care are stored. These data were extracted for analysis in the present study.
Intervention periodFrom January 2022 through September 2022, coinciding with the 6th and last wave of the pandemic.
Description of AASTREAASTRE is a validated proactive safety tool18,19 that allows unsafe situations to be detected and converted into safe ones in real-time. In its version 2.0,20,21 it checks a total of 32 mandatory safety measures, distributed across 8 different blocks: 1) mechanical ventilation, 2) hemodynamics, 3) renal function and continuous renal replacement teraphies, 4) analgesia and sedation, 5) treatment, 6) nutrition, 7) nursing care and structure, and 8) clinical information system. Each safety measure has a specific definition, evaluation criteria, and a specific methodology for its verification. AASTRE was scheduled twice weekly in each unit for a total of 9 months. On evaluation days, 30% of ICU patients and 50% of the safety measure blocks were randomized. All patients admitted to the ICU are eligible for AASTRE. However, only measures for which the selected patient meets the evaluation criteria will be assessed. The possible responses during audits are: (1) "Yes" - when the measure analyzed was performed/taken during the daily ICU round; (2) "Yes, after AASTRE" - when the safety audit detected an omission error that was corrected; (3) "No" - when the analyzed measure could not be changed despite the audit; (4) "Not applicable" - when the patient does not meet the evaluation criteria. The checklist and audit responses are entered into a web platform (https://v2.aastre.es/web/index.php). A senior professional (Prompter)—who was not directly responsible for the care of any of the selected patients on the evaluation day—conducted the AASTRE at the bedside using a mobile device (Tablet), along with the treating nurse and physician (attending or resident), acting as a facilitator and providing feedback to the professionals during the entire process. The amount of changes in the process of health care as a result of verification was considered.
Definition of variables and indicators- 1
Patient-days: Number of patients evaluated on all days when safety audits were conducted.
- 2
Feasibility: Number of patients for whom AASTRE was completed in relation to those scheduled and the mean evaluation time.
- 3
Utility: Number of changes in the health care processes implemented as a result of the verification. Specifically, for each safety measure, a quantitative variable was defined to analyze it (improvement proportion related to AASTRE, PMR-AASTRE). PMR-AASTRE is defined as a process indicator such that there can be a PMR-AASTRE for each measure, for each block of measures (PMR-AASTRE-B), or overall, for the entire set of measures (PMR-AASTRE-G). Its calculation was performed using the following formula:
A PMR-AASTRE ≥ 10% was considered clinically relevant.17,20
- 1
Outcome indicators: ICU mortality, mean length of stay, and rates of central venous catheter-related bacteremia (CRB), ventilator-associated pneumonia (VAP), ventilator-associated tracheobronchitis (VAT), catheter-associated urinary tract infections (CAUTI), self-extubation of the endotracheal tube (ETT), reintubations, or barotrauma, using definitions and metrics published in former studies.21,22
- 2
Multivariate analysis: A selection of variables was made to determine their independent impact on a significant PMR-AASTRE-G. These variables included demographics (sex, age, patient type, and admission type), severity (SOFA, APACHE-II, SAPS-3), care burden (Nursing/Patient and Doctor/Patient ratios in addition to NAS – Nursing Activities Score), and those derived from the disease, severity, and vital support during their stay (COVID, length of stay and days on mechanical ventilation, RASS scale, presence of shock or need for continuous renal replacement techniques, and nutritional risk).
Demographic data and variables necessary to assess care process measures and quality indicators were extracted from the CIS, using a previously defined extract, transform, and load process.21–23
Statistical analysisTo describe baseline characteristics, continuous variables were expressed as median (Q1-Q3 range) and categorical ones as number of cases (percentage).
For patient demographic characteristics, clinical characteristics, care process measures, and quality indicators, inter-group differences were evaluated using the chi-square test and Fisher's exact test for categorical variables, and the Mann-Whitney U test or Wilcoxon test for continuous variables. This was performed using Python and the Tableone module, applying the chi-square test for each variable.
We conducted a multivariate analysis to determine the relationship between selected independent variables and a significant PMR-AASTRE-G ≥ 10%. To adjust for potential confounding effects, multiple logistic regression analysis, fixed model, and likelihood ratio method were used for potential confounding effects. Results were expressed as odds ratios with 95% confidence intervals. The acceptable level of statistical significance was set at p < 0.05. Data analysis was performed using R software (cran.r-project.org).
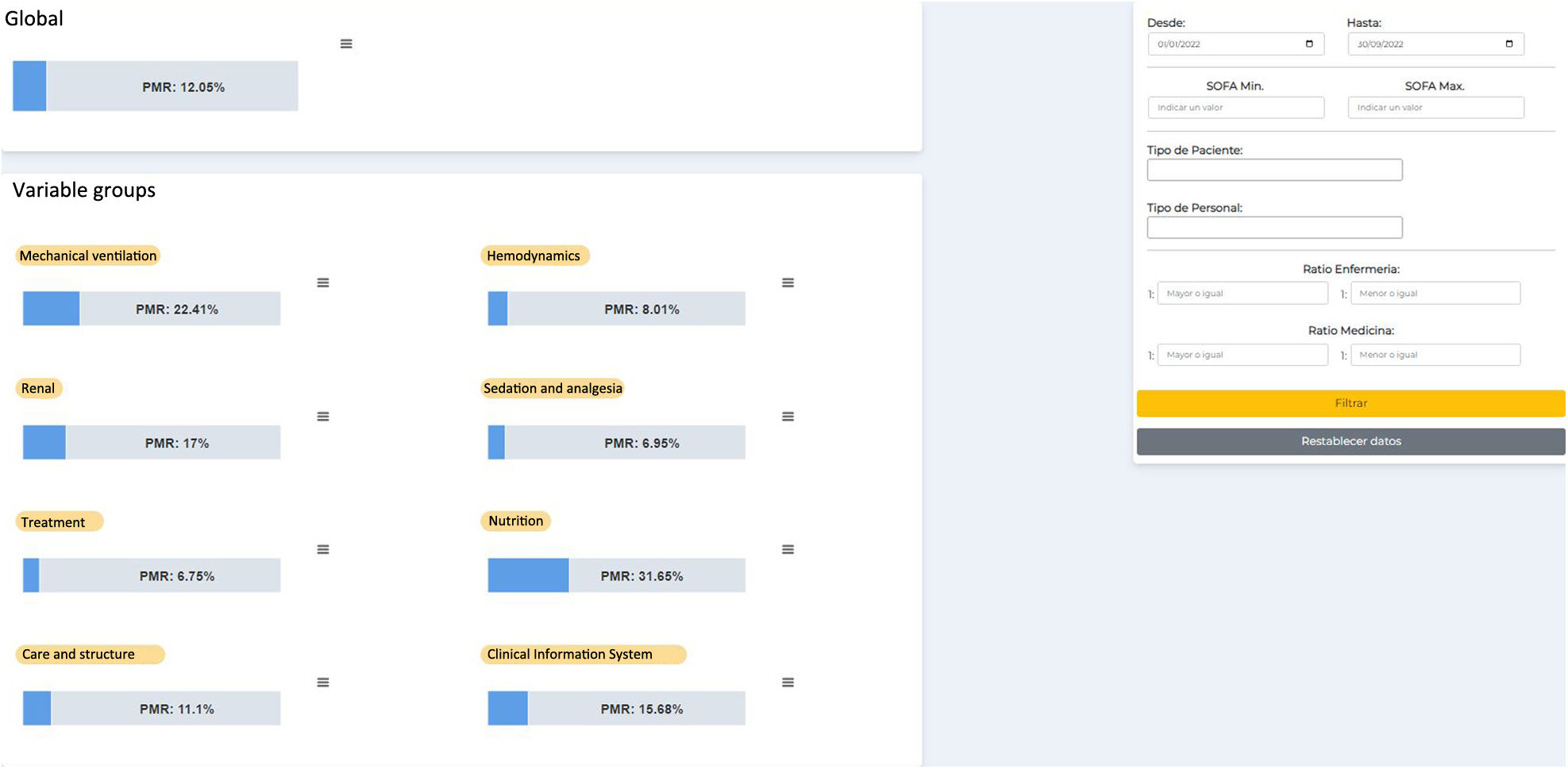
Construction and implementation of process indicators platformFor more efficient, automated, and real-time use of the data obtained from the AASTRE safety rounds, a web application was developed using free software (Python, Django, HTML, CSS, JavaScript) to access AASTRE results in the form of graphs that allow filtering by date, SOFA, patient type, staff type, nursing/patient ratio, and doctor/patient ratio, in addition to selecting PMR-AASTRE, whether for each variable, PMR-AASTRE-B, or PMR-AASTRE-G (Fig. 1).
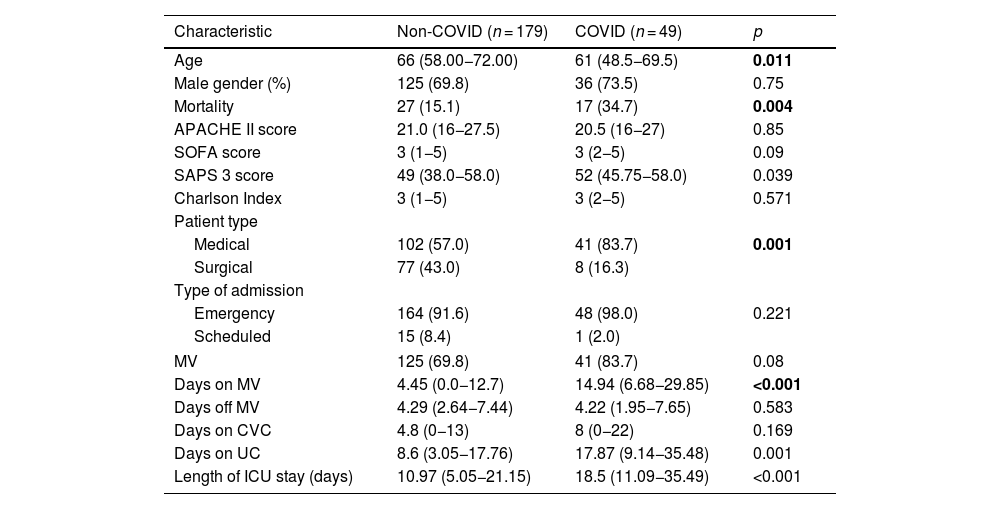
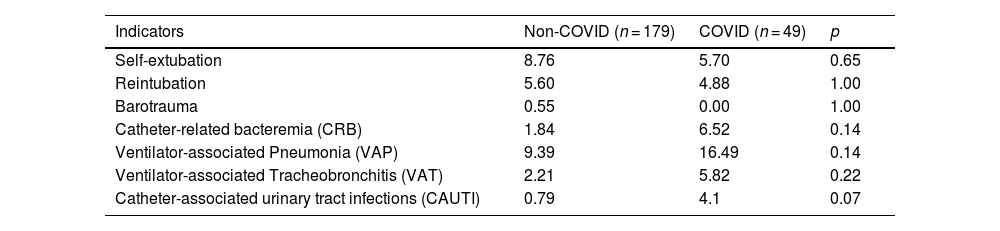
ResultsDuring the study period, a total of 390 patient-days were analyzed, 179 of whom were non-COVID patients and 49, COVID patients (Table 1). The latter were significantly younger [61 vs 66 years (p = 0.011)] than the non-COVID ones. Additionally, COVID patients had significantly longer lengths of stay, higher SAPS 3 scores, and higher ICU mortality rates vs non-COVID patients [18.5 vs 10.97 days (p < 0.001); 52 vs 49 (p = 0.039); and 34.7% vs 15.2% (p = 0.004), respectively]. Moreover, COVID patients remained many more days on mechanical ventilation and with a urinary catheter than no-COVID patients [14.94 vs 4.45 days (p < 0.001); 17.87 vs 8.6 days (p = 0.001), respectively]. No significant differences were found between the 3 groups in the outcome indicators under consideration (Table 2).
Patient characteristics. P < 0.05.
| Characteristic | Non-COVID (n = 179) | COVID (n = 49) | p |
|---|---|---|---|
| Age | 66 (58.00−72.00) | 61 (48.5−69.5) | 0.011 |
| Male gender (%) | 125 (69.8) | 36 (73.5) | 0.75 |
| Mortality | 27 (15.1) | 17 (34.7) | 0.004 |
| APACHE II score | 21.0 (16−27.5) | 20.5 (16−27) | 0.85 |
| SOFA score | 3 (1−5) | 3 (2−5) | 0.09 |
| SAPS 3 score | 49 (38.0−58.0) | 52 (45.75−58.0) | 0.039 |
| Charlson Index | 3 (1−5) | 3 (2−5) | 0.571 |
| Patient type | |||
| Medical | 102 (57.0) | 41 (83.7) | 0.001 |
| Surgical | 77 (43.0) | 8 (16.3) | |
| Type of admission | |||
| Emergency | 164 (91.6) | 48 (98.0) | 0.221 |
| Scheduled | 15 (8.4) | 1 (2.0) | |
| MV | 125 (69.8) | 41 (83.7) | 0.08 |
| Days on MV | 4.45 (0.0−12.7) | 14.94 (6.68−29.85) | <0.001 |
| Days off MV | 4.29 (2.64−7.44) | 4.22 (1.95−7.65) | 0.583 |
| Days on CVC | 4.8 (0−13) | 8 (0−22) | 0.169 |
| Days on UC | 8.6 (3.05−17.76) | 17.87 (9.14−35.48) | 0.001 |
| Length of ICU stay (days) | 10.97 (5.05−21.15) | 18.5 (11.09−35.49) | <0.001 |
COVID: COronaVIrus Disease; APACHE II: Acute Physiology and Chronic Health disease Classification System II at admission; SOFA: Sepsis-related Organ Failure Assessment in the first 24 h; SAPS 3: Simplified Acute Physiology Score III at admission; MV: mechanical ventilation; CVC: central venous catheter; UC: urinary catheter; UCI: intensive care unit.
Outcome indicators.
| Indicators | Non-COVID (n = 179) | COVID (n = 49) | p |
|---|---|---|---|
| Self-extubation | 8.76 | 5.70 | 0.65 |
| Reintubation | 5.60 | 4.88 | 1.00 |
| Barotrauma | 0.55 | 0.00 | 1.00 |
| Catheter-related bacteremia (CRB) | 1.84 | 6.52 | 0.14 |
| Ventilator-associated Pneumonia (VAP) | 9.39 | 16.49 | 0.14 |
| Ventilator-associated Tracheobronchitis (VAT) | 2.21 | 5.82 | 0.22 |
| Catheter-associated urinary tract infections (CAUTI) | 0.79 | 4.1 | 0.07 |
COVID: COronaVIrus Disease; CRB: catheter-related bacteremia; VAP: ventilator-associated pneumonia; VAT: ventilator-associated tracheobronchitis; CAUTI: catheter-associated urinary tract infections.
Audits were conducted in 93.8% of the patients for whom they were scheduled. The most common reasons for failing to conduct the audits in 6.2% of cases were lack of time from the Prompter and the patient's absence from the ICU at the time of the audit (e.g., in the operating room or undergoing imaging modalities). The median time spent on audits was 25 min ± 8 min.
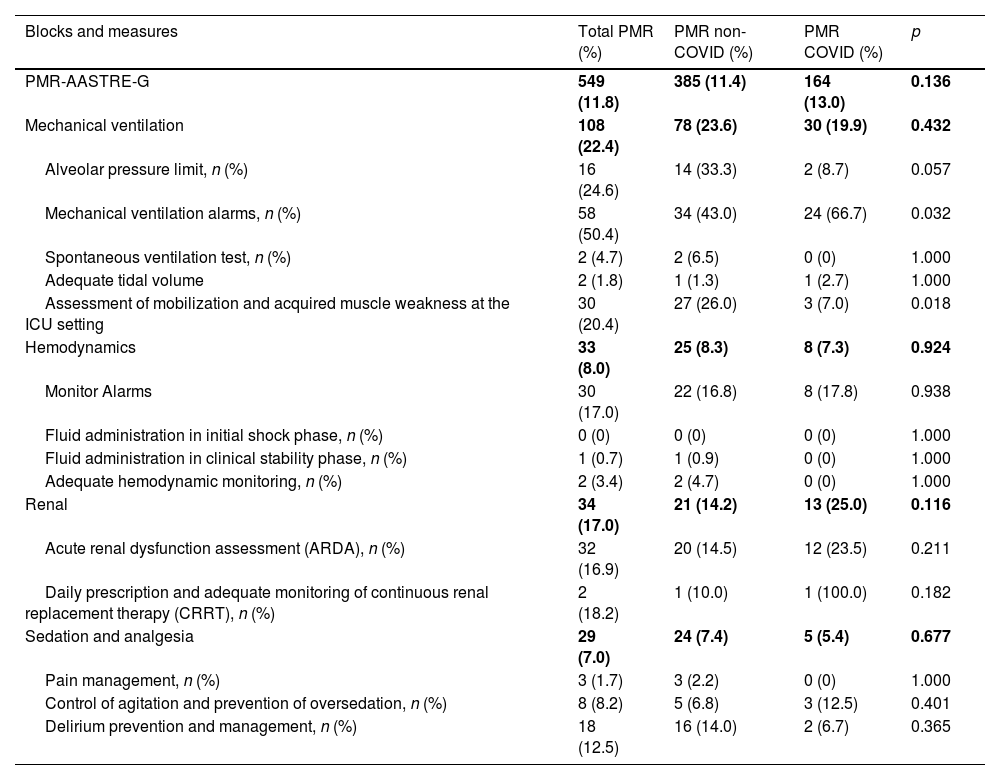
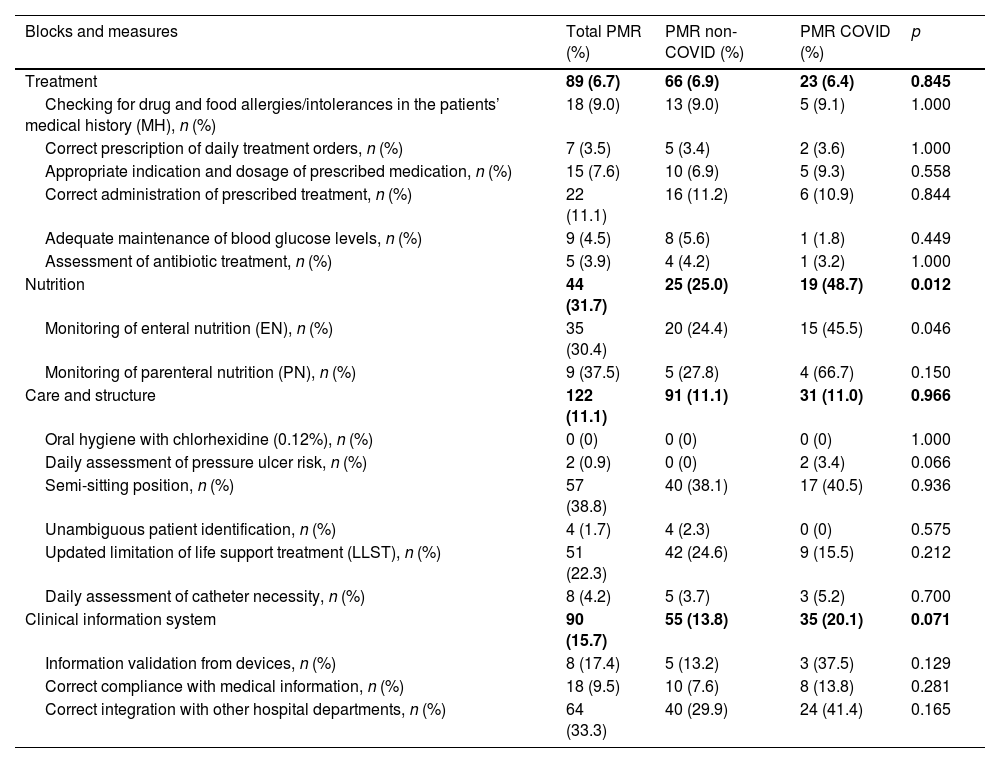
UtilityThe PMR-AASTRE-G was 11.8% (Tables 3a and 3b). No significant differences in PMR-AASTRE-G were observed between COVID and non-COVID patients. A PMR-AASTRE-B ≥ 10% was observed in the same measure blocks in both subgroups (5 out of 8 blocks). When the 2 patient subgroups were compared only the nutrition block showed significant differences, being PMR-AASTRE-B significantly higher in COVID patients (48.7% vs 25.0%; p = 0.012).
PMR-AASTRE results by evidence-based mandatory measures. PMR-AASTRE-B ≥ 10%. PMR-AASTRE ≥ 10%. P < 0.05.
| Blocks and measures | Total PMR (%) | PMR non-COVID (%) | PMR COVID (%) | p |
|---|---|---|---|---|
| PMR-AASTRE-G | 549 (11.8) | 385 (11.4) | 164 (13.0) | 0.136 |
| Mechanical ventilation | 108 (22.4) | 78 (23.6) | 30 (19.9) | 0.432 |
| Alveolar pressure limit, n (%) | 16 (24.6) | 14 (33.3) | 2 (8.7) | 0.057 |
| Mechanical ventilation alarms, n (%) | 58 (50.4) | 34 (43.0) | 24 (66.7) | 0.032 |
| Spontaneous ventilation test, n (%) | 2 (4.7) | 2 (6.5) | 0 (0) | 1.000 |
| Adequate tidal volume | 2 (1.8) | 1 (1.3) | 1 (2.7) | 1.000 |
| Assessment of mobilization and acquired muscle weakness at the ICU setting | 30 (20.4) | 27 (26.0) | 3 (7.0) | 0.018 |
| Hemodynamics | 33 (8.0) | 25 (8.3) | 8 (7.3) | 0.924 |
| Monitor Alarms | 30 (17.0) | 22 (16.8) | 8 (17.8) | 0.938 |
| Fluid administration in initial shock phase, n (%) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Fluid administration in clinical stability phase, n (%) | 1 (0.7) | 1 (0.9) | 0 (0) | 1.000 |
| Adequate hemodynamic monitoring, n (%) | 2 (3.4) | 2 (4.7) | 0 (0) | 1.000 |
| Renal | 34 (17.0) | 21 (14.2) | 13 (25.0) | 0.116 |
| Acute renal dysfunction assessment (ARDA), n (%) | 32 (16.9) | 20 (14.5) | 12 (23.5) | 0.211 |
| Daily prescription and adequate monitoring of continuous renal replacement therapy (CRRT), n (%) | 2 (18.2) | 1 (10.0) | 1 (100.0) | 0.182 |
| Sedation and analgesia | 29 (7.0) | 24 (7.4) | 5 (5.4) | 0.677 |
| Pain management, n (%) | 3 (1.7) | 3 (2.2) | 0 (0) | 1.000 |
| Control of agitation and prevention of oversedation, n (%) | 8 (8.2) | 5 (6.8) | 3 (12.5) | 0.401 |
| Delirium prevention and management, n (%) | 18 (12.5) | 16 (14.0) | 2 (6.7) | 0.365 |
PMR-AASTRE results by evidence-based mandatory measures. PMR-B-AASTRE ≥ 10%. PMR-AASTRE ≥ 10%. P < 0.05.
| Blocks and measures | Total PMR (%) | PMR non-COVID (%) | PMR COVID (%) | p |
|---|---|---|---|---|
| Treatment | 89 (6.7) | 66 (6.9) | 23 (6.4) | 0.845 |
| Checking for drug and food allergies/intolerances in the patients’ medical history (MH), n (%) | 18 (9.0) | 13 (9.0) | 5 (9.1) | 1.000 |
| Correct prescription of daily treatment orders, n (%) | 7 (3.5) | 5 (3.4) | 2 (3.6) | 1.000 |
| Appropriate indication and dosage of prescribed medication, n (%) | 15 (7.6) | 10 (6.9) | 5 (9.3) | 0.558 |
| Correct administration of prescribed treatment, n (%) | 22 (11.1) | 16 (11.2) | 6 (10.9) | 0.844 |
| Adequate maintenance of blood glucose levels, n (%) | 9 (4.5) | 8 (5.6) | 1 (1.8) | 0.449 |
| Assessment of antibiotic treatment, n (%) | 5 (3.9) | 4 (4.2) | 1 (3.2) | 1.000 |
| Nutrition | 44 (31.7) | 25 (25.0) | 19 (48.7) | 0.012 |
| Monitoring of enteral nutrition (EN), n (%) | 35 (30.4) | 20 (24.4) | 15 (45.5) | 0.046 |
| Monitoring of parenteral nutrition (PN), n (%) | 9 (37.5) | 5 (27.8) | 4 (66.7) | 0.150 |
| Care and structure | 122 (11.1) | 91 (11.1) | 31 (11.0) | 0.966 |
| Oral hygiene with chlorhexidine (0.12%), n (%) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Daily assessment of pressure ulcer risk, n (%) | 2 (0.9) | 0 (0) | 2 (3.4) | 0.066 |
| Semi-sitting position, n (%) | 57 (38.8) | 40 (38.1) | 17 (40.5) | 0.936 |
| Unambiguous patient identification, n (%) | 4 (1.7) | 4 (2.3) | 0 (0) | 0.575 |
| Updated limitation of life support treatment (LLST), n (%) | 51 (22.3) | 42 (24.6) | 9 (15.5) | 0.212 |
| Daily assessment of catheter necessity, n (%) | 8 (4.2) | 5 (3.7) | 3 (5.2) | 0.700 |
| Clinical information system | 90 (15.7) | 55 (13.8) | 35 (20.1) | 0.071 |
| Information validation from devices, n (%) | 8 (17.4) | 5 (13.2) | 3 (37.5) | 0.129 |
| Correct compliance with medical information, n (%) | 18 (9.5) | 10 (7.6) | 8 (13.8) | 0.281 |
| Correct integration with other hospital departments, n (%) | 64 (33.3) | 40 (29.9) | 24 (41.4) | 0.165 |
Regarding specific measures, the review of mechanical ventilation (MV) alarms (66.7% vs 43.0%; p = 0.032) and enteral nutrition monitoring (45.5% vs 24.4%; p = 0.046) were significantly higher in COVID patients. However, the evaluation of mobilization and evaluation of ICU-acquired muscle weakness (26.0% vs 7.0%; p = 0.018) was significantly higher in non-COVID patients.
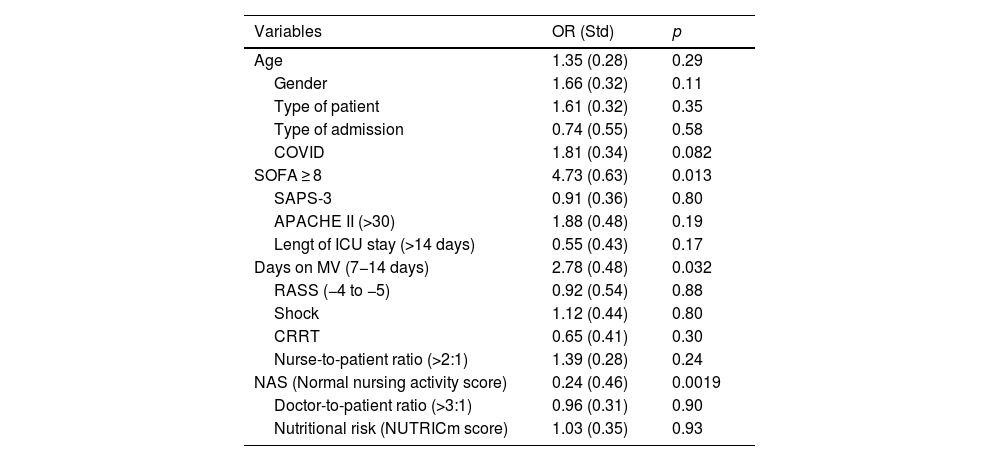
Table 4 shows the impact of selected independent variables on PMR-AASTRE-G. The Nursing Activities Score (NAS) < 50 (normal workload) was associated with a higher likelihood of meeting mandatory measures (OR, 0.24 -std 0.46-; p = 0.0019). On the other hand, both the days on MV (OR, 2.78 -std 0.48-; p = 0.032) and SOFA ≥ 8 (OR, 4.73 - std 0.63-; p = 0.013) were factors independently associated with higher overall tool utility (PMR-AASTRE-G ≥ 10%).
Multivariate analysis for PMR-AASTRE-G ≥ 10%. Data expressed as OR and std between brackets. P < 0.05.
| Variables | OR (Std) | p |
|---|---|---|
| Age | 1.35 (0.28) | 0.29 |
| Gender | 1.66 (0.32) | 0.11 |
| Type of patient | 1.61 (0.32) | 0.35 |
| Type of admission | 0.74 (0.55) | 0.58 |
| COVID | 1.81 (0.34) | 0.082 |
| SOFA ≥ 8 | 4.73 (0.63) | 0.013 |
| SAPS-3 | 0.91 (0.36) | 0.80 |
| APACHE II (>30) | 1.88 (0.48) | 0.19 |
| Lengt of ICU stay (>14 days) | 0.55 (0.43) | 0.17 |
| Days on MV (7−14 days) | 2.78 (0.48) | 0.032 |
| RASS (−4 to −5) | 0.92 (0.54) | 0.88 |
| Shock | 1.12 (0.44) | 0.80 |
| CRRT | 0.65 (0.41) | 0.30 |
| Nurse-to-patient ratio (>2:1) | 1.39 (0.28) | 0.24 |
| NAS (Normal nursing activity score) | 0.24 (0.46) | 0.0019 |
| Doctor-to-patient ratio (>3:1) | 0.96 (0.31) | 0.90 |
| Nutritional risk (NUTRICm score) | 1.03 (0.35) | 0.93 |
Age: ≤65; >65; Gender: Male/Female; type of patients: medical/surgical; type of admission: emergency/scheduled; COVID: Yes/No; SOFA: <4, 4–7, 8–12, >12; SAPS-3: <60, 60–80, >80; APACHE II: ≤ 14, 15–29, ≥ 30; Length of ICU stay (days): <7, 7−14, >14; Days on MV: <7, 7−14, >14; RASS: (−1, −2, −3) and (−4, −5); Shock: On noradrenaline, vasopressin, or dobutamine; CRRT: Yes/No; Nursing ratio: ≤2:1, >2:1; NAS (Nursing Activities Score): ≤50 (Normal workload), >50 (High workload); doctor ratio: ≤3:1, >3:1; nutritional risk: Low nutritional risk (0–4), high nutritional risk (5–9).
This is the first study ever conducted during the pandemic that evaluated the effect of a safety intervention that allowed converting unsafe situations into safe ones in real-time. Its feasibility and utility were demonstrated for both COVID and non-COVID patients.
The lack of studies on clinical safety during the pandemic is notable.24 Some authors describe patient safety incidents (PSIs) associated with treatment delays or the performance of inappropriate procedures, suggesting that proactive safety tools could have been useful in that context.25 Concurrently, the safety gap has been explained by the need to reallocate personal resources: many safety experts returned to purely clinical activities, preventing many functional units from maintaining or promoting their activities.26
Of note that AASTRE is a tool deeply embedded in the culture of our organization. This fact likely relates to the feasibility described in this study. AASTRE is based on the interaction between health care professionals (some responsible for patient care and others facilitating measure verification – prompters)16. This moves away from the idea of safety rounds led by managers or safety experts. In fact, in our own experience, the presence of trainees and nursing staff during audits makes interaction a space for organizational learning17 focused on processes. It has been reported that the one factor that significantly improves audit acceptance is building a shared sense of the results.27
A particularly striking result was the high utility of the tool during the analyzed period (PMR-AASTRE-G of 11.8%), which is significantly higher than the results obtained by AASTRE in previous pre-pandemic periods (5.4% and 6.7%).16,17 The utility of AASTRE is directly associated with real-time improvements in safety since the evaluated measures, if not performed at the time of the audit (omission errors), were taken immediately (real-time). Former studies17 associated AASTRE's utility with times of higher clinical workload. In this study, the health care team managed both COVID and non-COVID patients, so the clinical workload was evenly distributed among all professionals. During the pandemic, this sustained workload—translated into physical and cognitive fatigue—has been associated with omission errors through its influence on decision-making and task prioritization.28,29 In relation to utility, Arabi et al.30 concluded that, in situations like those experienced during the pandemic, a fundamental lesson that needed to be learnt was the need to prioritize the use of measures exclusively based on scientific evidence, an aspect guaranteed by AASTRE.
Reportedly, high clinical workload (high nursing and physician-to-patient ratios) has been associated with lower quality of care, a higher number of adverse events, longer lengths of stays, and higher mortality.31,32 Margadant et al.33 showed that a high NAS was associated with in-hospital mortality. Consequently, our study demonstrated that avoiding nursing workload overload (defined by NAS34) was associated with proper adherence to evidence-based measures and, therefore, fewer omission errors.
Consistent with what Ilan et al.35 described and what has been shown in former studies,16,17 our study found that patient severity was associated with significant tool utility. This is explained by the fact that high patient severity makes many medical care efforts to be focused on measures necessary for prompt patient resuscitation, while other less urgent but also important evidence-based measures are sidetracked.
It is of note that despite the COVID group being a more severe group, with longer lengths of stay, days on mechanical ventilation, and days with a urinary catheter, there were no significant differences in outcome indicators, such as rates of NAVM, tracheobronchitis, BRC, and catheter-associated urinary tract infections, as described by other authors.8–10
The goal of any health care system is to strive for excellence while prioritizing the patients’ best interests. For this reason, health care professionals should first aim to understand how they perform their roles, regardless of contextual complexity. In this regard, AASTRE has become a solid support for quality of care, not only because of its utility (as discussed) but also because it provides a form of quantitative feedback that has proven essential for driving any improvement in care quality.36
LimitationsThis study has several limitations: 1) It was conducted at a single center; 2) The study took place during the 6th wave of the pandemic, when clinical pressure was still high, though lower compared with other periods during the pandemic (although still far from normal clinical conditions, Moreno-Mulet et al.37). In any case, the reduced clinical pressure undoubtedly favored the implementation of AASTRE. 3) The study was conducted at a time when there was no longer collaboration with other specialties (Cardiology, Pediatrics, Emergency Medicine, Anesthesia) as had been the case in previous waves. This aspect would have been interesting to analyze as, under those circumstances, the use of AASTRE creates an interaction between the prompter and the trainee physician that allows for the formation of a learning space,20 which would have probably had more repercussions as a tool for information transmission and learning. 4) Finally, reactive tools, such as adverse event notifications were not considered, which would have helped analyze the safety situation of our ICU during the study period.
ConclusionsAASTRE proved to be a feasible and useful tool during the last phase of the pandemic (characterized by moderate clinical pressure) with only 2 weekly interventions of approximately 30 min each. The clinical complexity in an environment of COVID and non-COVID patients may explain the utility of AASTRE in both patient groups, with a notable increase in PMR-AASTRE-G compared with previously analyzed periods.
CRediT authorship contribution statementMB, GS, MS, and AR contributed to the study design. MB, GS, and MS conducted the safety rounds. JB and JC contributed to the configuration of the SIC and the calculation of metrics for indicators and process measures. JC, AR, and JB contributed to data analysis and statistical analysis. JC and MB developed and implemented the process indicators platform. All authors contributed to the drafting of the manuscript. All authors read and approved the final manuscript.
Ethical aspectsThis study was approved by the Ethics and Clinical Research Committee (CEIC) of Institut d’Investigació Sanitària Pere Virgili. Reference: 3/2021. Given the nature of the study and the anonymity of the data, obtaining informed consent was deemed unnecessary.
FundingThis study was funded by Instituto de Salud Carlos III (ISCIII) through the project "FIS PI20/01674" and co-financed by the European Union and the Ricardo Barri Casanovas Foundation grant.
We wish to thank the professionals working at the Intensive Care Medicine Department of Hospital Universitari de Tarragona Joan XXIII for their commitment to ensuring the quality of the data entered into the Clinical Information System.