Human albumin solutions are used in a number of disorders, though their indications are not clear in all circumstances. These solutions are costly, and their benefit has not been established in all settings. It is therefore interesting to assess the presence of albumin solutions in the daily clinical practice of critical care professionals.
ObjectivesTo report the standard clinical practices and to describe the variability of albumin solutions use in critically ill patients.
DesignA survey sent by e-mail to Spanish and South American Intensive Care Units (ICUs)
PeriodPlanning and execution were during the year 2012.
MethodsA questionnaire comprising of 35 questions.
ResultsFifty-seven surveys were analyzed. The use of albumin solutions was sporadic or negligible in critically ill patients (96.5%). The exceptions were patients with liver disease (87.7% of the responders administered albumin to these patients). A high percentage of professionals claimed to know the available scientific evidence on the use of albumin in patients with liver disease (82.5%) and in patients without liver disease (77.2%). Only 5.3% of the responders preferred to rely on their own experience to establish the indications of albumin use.
ConclusionsThe use of albumin solutions is infrequent in ICUs, except in patients with liver disease. Evidence-based knowledge on albumin use is declared to be extensive in ICUs. As a rule, opinions on the use of albumin solutions are based on the scientific recommendations, especially in patients with liver disease. Professional experience rarely prevails over the published clinical guidelines.
Las soluciones de albúmina humana se emplean en diversas enfermedades, aunque su indicación no es clara en todas. Presentan un coste elevado y su beneficio no se encuentra plenamente establecido. Resulta interesante conocer cuál es la presencia de las soluciones de albúmina en la práctica clínica diaria de los intensivistas.
ObjetivoDocumentar prácticas clínicas habituales y describir la variabilidad de las mismas en cuanto al empleo de soluciones de albúmina en enfermos críticos.
DiseñoEncuesta enviada mediante correo electrónico a unidades de cuidados intensivos (UCI) españolas y sudamericanas.
PeriodoPlanificación y realización durante el año 2012.
ÁmbitoUCI españolas y sudamericanas.
MétodosCuestionario de 35 preguntas.
ResultadosSe han analizado 57 encuestas. El empleo de las soluciones de albúmina fue esporádico o no se empleaba en el paciente crítico (96,5%). La excepción fueron los pacientes hepatópatas (un 87,7% de los encuestados la administraba). Un elevado porcentaje declaró conocer la evidencia científica disponible sobre el empleo de albúmina en pacientes hepatópatas (82,5%) y no hepatópatas (77,2%). El 5,3% de los encuestados prefería basarse en su experiencia para establecer las indicaciones del empleo de albúmina.
ConclusionesEl empleo de soluciones de albúmina no es frecuente en las UCI, salvo en pacientes hepatópatas. Los profesionales del enfermo crítico manifiestan tener un amplio conocimiento de la evidencia científica. Las opiniones emitidas, acerca del empleo de albúmina, son acordes con las recomendaciones establecidas, sobre todo, en pacientes hepatópatas. La experiencia profesional prevalece en escasas ocasiones sobre las recomendaciones publicadas.
Human albumin solutions are obtained from healthy donor plasma after pasteurization at 60°C for 10h, and are used in a broad range of clinical and surgical illnesses, though only some of their indications are authorized. In other cases, the use of albumin solutions must be decided on an individualized basis.1–14
Although many studies have examined the effects of albumin solutions upon mortality in critical patients, the conclusions have been contradictory1. A metaanalysis carried out in 1998 with 30 randomized studies, and two Cochrane reviews,3,4 suggested the use of albumin to be associated to increased mortality among critical patients. Posteriorly, another metaanalysis published in the year 2001,5 as well as more recent studies,6–11 have failed to confirm such an increase in mortality, except in patients with severe traumatic brain injury (TBI).12 Furthermore, the antioxidant actions of albumin and its effects upon maintenance of the plasma redox state must be taken into account for the adequate designing of future clinical studies.15
Albumin solutions are more expensive than other colloids and crystalloids. In addition, although such solutions are usually well tolerated, they are not without risks. In effect, albumin solutions can cause allergic reactions, and very rapid infusion can induce sudden hypotension and, in some cases, heart failure–particularly when used at high concentrations. Moreover, although albumin solutions are blood products that are considered to be safe in terms of the transmission of infections, there are doubts regarding the possibility of prion transmission through such solutions.16
The limited availability of albumin solutions, the risk of adverse effects, and their high cost mean that such solutions must be used only in situations where they are known to be effective. However, the level of evidence on which the recommendations are based is limited, and this is probably reflected in daily clinical practice by a broad range of interventions in which such solutions are used among critical care professionals. With the purpose of assessing the clinical practice in Intensive Care Units (ICUs), the Transfusions and Blood Products and Metabolism and Nutrition work groups of the Spanish Society of Intensive Care and Coronary Units (Sociedad Española de Medicina Intensiva y Unidades Coronarias, SEMICYUC) have conducted a survey to evaluate the knowledge and behaviors referred to the use of albumin solutions in ICUs.
Patients and methodsA survey was developed on the use of albumin solutions in critical patients. The Transfusions and Blood Products and Metabolism and Nutrition work groups of the SEMICYUC designed the questionnaire in 2012, and invited Spanish and South American ICUs belonging to both public and private centers to participate via e-mail. The professionals who received the questionnaire were personal contacts of the authors, no preference being assigned to members of the work groups of the SEMICYUC. Within each ICU, only a critical care professional answered the questionnaire.
Fifty-seven ICUs were invited, and all agreed to answer the questionnaire (Appendix A).
The submitted questionnaire and results obtained are shown in Table 1. The questionnaire consisted of 35 questions, most with multiple and closed answers, and some with open answers. The questions were grouped into different sections covering data referred to the responders and their work centers, albumin use in different disease conditions; and aspects referred to clinical practice/evidence. The aim of the questionnaire was to record the following aspects: general characteristics of the ICU and of the professionals (specialty, years of experience, and work setting: type of hospital center, number of hospital and ICU beds, and annual admissions to the ICU), usual clinical practice referred to the use of albumin solutions in different disease conditions (nutritional-metabolic, hypovolemia, septic shock/sepsis, and hepatorenal disorders), degree of knowledge of the available scientific evidence, type of albumin solutions used, and cost.
Full-submitted questionnaire and answers received.
| Questionnaire | ||
| Questions | Answers | % |
| (1) Do you regularly use albumin solutions in the treatment of critical patients? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 03.549.147.4 |
| Nutritional-metabolic | ||
| (2) Do you regularly use albumin solutions in the case of critical patients with protein denutrition? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 01.819.378.9 |
| (3) Do you regularly use albumin solutions in the case of critical patients with hypoalbuminemia? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 05.335.159.6 |
| (4) If you use albumin solutions in patients with hypoalbuminemia, from what serum albumin concentration do you decide to administer them? (g/dl) | 3–3.52.5–32–2.51.5–21–1.5<1 | 008.821.110.50 |
| (5) Do you administer albumin with critical patient nutrition? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 005.394.7 |
| (6) If you administer albumin with nutrition, what types of nutrition do you use? | Enteral nutritionParenteral nutritionBoth | 03.51.8 |
| (7) If you use albumin solutions in these cases, do you do so added to insulin for improved fixation? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 0003.5 |
| Hypovolemia/hypovolemic shock | ||
| (8) Do you regularly use albumin solutions in the case of critical patients requiring volume replacement? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 01.810.586 |
| (9) Do you regularly use albumin solutions in the case of critical patients with hypovolemic shock? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 01.810.586 |
| (10) If you use albumin solutions in hypovolemia/hypovolemic shock, the volume you usually administer is (ml) | <200200–400400–600600–800800–1000>1000 | 3.57001.80 |
| (11) At what concentration do you administer albumin in these cases? (%) | 52025Diluted | 3.5 10.500 |
| (12) The total albumin dose over 24h which you usually use in patients with hypovolemia/hypovolemic shock is (g) | ≤5050–100100–200>200 | 3.53.5 3.51.8 |
| Sepsis/septic shock | ||
| (13) Do you regularly use albumin solutions in the case of critical patients with sepsis/septic shock? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 01.810.586 |
| (14) If you use albumin solutions in sepsis/septic shock, the volume you usually administer is (ml) | <200200–400400–600600–800800–1000>1000 | 5.35.31.8000 |
| (15) At what concentration do you administer albumin in these cases? (%) | 52025Diluted | 1.810.500 |
| (16) The total albumin dose over 24h which you usually use in patients with sepsis/septic shock is (g) | ≤5050–100100–200>200 | 1.871.81.8 |
| Hepatorenal | ||
| (17) Do you regularly use albumin solutions in the case of critical patients with liver disease and hypoalbuminemia? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 10.521.156.112.3 |
| (18) Do you regularly use albumin solutions in the case of critical patients with liver disease subjected to evacuating paracentesis? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 52.631.6141.8 |
| (19) Do you regularly use albumin solutions in the case of critical patients with liver disease and hepatorenal syndrome? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 26.324.640.48.8 |
| (20) You use albumin solutions in patients with liver disease considering the following parameters | Serum albuminTotal blood proteinsAge and basal conditionPatient nutritional conditionVolemiaDiuresisVolume of ascitic fluid at paracentesisOthers | 50.910.512.31428.149.189.50 |
| (21) Do you regularly use albumin solutions in the case of critical patients with anasarca before the administration of furosemide? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 5.3736.850.9 |
| Other uses | ||
| (22) Is plasmapheresis performed in your center? | YesNo | 73.726.3 |
| (23) If your center performs plasmapheresis, is replacement made with albumin solutions? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 28.1722.814 |
| (24) If you use albumin solutions in relation to other indications not mentioned in this questionnaire, please specify which indications | MARS®Resuscitation in major burnsOligoanuric renal failure with severe hypoalbuminemia (<1.5g/dl)Non-continuous hemodialysis with hypotensive episodes and the intent to achieve negative water balanceHypoproteinemia | 73.51.81.81.8 |
| Clinical practice/evidence | ||
| (25) Do you know the available scientific evidence referred to the use of albumin solutions in critical patients without liver disease? | Yes, it is clear in this respectYes, but it does not clarify the usual doubtsYes, but I prefer to rely on my own experienceNo | 26.347.43.517.5 |
| (26) Do you think the use of albumin solutions can have a positive effect upon the prognosis of critical patients without liver disease? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 01.8035.157.9 |
| (27) The routine use of albumin solutions can have a negative impact upon critical patients without liver disease | I fully agreeI generally agreeI neither agree nor disagreeI sometimes agreeI rarely agreeI disagree | 12.319.329.821.11.810.5 |
| (28) Do you know the available scientific evidence referred to the use of albumin solutions in critical patients with liver disease? | Yes, it is clear in this respectYes, but it does not clarify the usual doubtsYes, but I prefer to rely on my own experienceNo | 43.933.35.312.3 |
| (29) Do you think the use of albumin solutions can have a positive effect upon the prognosis of critical patients with liver disease? | Yes, always or almost alwaysYes, oftenYes, sometimesNever or almost never | 8.817.559.68.8 |
| (30) The routine use of albumin solutions can have a negative impact upon critical patients with liver disease | I fully agreeI generally agreeI neither agree nor disagreeI sometimes agreeI rarely agreeI disagree | 010.510.533.312.328.1 |
| (31) If you administer albumin solutions, the volume of the vials you use is (ml) | 1050100250500Others | 084.275.31.80 |
| (32) If you administer albumin solutions, the albumin concentration you usually use is (%) | 35152025Diluted | 1.810.51.8863.50 |
| (33) Do you know the price of the albumin solutions? | YesNo | 33.361.4 |
| (34) If you do not know the price of the albumin solutions, you estimate that a vial containing 50ml of albumin 20% costs (€) | ≤1010–3030–50>50 | 1.85.315.831.6 |
| (35) Do you know any substitutes of albumin solutions? | Yes. Which?– Colloids– FFP– Ig– EN and PNNo | 47.443.98.81.81.847.4 |
Ig: immunoglobulins; EN: enteral nutrition; PN: parenteral nutrition; FFP: fresh frozen plasma.
The survey was carried out before the publication of the latest update on the Surviving Sepsis Campaign.17
The data are expressed as frequencies and percentages in the case of qualitative variables, and as means and standard deviations in the case of quantitative variables.
ResultsA total of 57 questionnaires from 54 Spanish ICUs and three South American ICUs (Brazil, Chile and Venezuela) were analyzed. All the invited ICUs answered the questionnaire. In turn, all the items of the questionnaire were answered, except three questionnaires that failed to answer the section referred to clinical practice/evidence, and one questionnaire that failed to answer the sections referred to hypovolemia and sepsis/septic shock.
Data referred to the professionalThe first part of the questionnaire explored data referred to the professionals and their working institutions. Most of those interviewed were intensivists (96.5%) and mostly specialists (96.5%, of which 21.1% were Section Chiefs or Heads of Department), with an average of 15 years of experience. Only two residents in Intensive Care Medicine participated in the study. In 98.2% of the hospital centers, the intensivists were in charge of critical patient care.
Data referred to the hospital centerMost of the participating hospitals were secondary (n=23; 40.4%) or tertiary centers (n=26, 45.6%), and almost all (91.2%) registered more than 300 ICU admissions a year. The mean number of beds in the ICU was 18.7±14, with an average of 500.6±349.5 hospital beds. In over one-half of the cases (54.4%), these data came from documented reliable sources, while in 45.6% of the cases they represented estimates by the responders.
QuestionnaireThe answers to the questionnaire regarding albumin use are shown in Table 1. Most of the professionals used albumin solutions either sporadically or not at all in the critical patient (n=55, 96.5%).
“Nutritional-metabolic” sectionWith regard to the different diseases, the use of albumin solutions in critical patients with protein denutrition was either sporadic or inexistent in 98.2% of the cases (n=56). In the patients with hypoalbuminemia, the use of albumin was likewise scarce or inexistent in most of the cases (n=54, 94.7%). Some professionals administered albumin when albuminemia was found to be within the following ranges: 2–2.5g/dl (n=5 8.8%); 1.5–2g/dl (n=12, 21.1%); and 1–1.5g/dl (n=6, 10.5%).
Only three physicians used albumin solutions with nutrition: two of them with parenteral nutrition and the other with both types of artificial nutrition (enteral and parenteral). Of these professionals, only one occasionally used albumin solutions with insulin with the purpose of ensuring greater insulin fixation.
“Hypovolemia/hypovolemic shock” sectionIn this section, 49 responders (86%) claimed to never or almost never use albumin for volume replacement or expansion, not even in cases of hypovolemic shock. Of the 7 responders who often or sometimes used albumin for volume replacement in the critical patient with hypovolemia/hypovolemic shock, the volume of albumin commonly administered was <200ml in two questionnaires (3.5%), 200–400ml in four (7%), and 800–1000ml according to one questionnaire (1.8%). The concentration of the administered albumin solutions was 5% in two questionnaires (3.5%) and 20% in 6 (10.5%). The total albumin dose usually administered over 24h to patients with hypovolemia/hypovolemic shock was <50g in two questionnaires (3.5%), 50–100g in two (3.5%), 100–200g in two (3.5%), and >200g according to one questionnaire (1.8%).
“Sepsis/septic shock” sectionAlbumin solutions were never or almost never used in sepsis/septic shock according to 49 responders (86%). Only 7 physicians (12.3%) used albumin solutions occasionally or often in such situations (Table 1). In these cases, the volume administered was usually <200ml according to three questionnaires (5.3%), 200–400ml in three (5.3%), and 400–600ml in one questionnaire (1.8%). The concentration of the albumin solutions was 5% according to one questionnaire (1.8%) and 20% in 6 (10.5%). The total albumin dose usually administered by these professionals over 24h in cases of sepsis/septic shock was <50g according to one questionnaire (1.8%), 50–100g in four questionnaires (7%), 100–200g in one (1.8%), and >200g in one questionnaire (1.8%).
“Hepatorenal” sectionFifty ICUs (87.7%) used albumin in critical patients with liver disease and hypoalbuminemia. Fifty-six responders (98.2%) administered albumin after evacuating paracentesis, while 52 (91.2%) did so in patients with hepatorenal syndrome. The aspects most taken into account by the professionals in administering albumin to critical patients with liver disease were: volume of ascitic fluid evacuated at paracentesis (51 responders, 89.5%), serum albumin (29 responders, 50.9%) and diuresis (28 responders, 49.1%). Twenty-eight physicians (49.1%) administered albumin to critical patients with anasarca before the administration of furosemide.
“Other uses” sectionThis section explored the use of plasmapheresis in the different hospital centers. Forty-two centers (73.7%) claimed to use the technique. One of the responders did not know whether plasmapheresis was performed with albumin in his center. In the rest of the cases, albumin solutions were used in the plasmapheresis sessions at least occasionally in 57.9% of the cases (33 centers).
Among the indications of albumin not reflected in the questionnaire, the options cited by the responders were liver dialysis with MARS® (molecular adsorbent recirculating system) in four questionnaires (7%), oligoanuric renal failure with severe hypoalbuminemia (<1.5g/dl) in one (1.8%), non-continuous hemodialysis with hypotensive episodes and the intent to achieve negative water balance in one questionnaire (1.8%), hypoproteinemia in one (1.8%), and resuscitation in major burn victims in two questionnaires (3,5%).
“Clinical practice/evidence” sectionThis section was answered by 54 responders. A large percentage of the professionals (77.2%) claimed to know the available scientific evidence regarding the use of albumin in critical patients without liver disease. Nevertheless, 3.5% preferred to rely on their own professional experience to establish the indication.
On the other hand, 57.9% of the responders considered that the use of albumin solutions never or almost never has a positive impact upon critical patients without liver disease, while 31.6% (n=18) agreed that albumin use always or generally could have a negative impact upon such patients.
The majority of the professionals (82.5%) claimed to know the available scientific evidence regarding the use of albumin in critical patients with liver disease. Three physicians preferred to rely on their own professional experience to establish the indication.
The prevalent opinion (n=49, 86%) was that the use of albumin in patients with liver disease could exert a beneficial effect upon the clinical course, and few considered that albumin in general could exert a negative effect (n=6, 10.5%).
The most frequently used albumin vial volume was 50ml according to 48 responders (84.2%), while the most commonly administered concentration was 20% according to 49 responders (86%).
Most of the responders were unaware of the price of the albumin solutions (n=35, 61.4%), and only 15.8% correctly estimated the price of a 50-ml vial of human albumin at a concentration of 20%.
Almost one-half of the responders knew of no substitute for albumin solutions. The most frequently identified substitutes were colloids (43.9% of the responders).
DiscussionThe number of completed questionnaires constitutes a large sample reflecting routine practice in Spain, though it might not be fully representative of the true situation, since the ICUs were not selected on a random basis. Nevertheless, the answers were very homogeneous in practically all the analyzed areas, and this in turn suggests that the survey effectively reflects routine practice regarding the use of albumin solutions. The South American sample is of course too small to allow us to draw such conclusions.
It would be interesting to repeat this study some years after its publication, with a larger number of ICUs, in order to determine whether the recent studies17,18 on the utilization of albumin in septic patients lead to changes in the way in which albumin is used.
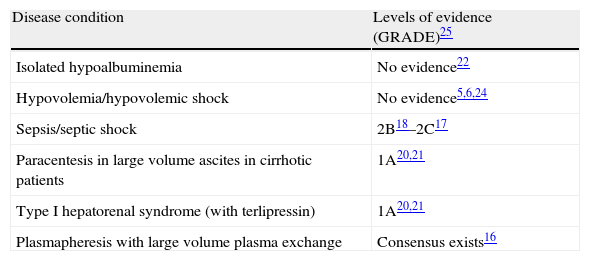
The questionnaires were mainly answered by experienced intensivists working in medium to large centers and with a considerable number of ICU admissions each year. In this setting, albumin solutions were used sporadically or were not used in critical patients. This observation persisted in the different sections of the questionnaire, with the sole exception of the hepatorenal section. Thus, albumin was little used or not used at all in denutrition, hypoalbuminemia, hypovolemia and sepsis/septic shock. This is consistent with the general recommendations on albumin use. Table 2 shows the level of evidence referred to the use of albumin solutions in the different sections of the questionnaire.
Levels of evidence referred to the use of albumin solutions in the different sections of the questionnaire, according to the available recommendations.
| Disease condition | Levels of evidence (GRADE)25 |
| Isolated hypoalbuminemia | No evidence22 |
| Hypovolemia/hypovolemic shock | No evidence5,6,24 |
| Sepsis/septic shock | 2B18–2C17 |
| Paracentesis in large volume ascites in cirrhotic patients | 1A20,21 |
| Type I hepatorenal syndrome (with terlipressin) | 1A20,21 |
| Plasmapheresis with large volume plasma exchange | Consensus exists16 |
GRADE system25: Evaluates the strength of recommendation as A (strong) or B (weak), and the quality of evidence as high (it is unlikely for the results of new studies to modify confidence in estimation of the effect), moderate (estimation of the effect is probably close to the real effect, but there might be substantial differences), low (new studies are likely to modify confidence in estimation of the effect and its magnitude), or very low (any estimation of effect is very uncertain).
The recently published consensus document of the European Society of Intensive Care on the use of colloids in the critical patient contemplates the possibility of administering albumin solutions in septic patients, though their efficacy has not been firmly established.18 The more recent review of the management guides of the Surviving Sepsis Campaign recommend the administration of albumin in patients with septic shock who require large amounts of crystalloids, with level of evidence 2C according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE).17 This document has been published after the present survey, and therefore could not be taken into account by the responders of the questionnaire. In any case, the evidence on the effectiveness of albumin in sepsis lacks the quality needed to allow its recommendation in severe sepsis/septic shock.19
The indications of albumin are supported by a greater level of evidence in the case of hepatorenal disease.20,21 This is also reflected by the findings of our study. In fact, most of the responders used albumin solutions in patients with liver disease and hypoalbuminemia, following evacuating paracentesis or in hepatorenal syndrome. In deciding to use albumin, the professionals mainly considered albuminemia, diuresis and the volume of ascitic fluid collected at paracentesis. Indeed, the volume of ascitic fluid collected at paracentesis establishes the indication of albumin administration. Cirrhotic patients with spontaneous bacterial peritonitis or type I hepatorenal syndrome could also benefit from the administration of albumin solutions, though there are no other indications for albumin use in these patients.19,20 However, although there is no scientific evidence warranting the indication of albumin associated to furosemide in patients with anasarca, the results of our study show that almost one-half of the responders used this combination.
Plasmapheresis was available in a large percentage of the centers in our study, and in over one-half of the cases albumin solutions were used to replace the extracted plasma. Based on the available evidence, the use of albumin during plasmapheresis involving the extraction of over 20ml of plasma/kg body weight in a single session, or 20ml/kg/week in several consecutive sessions, is considered adequate. When the volumes are smaller, the indication of albumin should be established on an individualized basis.16,22 In our survey we requested no information on the indications of plasmapheresis with albumin in relation to the needs referred to volume exchange. However, over 28% of the responders claimed that plasmapheresis sessions were always or almost always performed with albumin. We do not know whether there was precise knowledge of the technique on the part of the responders in each center.
Most of the responders considered that albumin use complies with the current recommendations referred to critical patients without liver disease.18 Furthermore, most of the responders were of the opinion that albumin has no positive impact upon the patients, and indeed could even have a negative effect. The available literature does not offer conclusive proof that albumin reduces the mortality rate among patients with hypovolemia, burns and hypoproteinemia, although there is also no proof that it might increase the mortality risk. Consequently, the scant use of albumin in these patients is consistent with the scientific evidence. The degree of dissatisfaction on the part of the professionals regarding the scientific literature supports the need to conduct well-designed studies with homogeneous populations and adequate sample sizes.
Although the studies conducted to date have not confirmed the positive or negative effect which albumin use might have, in the subgroup of patients with severe traumatic brain injury (TBI) of the SAFE study,12 the administration of albumin was associated to greater mortality than resuscitation performed with physiological saline solution (OR 1.8; 95%CI 1.31–2.70; p<0.001). In our survey we did not ask about the management of patients with TBI, though considering the scant use of albumin in the resuscitation of critical patients, similar considerations probably could also be extrapolated to severe TBI.
In critical patients with liver disease, the levels of evidence referred to albumin use are higher.20,21 This is related to the fact that in this case there are more studies with an adequate design and with more homogeneous populations. In this regard, our study shows that in the case of critical patients with liver disease, the evidence was more widely known among the responders and guided albumin use more often. The belief that at least in some cases albumin use could have a positive effect was more prevalent in the case of these patients, and the belief that it might have a negative impact was less prevalent.
The need for more studies with adequate designs, the low levels of evidence referred to albumin use in most critical situations, and the lack of knowledge of the effects of albumin upon mortality undoubtedly condition the habits regarding albumin use and which are still found in the routine practice of some ICUs, despite the absence of adequate degrees of recommendation.
In order to obtain more information on routine clinical practice, we asked about the albumin vials commonly used in the ICUs. In this respect, the vials were found to contain 50ml of albumin solution at a concentration of 5 or 20%. In general, the available presentations and concentrations of albumin were known to the professionals. Few of the responders in our study used concentrations not found on the market.
Regarding knowledge of the cost of albumin use, the questionnaire asked about the price of the vials. The price of a 50-ml vial containing 20% albumin ranges from 37.45 to 44.87 euros (updated prices in Medimecum 201023). A large percentage of the responders did not know the cost of albumin, and only 15.8% offered an adequate price estimate of between 30 and 50 euros. The cost of the albumin solutions therefore does not seem to be particularly important in deciding to use such products, since most of the professionals were unaware of the price of the vials.
Among the substitutes of albumin solutions, a large percentage of the responders (43.9%) cited colloids (starches, dextrans and gelatins) as an alternative. Colloids are used in routine clinical practice mainly for resuscitation in hypovolemia (absolute or relative), although there is controversy regarding the type of fluid indicated in such cases, and at present colloids are not considered to be better than crystalloids. This is another issue requiring further clarification.18
ConclusionsThe use of human albumin solutions is not recognized as a common practice among physicians attending critical patients, except in the case of subjects with liver disease. Knowledge of the available scientific evidence is reported to be widespread among these physicians. In general, the opinions on the use of albumin solutions are consistent with the established recommendations, particularly in patients with liver disease, and their utilization in the ICU is guided by the available evidence. Professional experience conditions the decision to use albumin–the mentioned recommendations prevailing only among a minority of professionals.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Andalusia: Pilar Martínez (Hospital de Puerto Real, Cádiz); Antonio Valverde (Hospital Virgen de la Victoria, Málaga); Manuel Herrera, Juan Fernández (Hospital Carlos Haya, Málaga); Carlos Ortiz, Juan José Egea (Hospital Virgen del Rocío, Sevilla); Juan Márquez (Hospital de la Macarena, Sevilla). Aragón: Carlos González (Hospital de Barbastro, Barbastro); Carlos Serón (Hospital San Jorge, Huesca). Asturias: José Antonio Gonzalo (Hospital Central de Asturias, Oviedo). Brasil: Valéria Abrahão (Hospital Ipanema Plus, Río de Janeiro). Castilla-La Mancha: Ana Bueno (Hospital General de Ciudad Real, Ciudad Real); Elena González (Hospital Virgen de la Luz, Cuenca); Miguel Ángel Taberna (Hospital Nuestra Señora del Prado, Talavera de la Reina); Marcelino Sánchez (Hospital Virgen de la Salud, Toledo); Francisca Prieto (Hospital de Santa Bárbara, Puertollano); Jaime Serrano (Hospital General La Mancha-Centro, Alcázar de San Juan); Sergio Sáez, Concepción García (Hospital Provincial de la Misericordia, Toledo). Castilla-León: Miriam Ochoa (Hospital Clínico Universitario de Salamanca, Salamanca); Sopetrán Rey, María Jesús López, Santiago Macías, Patricia Jimeno (Hospital General de Segovia, Segovia); Vicente Morán (Complejo Hospitalario de León, León); Teresita Loreto (Hospital Virgen de la Concha, Zamora). Catalonia: Alberto Sanduimenge (Hospital Universitari Joan XXIII, Tarragona); Luis Serviá (Hospital Arnau de Vilanova, Lérida); Carol Lorencia, Alfonso Bonet (Hospital Universitari Doctor Josep Trueta, Gerona); Ángel Robles (Hospital Vall d¿Hebron, Barcelona); Ferrán Roche (Hospital Sant Pau, Barcelona); Carles Ferrer (Hospital Mútua de Terrasa, Tarrasa); Luisa Bordel (Hospital Universitari Germans Trias i Pujol, Badalona). Chile: Darwin Acosta (Hospital Clínico Mutual, Iquique). Extremadura: Marta Montans (Hospital San Pedro de Alcántara, Cáceres). Balearic Islands: Carlos Antón (Hospital de Manacor, Manacor); Isabel Ceniceros (Clínica ISP Palmaplanas, Palma de Mallorca). La Rioja: María Macías (Hospital de San Pedro, Logroño). Madrid: Blanca López Matamala (Hospital Universitario del Tajo, Aranjuez); Fernando Monasterio, Manuel Sánchez (Hospital Universitario de la Princesa, Madrid); Amalia de la Gándara (Hospital Infanta Leonor, Madrid); Vicente Gómez (Hospital Moncloa, Madrid); Juan Carlos Montejo (Hospital 12 de Octubre, Madrid); Antonio Blesa (Hospital Clínico de San Carlos, Madrid); José Ángel Lorente (Hospital Universitario de Getafe, Getafe); Clara Vaquerizo (Hospital Fundación Fuenlabrada, Fuenlabrada); Pedro Galdós (Hospital Universitario Puerta de Hierro, Majadahonda); Patricia Albert (Hospital Universitarios del Sureste, Arganda); Alberto Hernández (Hospital Universitario Fundación Alcorcón, Alcorcón); Ángela Algaba (Hospital Universitario de Torrejón, Torrejón de Ardoz); Jorge López (Hospital Severo Ochoa, Leganés). Murcia: Francisco García (Hospital General Universitario Los Arcos del Mar Menor, San Javier); Carmen Sánchez (Hospital General Universitario Reina Sofía, Murcia). Navarre: Laura Macaya (Hospital General de Navarra, Pamplona). Basque Country: María Mercedes Zabarte (Hospital Donostia, San Sebastián). Valencia: Manuel Cervera (Hospital Arnau de Vilanova, Valencia); Alfonso Mesejo (Hospital Clínico, Valencia); Miguel Ángel García (Hospital de Torrevieja, Torrevieja); José Antonio Acosta (Hospital General de Alicante, Alicante); Begoña Balerdi, Remedios Clemente (Hospital La Fe, Valencia); Lidón Mateu (Hospital General de Castellón, Castellón). Venezuela: Tully Baptista (Hospital Universitario, Caracas).
Please cite this article as: Estébanez-Montiel MB, Quintana-Díaz M, García de Lorenzo y Mateos A, Blancas Gomez-Casero R, Acosta-Escribano J, Marcos-Neira P, et al. Resultados de una encuesta sobre la práctica clínica habitual en el empleo de albúmina en UCI. Med Intensiva. 2014;38:403–412.