Edited by: Juan Antonio Llompart-Pou - Specialist in Intensive Care Medicine, Doctorate in Health Sciences, Universitat Illes Balears. Department of Intensive Care Medicine, Trauma and Neurocritical ICU, Hospital Universitari Son Espases. Palma, Spain
Last update: November 2025
More infoTo determine whether the Reverse Shock Index multiplied by the Glasgow Coma Scale (rSIG) is a predictor of in-hospital mortality in patients with traumatic brain injury (TBI).
DesignThis is a systematic review and meta-analysis.
SettingA comprehensive search was conducted in five databases for studies published up to May 22, 2024, using a PECO strategy. Eight studies were identified for quantitative analysis and included in our meta-analysis.
ParticipantsThe participants of the included primary studies.
InterventionsPatients with a low rSIG as a predictor of in-hospital mortality in TBI.
Main variables of interestrSIG, in-hospital mortality, TBI.
ResultsOur meta-analysis evaluated a total of eight observational studies encompassing 430,000 patients with TBI, observing 6,417 deaths (15%). After performing a sensitivity analysis, we found that patients with TBI and a low value of the reverse shock index multiplied by the Glasgow Coma Scale (rSIG) had a 24% higher risk of death (OR 1.24; 95% CI 1.12–1.38; I²: 96%). Furthermore, rSIG values were significantly higher in survivors compared to those who died (MD 7.72; 95% CI 1.86–13.58; I²: 99%).
Determinar si el rSIG Índice de shock inverso multiplicado por la escala de coma de Glasgow (rSIG) es un predictor de mortalidad intrahospitalaria en pacientes con trauma craneoencefálico (TBI).
DiseñoSe presenta una investigación tipo Revisión sistemática y Metaanálisis.
ÁmbitoRealizamos una búsqueda exhaustiva en cinco bases de datos de estudios publicados hasta el 22 de mayo de 2024, utilizando una estrategia PECO. Se identificaron ocho estudios para el análisis cuantitativo, los cuales fueron incluidos en nuestro metaanálisis.
PacientesLos participantes de los estudios primarios incluidos.
IntervencionesPacientes con rSIG alto como predictor de mortalidad intrahospitalaria en TBI.
Variables de interés principalesrSIG, mortalidad intrahospitalaria, TBI.
ResultadosNuestro metaanálisis evaluó un total de ocho estudios observacionales, que abarcaban 430,000 pacientes con TCE y observaron 6,417 muertes (15%). Tras realizar un análisis de sensibilidad, encontramos que los pacientes con TCE y un valor bajo del índice de shock inverso multiplicado por la escala de coma de Glasgow (rSIG) presentaban un 24% mayor riesgo de muerte (OR 1.24; IC 95% 1.12–1.38; I²: 96%). Además, los valores de rSIG fueron significativamente más altos en los sobrevivientes en comparación con aquellos que fallecieron (DM 7.72; IC 95% 1.86–13.58; I²: 99%).
ConclusionesEn los pacientes con TBI, el rSIG actúa como un predictor de mortalidad, con un riesgo de muerte un 24% mayor en aquellos con valores bajos.
Traumatic Brain Injury (TBI) remains as a significant public health challenge, affecting millions of individuals worldwide and resulting in substantial morbidity and mortality.1 As one of the leading causes of death, particularly among young adults,1,2 TBI presents a complex clinical picture that requires effective assessment tools for predicting outcomes. Accurate prognostication is essential for guiding clinical decisions, optimizing resource allocation, and improving patient care.2,3
The pathophysiology of TBI involves several mechanisms, including primary injury from mechanical impact and secondary injury due to inflammation, oxidative stress, and metabolic dysfunction.4–6 Cardiovascular complications frequently arise following TBI, including cardiac dysfunction.7,8 Research has shown that traumatic brain injury (TBI) induces a systemic catecholamine storm, which increases sympathetic outflow and may potentially lead to ischemia, cardiac arrhythmias, ventricular dysfunction, cardiogenic pulmonary edema, and systemic hypotension.9–11
Understanding the complex nature of TBI is essential for developing effective prognostic tools. In this context, rSIG provides a novel method for predicting outcomes in TBI patients by integrating two critical metrics. The Reverse Shock Index (rSI) assesses the circulatory status by quatifiying the relationship between the systolic blood pressure to hearth rate, offering insights into the patient’s hemodynamic stability.12 The Glasgow Coma Scale (GCS), a well-established measure of consciousness and neurological function, complements this assessment.13 Together, both can provide a comprehensive assessment of a patient's overall condition, highlighting those at higher risk for adverse outcomes.14,15
Despite its theoretical advantages, the practical application and validation of rSIG in clinical settings warrant further investigation. A systematic review and meta-analysis of existing literature is essential to clarify the effectiveness of rSIG across diverse patient populations and clinical contexts. This study aims to assess the utility of rSIG as a reliable predictor of mortality among TBI patients through a comprehensive analysis of available literature.
MethodsSearch strategyOur systematic review adheres to the methodological standards outlined in the Cochrane Handbook for Systematic Reviews,16 PRISMA guidelines,17 and AMSTAR 2 criteria.18 The study protocol was registered in PROSPERO (CRD42024549613). We conducted a comprehensive search of major databases, including MEDLINE (PubMed), Scopus, EMBASE, Web of Science, Science Direct, and the Cochrane Library from inception to 22 mayo 2024. Our search strategy incorporated both controlled vocabulary (e.g., MeSH, Emtree) and free terms, combined with Boolean operators, to implement our PECOS strategy (Population: adult patients with traumatic brain injury; Exposition: Reverse shock index multiplied by Glasgow Coma Scale (rSIG) < 14 points; Comparator: Reverse shock index multiplied by Glasgow Coma Scale (rSIG) > or = 14 points; Outcome: in-hospital mortality; Study design: Observational studies, case control, cohort, transversal studies). Keywords were carefully selected to encompass relevant exposures ("Reverse shock index multiplied by Glasgow Coma Scale” OR “rSIG") AND “traumatic brain injury”. A detailed outline of the search strategy is provided in the Supplementary Materials (refer to Table 1SA in supplementary material).
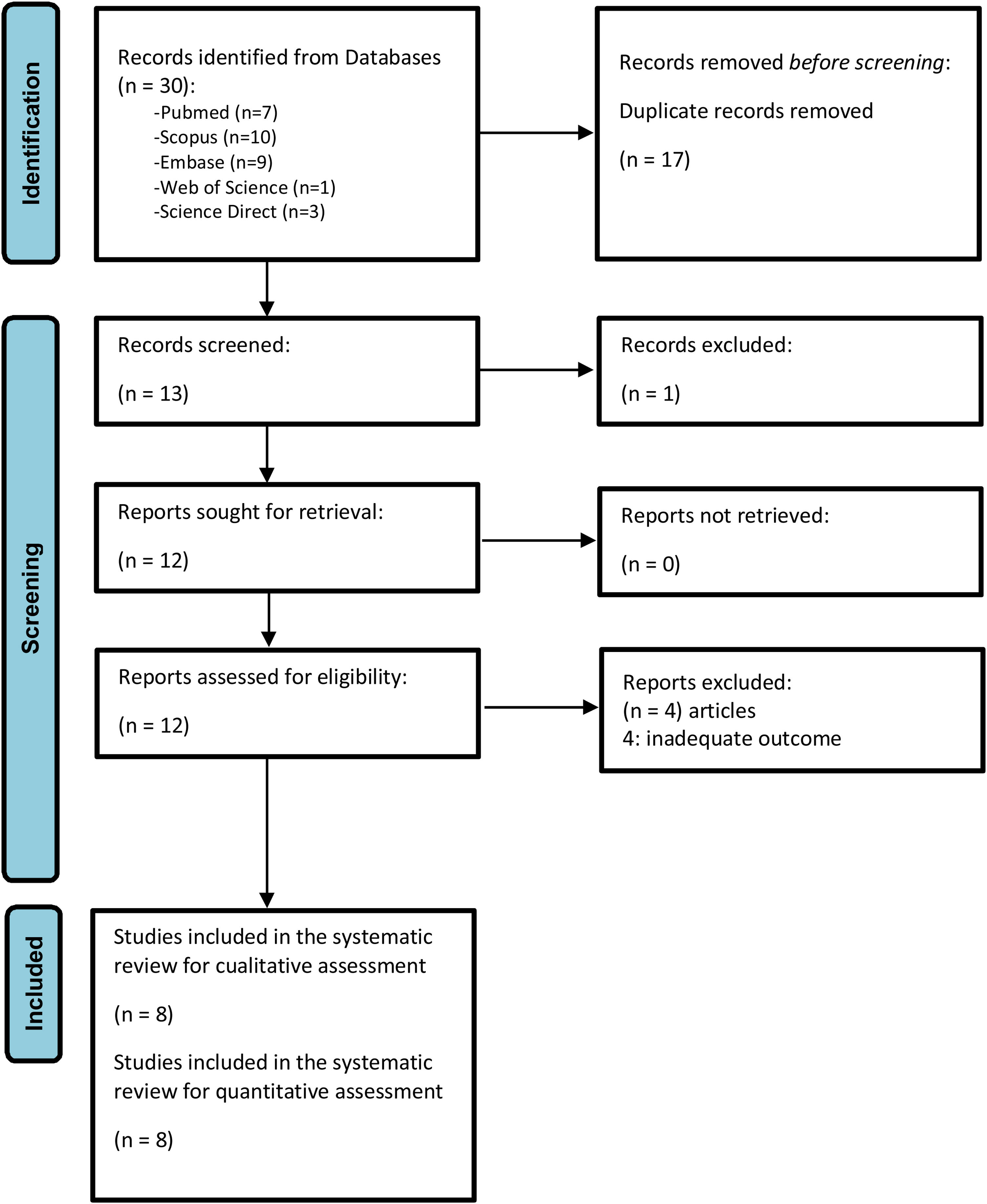
All articles identified during the primary and secondary screenings were cataloged using Zotero® 6.0.15. After removing duplicates, the documents were transferred to the Rayyan® platform. Two authors (GAVT and MCC) independently and blindly screened the titles and abstracts. Studies were selected by mutual agreement, with a third researcher (EDMR) mediating any disagreements. The selected articles then underwent a thorough full-text review to assess their eligibility. Additionally, reference lists and citations of the included studies were manually reviewed to identify any further relevant publications. For clarity, the selection process is outlined in Fig. 1.
Selection criteriaWe included observational studies (case-control, cohort, or cross-sectional studies) that evaluated rSIG as a predictor of in-hospital mortality. The studies enrolled adult patients (≥18 years old) of both sexes. We considered articles published up to May 22, 2024, without restrictions on language or publication date. We excluded case reports, systematic reviews, and duplicates. Additionally, studies that did not report association measures, such as odds ratio (OR), but rather presented sensitivity, specificity, positive predictive value, and negative predictive value, were included.
OutcomesThe primary outcome was in-hospital mortality.
Data extractionTwo independent researchers collected and extracted relevant information from each included study using a standardized, blinded spreadsheet. This information included authors’ names, country and year of publication, clinical and epidemiological characteristics of the population, number of participants and cases, measures of association, confounding factors, and outcomes. For dichotomous variables (mortality), adjusted odds ratios (OR) with 95% confidence intervals (CI 95%) were collected based on events in exposed and non-exposed groups. Missing data were reported when appropriate. For continuous variables, we gathered the mean and standard deviation (SD) for both exposed and non-exposed groups. When studies presented these variables as medians and interquartile ranges (IQR), we converted them to means and SDs using appropriate methods.19,20 Some studies reported the strength of association, such as odds ratio (OR), while others provided diagnostic accuracy metrics like sensitivity (Se), specificity (Sp), positive likelihood ratio (LR+), negative likelihood ratio (LR−), and ROC curves for mortality prediction. However, we opted to conduct the meta-analysis on both the studies reporting OR and those presenting diagnostic accuracy metrics.
Statistical analysisWe employed the Inverse Variance (IV) method21 in the meta-analysis to combine adjusted odds ratios (ORs) and their 95% confidence intervals (CIs). This analysis was performed using R® 4.2.226 software. Forest plots were used to summarize the quantitative synthesis, utilizing the meta library, and the metabin22 (for dichotomous variables) and metacont23 (for continuous variables) functions. The IV method with DerSimonian-Laird (DL) for tau2 was applied. Heterogeneity among studies was assessed using Cochran’s Q test and Higgins I2 statistic. In cases of statistically significant heterogeneity (I2 statistics > 40%), a random effects model without Hartung-Knapp (HK) adjustment was employed. Sensitivity analysis was conducted using Influence Analysis with the InfluenceAnalysis function, and Graphic Display of Heterogeneity (GOSH) with the gosh.diagnostics function.24 Meta-regression was performed using variables that may clinically influence heterogeneity, conducted by “cutoff values” of rSIG and risk of bias (RoB) of studies. The metareg function was utilized, employing mixed-effects with REML as the method of estimation.25
Quality assessmentWe assessed the potential risk of bias using Newcastle-Ottawa Scale (NOS).26 Two researchers (GAVT and MCC) independently evaluated the certainty of the evidence (CoE) for each outcome based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria.27,28 Any discrepancies between reviewers were resolved through discussion with the lead researcher (EDMR).
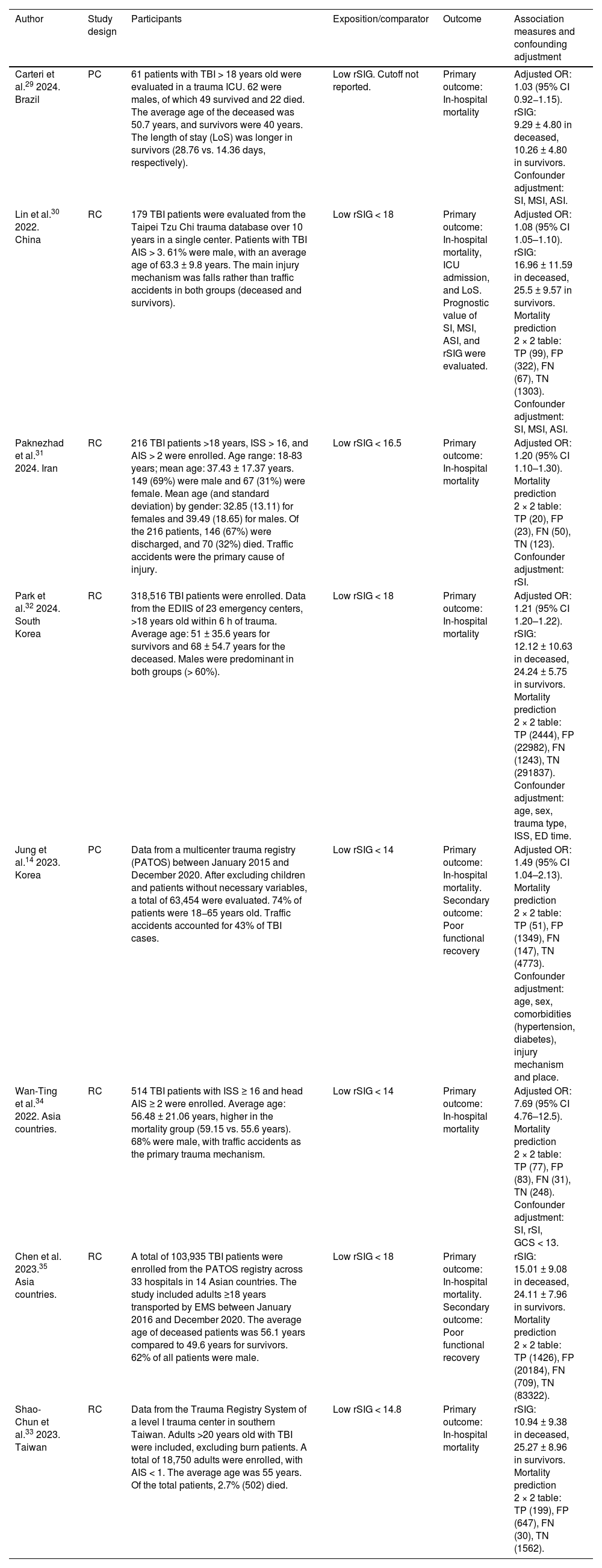
ResultsSearch results and study characteristicsEight observational studies were included in the qualitative evaluation and meta-analysis. All studies were conducted in various countries and continents, including Brazil,29 China,30 Iran,31 Korea,14 South Korea,32 Taiwan33 and varios Asian countries.33–35 Approximately 430 000 patients with TBI were evaluated, among whom there were 6,417 deaths (1.5%). Of the nine studies included, six14,29–32,34 reported adjusted association measures (OR) for the meta-analysis, and other six29,30,32–35 too reported rSIG values as a numerical variable in both the mortality and survival groups due to TBI. Additional demographic characteristics of the study population are detailed in Table 1. The patients' site of care was in the intensive care unit (ICU) and emergency department (ED). Chen35 and Park's32 studies were based on data reports (PATOS and EDIIS), contributing a large number of patients to this meta-analysis. Additional demographic characteristics of the study population are detailed in Table 1.
General characteristics of included studies.
| Author | Study design | Participants | Exposition/comparator | Outcome | Association measures and confounding adjustment |
|---|---|---|---|---|---|
| Carteri et al.29 2024. Brazil | PC | 61 patients with TBI > 18 years old were evaluated in a trauma ICU. 62 were males, of which 49 survived and 22 died. The average age of the deceased was 50.7 years, and survivors were 40 years. The length of stay (LoS) was longer in survivors (28.76 vs. 14.36 days, respectively). | Low rSIG. Cutoff not reported. | Primary outcome: In-hospital mortality | Adjusted OR: 1.03 (95% CI 0.92−1.15). rSIG: 9.29 ± 4.80 in deceased, 10.26 ± 4.80 in survivors. Confounder adjustment: SI, MSI, ASI. |
| Lin et al.30 2022. China | RC | 179 TBI patients were evaluated from the Taipei Tzu Chi trauma database over 10 years in a single center. Patients with TBI AIS > 3. 61% were male, with an average age of 63.3 ± 9.8 years. The main injury mechanism was falls rather than traffic accidents in both groups (deceased and survivors). | Low rSIG < 18 | Primary outcome: In-hospital mortality, ICU admission, and LoS. Prognostic value of SI, MSI, ASI, and rSIG were evaluated. | Adjusted OR: 1.08 (95% CI 1.05–1.10). rSIG: 16.96 ± 11.59 in deceased, 25.5 ± 9.57 in survivors. Mortality prediction 2 × 2 table: TP (99), FP (322), FN (67), TN (1303). Confounder adjustment: SI, MSI, ASI. |
| Paknezhad et al.31 2024. Iran | RC | 216 TBI patients >18 years, ISS > 16, and AIS > 2 were enrolled. Age range: 18-83 years; mean age: 37.43 ± 17.37 years. 149 (69%) were male and 67 (31%) were female. Mean age (and standard deviation) by gender: 32.85 (13.11) for females and 39.49 (18.65) for males. Of the 216 patients, 146 (67%) were discharged, and 70 (32%) died. Traffic accidents were the primary cause of injury. | Low rSIG < 16.5 | Primary outcome: In-hospital mortality | Adjusted OR: 1.20 (95% CI 1.10–1.30). Mortality prediction 2 × 2 table: TP (20), FP (23), FN (50), TN (123). Confounder adjustment: rSI. |
| Park et al.32 2024. South Korea | RC | 318,516 TBI patients were enrolled. Data from the EDIIS of 23 emergency centers, >18 years old within 6 h of trauma. Average age: 51 ± 35.6 years for survivors and 68 ± 54.7 years for the deceased. Males were predominant in both groups (> 60%). | Low rSIG < 18 | Primary outcome: In-hospital mortality | Adjusted OR: 1.21 (95% CI 1.20–1.22). rSIG: 12.12 ± 10.63 in deceased, 24.24 ± 5.75 in survivors. Mortality prediction 2 × 2 table: TP (2444), FP (22982), FN (1243), TN (291837). Confounder adjustment: age, sex, trauma type, ISS, ED time. |
| Jung et al.14 2023. Korea | PC | Data from a multicenter trauma registry (PATOS) between January 2015 and December 2020. After excluding children and patients without necessary variables, a total of 63,454 were evaluated. 74% of patients were 18−65 years old. Traffic accidents accounted for 43% of TBI cases. | Low rSIG < 14 | Primary outcome: In-hospital mortality. Secondary outcome: Poor functional recovery | Adjusted OR: 1.49 (95% CI 1.04–2.13). Mortality prediction 2 × 2 table: TP (51), FP (1349), FN (147), TN (4773). Confounder adjustment: age, sex, comorbidities (hypertension, diabetes), injury mechanism and place. |
| Wan-Ting et al.34 2022. Asia countries. | RC | 514 TBI patients with ISS ≥ 16 and head AIS ≥ 2 were enrolled. Average age: 56.48 ± 21.06 years, higher in the mortality group (59.15 vs. 55.6 years). 68% were male, with traffic accidents as the primary trauma mechanism. | Low rSIG < 14 | Primary outcome: In-hospital mortality | Adjusted OR: 7.69 (95% CI 4.76–12.5). Mortality prediction 2 × 2 table: TP (77), FP (83), FN (31), TN (248). Confounder adjustment: SI, rSI, GCS < 13. |
| Chen et al. 2023.35 Asia countries. | RC | A total of 103,935 TBI patients were enrolled from the PATOS registry across 33 hospitals in 14 Asian countries. The study included adults ≥18 years transported by EMS between January 2016 and December 2020. The average age of deceased patients was 56.1 years compared to 49.6 years for survivors. 62% of all patients were male. | Low rSIG < 18 | Primary outcome: In-hospital mortality. Secondary outcome: Poor functional recovery | rSIG: 15.01 ± 9.08 in deceased, 24.11 ± 7.96 in survivors. Mortality prediction 2 × 2 table: TP (1426), FP (20184), FN (709), TN (83322). |
| Shao-Chun et al.33 2023. Taiwan | RC | Data from the Trauma Registry System of a level I trauma center in southern Taiwan. Adults >20 years old with TBI were included, excluding burn patients. A total of 18,750 adults were enrolled, with AIS < 1. The average age was 55 years. Of the total patients, 2.7% (502) died. | Low rSIG < 14.8 | Primary outcome: In-hospital mortality | rSIG: 10.94 ± 9.38 in deceased, 25.27 ± 8.96 in survivors. Mortality prediction 2 × 2 table: TP (199), FP (647), FN (30), TN (1562). |
RC, retrospective cohort studye; PC, prospective cohort study; TBI, trauma brain injury; rSIG, reverse shock index multiplied by Glasgow coma scale; ICU, intensive care unit; LoS, length of hospitalization days; AIS, Abbreviated Injury Scale; ISS, Injury Severity Score; PATOS, Pan-Asian Trauma Outcome Study; EDIIS, Emergency Department-based Injury In-depth Surveillance; EMS, emergency medical service; aOR, adjusted Odds Ratio, TP, true positive; FP, salse positive; FN, false negative; TN, true negative.
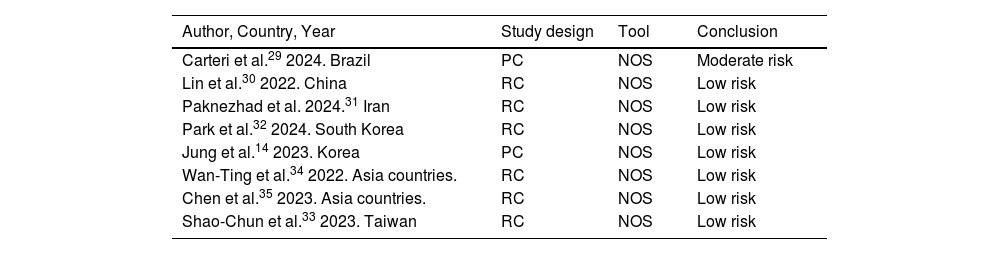
The Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias in the included observational studies. Of the eight studies included, only one, Carteri et al.,29 presented a moderate risk of bias, while the rest showed a low risk of bias.14,30–35 For further details, see Table 2.
Risk of bias of the included studies.
| Author, Country, Year | Study design | Tool | Conclusion |
|---|---|---|---|
| Carteri et al.29 2024. Brazil | PC | NOS | Moderate risk |
| Lin et al.30 2022. China | RC | NOS | Low risk |
| Paknezhad et al. 2024.31 Iran | RC | NOS | Low risk |
| Park et al.32 2024. South Korea | RC | NOS | Low risk |
| Jung et al.14 2023. Korea | PC | NOS | Low risk |
| Wan-Ting et al.34 2022. Asia countries. | RC | NOS | Low risk |
| Chen et al.35 2023. Asia countries. | RC | NOS | Low risk |
| Shao-Chun et al.33 2023. Taiwan | RC | NOS | Low risk |
RC, retrospective cohort study; PC, prospective cohort study; NOS, Newcastle–Ottawa Scale.
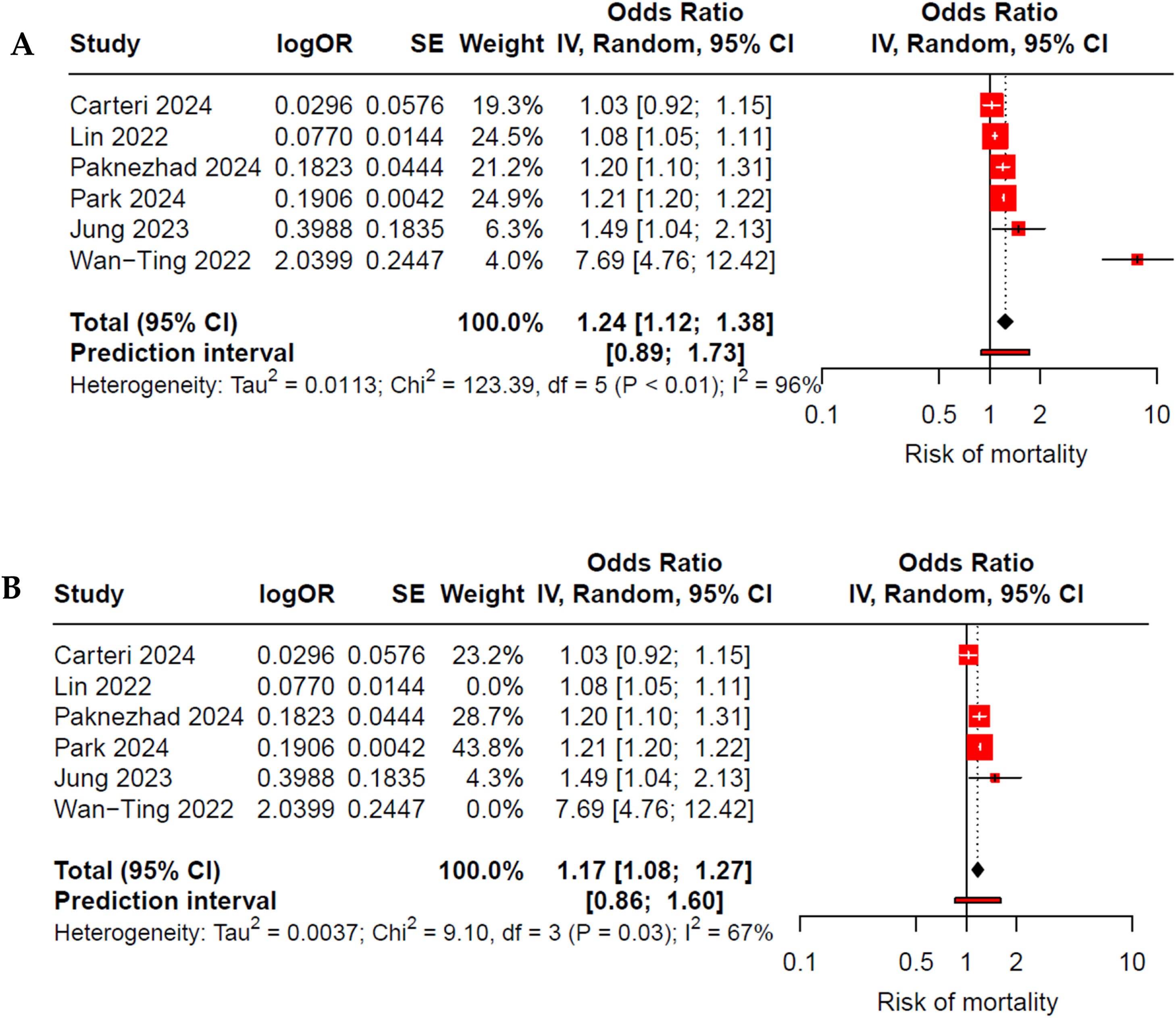
The initial meta-analysis of six studies indicates that patients with TBI and low rSIG have a higher risk of mortality, with a 24% increase in the risk of death (OR 1.24; 95% CI 1.12–1.38; I²: 96%) (Fig. 2A); however, there was very high heterogeneity. When performing a sensitivity analysis using the InfluenceAnalysis function and GOSH, we identified Lin et al.30 and Wan-Ting et al.34 as outliers. After excluding these studies, the increase in the risk of death among those with elevated rSIG remained significant (OR 1.17; 95% CI 1.08–1.27; I²: 67%), with a reduction in heterogeneity to a moderate level (Fig. 2B).
(A) Forest plot of low rSIG and mortality in adults with traumatic brain injury (TBI). All studies were included in the initial meta-analysis, which demonstrated high heterogeneity. After identifying outliers through Influence Analysis and GOSH, Lin et al.30 and Wan-Ting et al.34 were eliminated. (B) Forest plot after exclusion of outliers, illustrating the effect of low rSIG on mortality in adults with TBI, showing moderate heterogeneity.
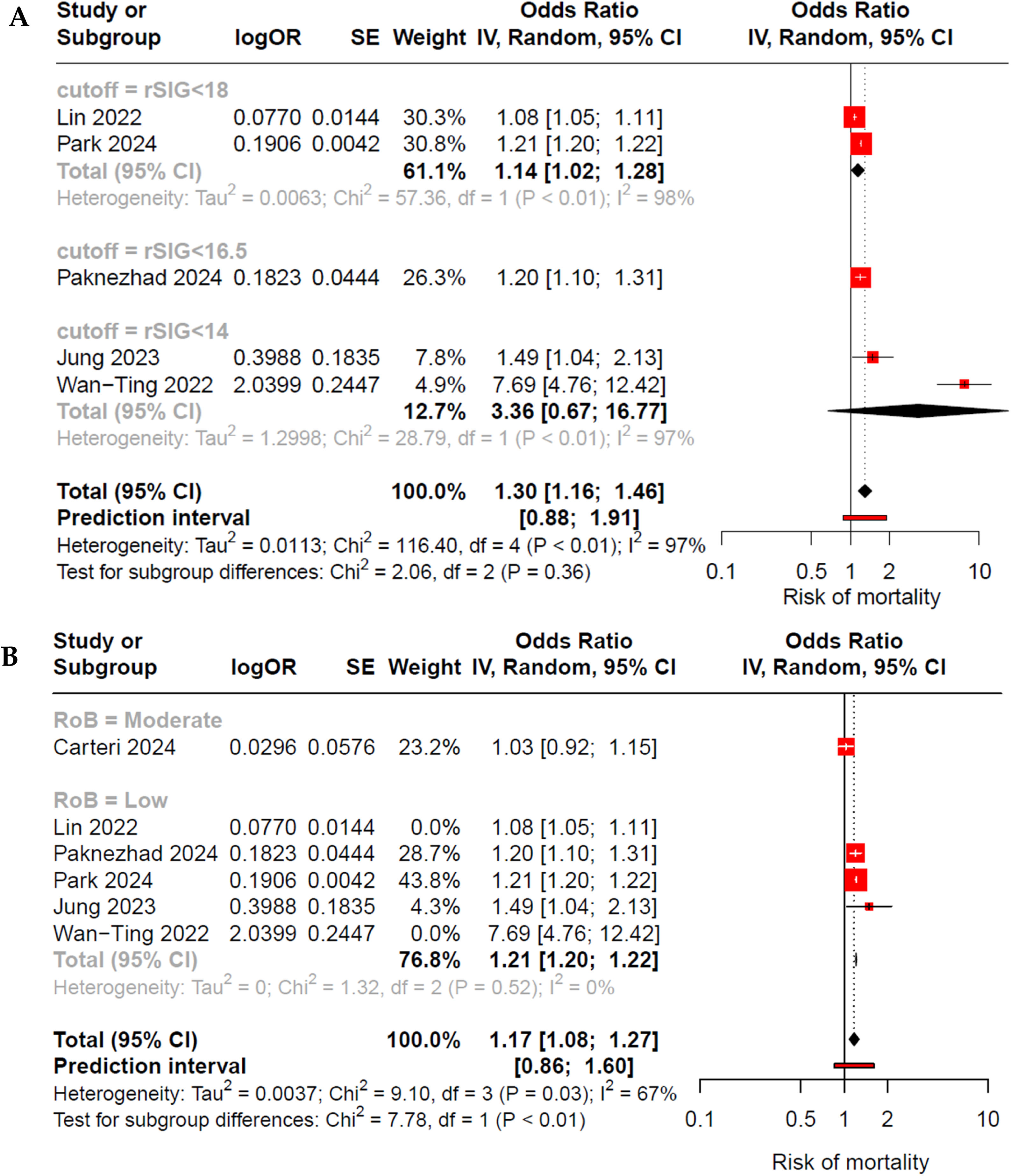
When evaluating subgroups based on the assessed rSIG cutoff points (<14, <16.5 or <18) across all studies, we observed an increase in the risk of mortality only in cutoff rSIG <18 subgroup; however, high heterogeneity persisted (Fig. 3A). Conversely, when evaluating by subgroups according to risk of bias (RoB) and excluding the outliers, we found a reduction in heterogeneity within the low RoB subgroup, accompanied by an increased risk of mortality (OR 1.21; 95% CI 1.20–1.22; I²: 0%) (Fig. 3B). Meta-regression based on the cutoff variable did not reveal it as an explanation for the heterogeneity (cutoff β: −1.7; p: 0.05); however, it did demonstrate a significant role when evaluated by RoB (RoB β: −0.23; p: 0.03) (Figs. 1S and 2S of Supplementary Material)
Forest plot by subgroups: (A) According to the rSIG cutoff point, heterogeneity remains high; however, statistical significance persists only in the group with rSIG <18. (B) According to risk of bias and outliers excluded, it shows that in the low risk of bias subgroup, there is an association between low rSIG and mortality in patients with TBI, with nule heterogeneity observed.
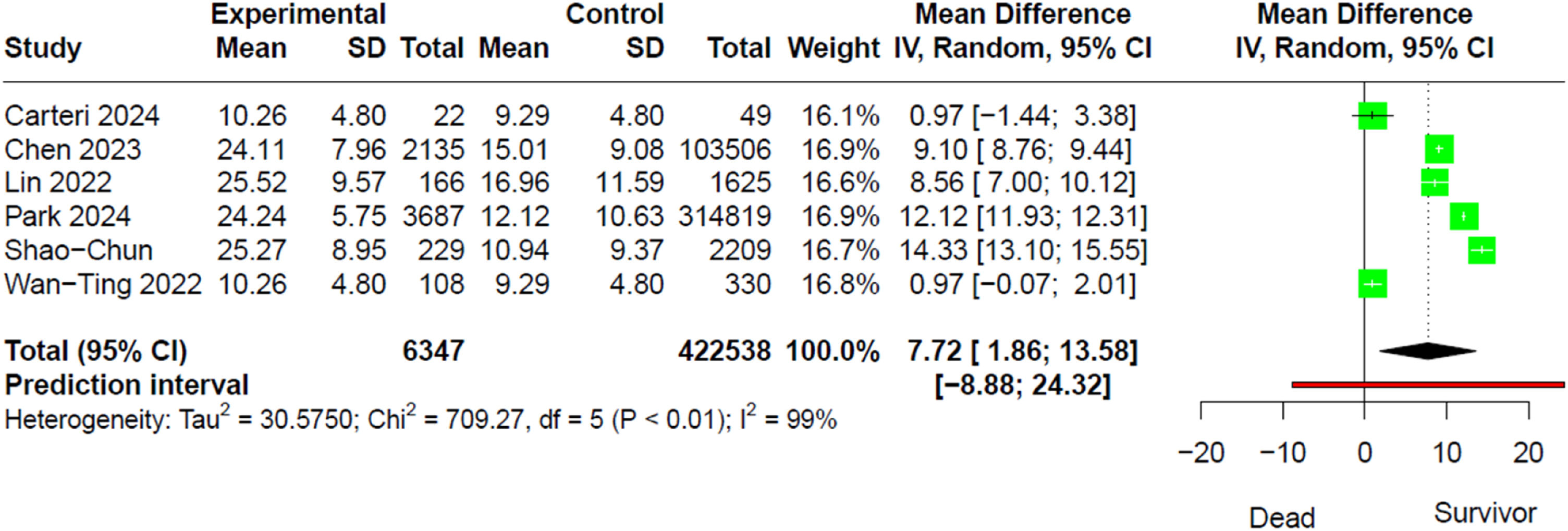
When evaluating rSIG as a continuous variable in both the deceased and survivor groups, we found that the survivor group had a higher mean difference (MD) compared to the deceased (MD 7.72; 95% CI 1.86–13.58; I²: 99%) (Fig. 4).
When performing meta-regression based on subgroups according to rSIG cutoff point (<14 vs. <18) and risk of bias (moderate vs. low), we found that these variables did not explain the heterogeneity (cutoff β: 4.7; 95% CI −8.3–17.2; p > 0.05 and RoB β: −8.04; 95% CI −23.8–7.7; p > 0.05, respectively)
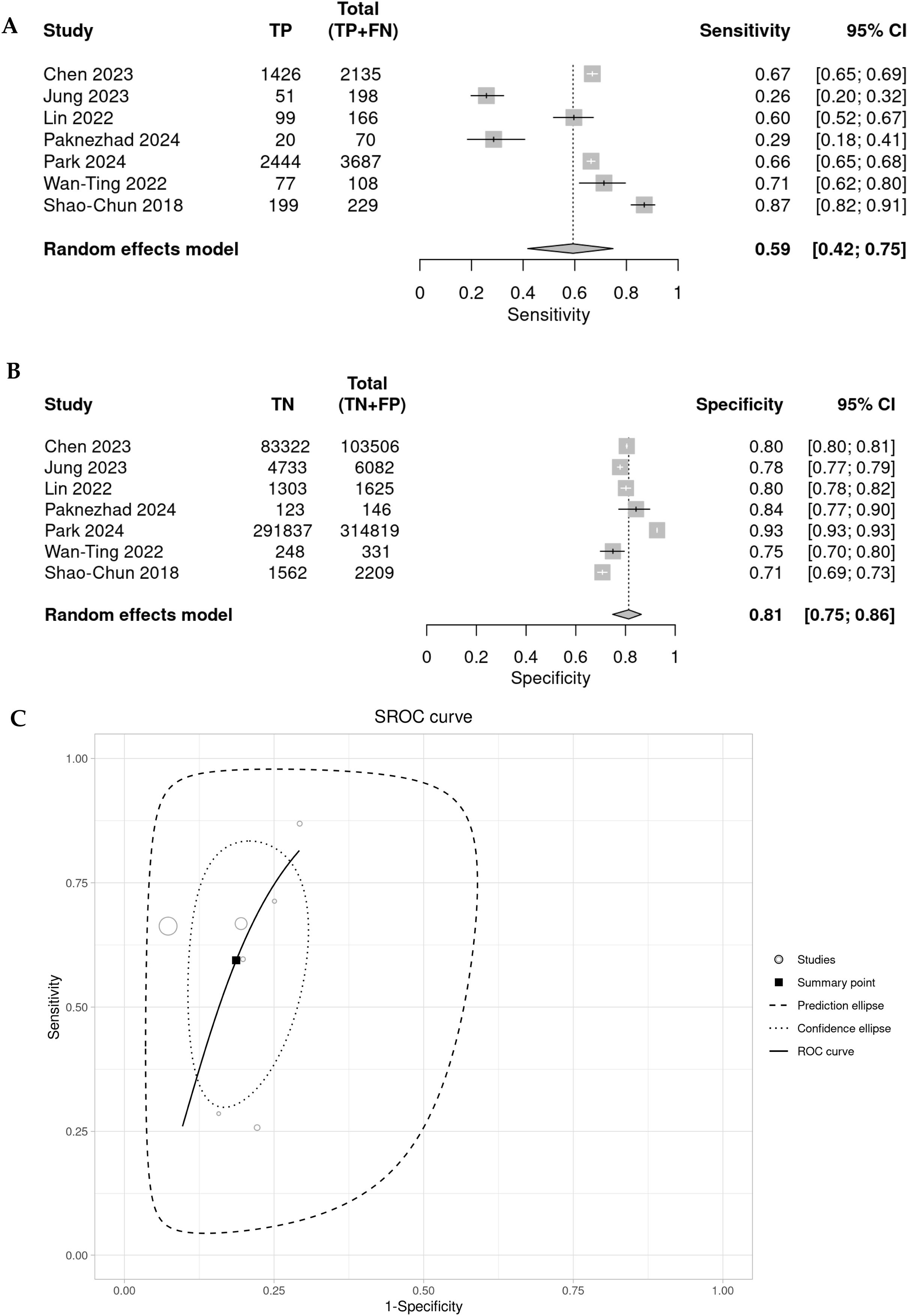
Diagnostic accuracy of rSIG to predict de mortalidad en TBISeven of the reported studies present results based on sensitivity (Se), specificity (Sp), likelihood ratio + (LR+) and likelihood ratio (LR−). When meta-analyzing these results, we found that rSIG, in predicting mortality in TBI, has a pooled sensitivity of 0.59 (95% CI 0.42–0.75; I2: 96%), a pooled specificity of 0.81 (95% CI 0.75–0.86; I²: 100%), a pooled LR+ of 3.179 (95% CI 2.178–4.63), a pooled LR− of 0.499 (95% CI 0.33–0.756), a pooled diagnostic OR of 6.365 (95% CI 3.05–13.282), and a false positive rate (FPR) of 0.187 (95% CI 0.136–0.251). Finally, the pooled diagnostic performance of rSIG, based on the area under the ROC curve, is 0.85. (Fig. 5).
The subgroup evaluation of the various indices according to rSIG cutoff point shows better performance when the cutoff point is rSIG <14 vs. 18 points (pooled S, E, and diagnostic OR for rSIG <14 are: 0.55, 0.77, and 4.14, respectively, and for rSIG <18 they are 0.65, 0.86, and 10.86, respectively). However, in meta-regression, using this cutoff point does not explain the level of heterogeneity (estimate for Se: 1.17, p: 0.6, and estimate for Sp: 1.11, p: 0.1) (Fig. 2S of Supplementary Material).
GRADE assessment and Funnel plotWe used GRADE to assess the certainty of evidence (CoE) regarding the association between rSIG and in-hospital mortality in TBI. Publication bias was not assessed due to the limited number of studies. Table 3 shows a 24% increase in the risk of death in TBI patients with low rSIG, with a moderate certainty of evidence.
Certainty of Evidence through GRADE. Patients with traumatic brain injury and low rSIG values have a 24% increased risk of in-hospital mortality, with a moderate level of certainty.
| Outcome № of participants (studies) | Relative effect (95% CI) | Certainty |
|---|---|---|
| In-hospital mortality № of participants: (6 non-randomised studies) | OR 1.24 (1.12–1.38) | ⨁⨁⨁◯ Moderate |
CI: confidence interval; OR: odds ratio.
Our meta-analysis of eight observational studies involving over 430,000 patients with TBI demonstrates a significant association between low rSIG levels and mortality, with a 24% increased risk of death in TBI patients (OR 1.24; 95% CI 1.12–1.38; I2: 96%). Similarly, sensitivity analyses and subgroup evaluations indicate that in studies with low risk of bias, the trend of increased mortality risk persists, with no heterogeneity observed (OR 1.21; 95% CI 1.20–1.22; I2: 0%). Furthermore, survivor groups exhibit significantly higher rSIG values compared to deceased patients (MD 7.72; 95% CI 1.86–13.58; I2: 99%). Therefore, rSIG is a significant predictor that should be used alongside other indicators to stratify risk in these patients and could be considered a standard tool for assessing the risk of mortality.
This is the first systematic review and meta-analysis investigating the predictive role of rSIG in mortality among patients with TBI. Although there are reports of several mortality predictors such as shock index (SI) and reversal shock index (rSI), rSIG emerges as a significant variable encompassing the prior hemodynamic complications of TBI and neurological deterioration as assessed by the GCS.1,15
During TBI, primary neurological changes resulting from trauma lead to deleterious secondary effects, including hemodynamic alterations that cause fluctuations in blood pressure, cardiac arrhythmias, and left ventricular dysfunction. These factors are associated with an increased risk of mortality, implicating the so-called "neuro-cardiac axis." Consequently, the SI relates physiological parameters such as heart rate (HR) and systolic blood pressure (SBP), playing a crucial role as a predictor of complications not only in TBI but also in other medical conditions.14,29,30,33 Normal values of SI range from 0.5 to 0.7, while values greater than 1 indicate uncompensated shock. It is noteworthy that SBP tends to decline later in patients with hemorrhagic shock, leading to the proposal of the rSI for patients in whom SBP remains compensated, at least initially.14
Although the GCS has been associated with neurological outcomes and mortality, a Japanese study group incorporates this score into rSIG, identifying it as a superior predictor of in-hospital mortality compared to other scores such as TRISS, ISS, and RTS in the initial screening and stratification for early interventions, including orotracheal intubation, transfusions, or admission to the ICU.15,32
Further analysis indicates that the rSIG displays a adequate pooled sensitivity (0.59; 95% CI 0.42−0.75), suggesting that while it can identify a substantial proportion of patients at risk, there is a concerning likelihood of false negatives. Meanwhile, the pooled specificity of rSIG was found very adequate (0.81; 95% CI 0.75−0.86), indicating that it is effective at ruling out patients who are unlikely to have high mortality.
In clinical practice, rSIG's high specificity allows for the identification of patients with favorable prognoses who may not require aggresive interventions, while its low sensitivity underscores the need for comprehensive assessments in high-acuity situations. This highlights the importance of establishing well-defined protocols that incorporate rSIG as part of a broader set of diagnostic tools, thereby standardizing care practices and enhancing the overall quality of care. By establishing precise guidelines for the application of rSIG, healthcare providers can ensure timely and appropriate patient care, reducing variability stemming from subjective clinical judgments and facilitating informed decision-making.36,37
The SROC curve analysis demonstrates an area under the curve (AUC) of 0.8497, suggesting that the rSIG performs adequately as a predictive tool in this clinical context. Although sensitivity and specificity are not perfectly balanced, rSIG is particularly skilled at differentiating between patients likely to survive and those at higher risk of mortality. This discrepancy emphasizes the importance of using rSIG in conjunction with other diagnostic methods to enhance predictive accuracy.15,38
However, the substantial heterogeneity observed across the studies (I2: 99%) raises concerns about the consistency of rSIG’s efficacy, it also presents an opportunity for refinement. Variability in patient populations, inclusion criteria, and methodologies can significantly impact the findings, suggesting that rSIG's predictive capacity may be influenced by factors like age, sex, comorbidities, and mechanisms of injury, cutoff of rSIG. While this heterogeneity complicates interpretation, it also emphasizes the need for rSIG to be assessed in more homogeneous clinical settings to ensure consistency and generalizability of the results, underscoring the the need for standardization in study protocols and patient selection.15,38–40
Moreover, our analysis calls for future research to focus not only on data aggregation from different studies but also on ensuring homogeneity in the populations and research methodologies employed. This approach could yield more reliable insights into the true impact of rSIG and its interactions with other clinical markers. Rigorous evaluation of rSIG's implementation in clinical settings is essential to translate its prognostic advantages into tangible improvements in TBI management and overall mortality reduction.
StrengthsOur study has several strengths. First, we implemented a comprehensive search strategy that encompassed six essential databases, facilitating a robust epidemiological evaluation of cause and effect. Second, we employed a rigorous methodology for our review and meta-analysis, which included a thorough quality assessment of the studies and a statistical analysis to address heterogeneity. Third, our results are both robust and reliable, as demonstrated by our careful evaluation of heterogeneity and identification of outliers, with consistent findings across individual studies. Fourth, this is the first systematic review and meta-analysis of sufficient quality that incorporates the most recent observational studies on rSIG based on Asian databases, enrolling a large number of patients.
LimitationsHowever, it is important to acknowledge several limitations in our study. First, the primary limitation is that the studies included use different cutoff points for rSIG and mortality. To address this, we analyzed the pooled OR by subgroups and also examined it as a continuous numerical variable in both the mortality and survival groups. Second, only a limited number of completed studies directly addressed our PECO question, with the majority focusing on Asian populations, which may affect the generalizability of our findings. Third, the high heterogeneity observed among the included studies can largely be attributed to the risk of bias inherent in each study. Furthermore, the diverse clinical characteristics of patients and various confounding factors identified in the studies contribute to the overall high level of heterogeneity. Nevertheless, the finding of an increased risk of mortality remains consistent within the low risk of bias subgroup.
Conclusions- -
Our study suggests that rSIG is a predictor of mortality in patients with traumatic brain injury (TBI), finding that patients with low rSIG values have a 24% increased risk of death with moderate evidence level.
- -
This association remains significant when conducting sensitivity analyses (excluding outliers and performing subgroup analyses based on the risk of bias and cutoff points for rSIG of the studies).
- -
Although there are limited studies evaluating the role of rSIG, it may be considered an early predictor for screening patients at risk of in-hospital complications.
GV-T: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
CQ-C: Methodology, Supervision, Writing – original draft.
MC-C: Methodology, Supervision, Writing – original draft.
EM-R: Conceptualization, Formal analysis, Methodology, Project administration, Writing – original draft. MC-C: Validation, Visualization, Writing – review & editing.
PA-M: Data curation, Formal analysis, Writing – original draft.
LL-D: Data curation, Formal analysis, Investigation, Writing – original draft.
HA-G: Funding acquisition, Project administration, Resources, Writing – review & editing.
WG-A: Data curation, Methodology, Writing – original draft.
LR-C: Funding acquisition, Project administration, Writing – review & editing.
FundingOwn resources.
The authors declare that they have no conflicts of interest or non-financial competing interests.