To evaluate the risk factors associated to noninvasive mechanical ventilation (NIV) failure in patients with primary pneumonia due to influenza A (H1N1)pdm09 virus admitted to the intensive care unit (ICU), and to demonstrate the association of NIV failure to increased mortality and longer stays.
DesignA cohort study was carried out.
ScopeA mixed ICU (16 beds) in a teaching hospital.
PatientsAdult patients admitted to the ICU with a diagnosis of pneumonia due to influenza A (H1N1)pdm09 virus requiring mechanical ventilation.
MeasurementsAge, sex, severity scores, administration of corticosteroids, oseltamivir within 72h of symptoms onset, days of symptoms prior to admission, affected quadrants, hemodynamic parameters, renal failure, laboratory test data on admission, mortality and stay in ICU and in hospital.
ResultsA total of 54 patients were admitted to the ICU and 49 were ventilated; 29 were females (59.2%), and the mean age±standard deviation was 66.77±14.77 years. Forty-three patients (87.75%) were ventilated with NIV, and 18 (41.9%) of them failed. Patients with NIV failure were younger (63 vs. 74 years; p=0.04), with a higher SOFA score (7 vs. 4; p=0.01) and greater early hemodynamic failure (61.1 vs. 8%; p=0.01). In addition, they presented longer ICU (26.28 vs. 6.88 days; p=0.01) and hospital stay (32.78 vs. 18.8 days; p=0.01). The ICU mortality rate was also higher in the NIV failure group (38.9 vs. 0%; p=0.02). In the multivariate analysis, corticosteroid therapy (OR 7.08; 95% CI 1.23–40.50) and early hemodynamic failure (OR 14.77; 95% CI 2.34–92.97) were identified as independent risk factors for NIV failure.
ConclusionsTreatment with corticosteroids and early hemodynamic failure were associated to NIV failure in patients with primary pneumonia due to influenza A (H1N1)pdm09 virus infection admitted to the ICU. The failure of NIV was associated to increased mortality.
Analizar factores de riesgo de fracaso de la ventilación no invasiva (VNI) en pacientes que ingresan en una unidad de cuidados intensivos (UCI) por neumonía primaria por virus influenza A (H1N1)pdm09. Demostrar que los pacientes que fracasan con la VNI tienen mayor mortalidad y estancias más largas.
DiseñoEstudio de cohorte.
ÁmbitoUCI polivalente de un hospital universitario de 16 camas.
PacientesPacientes adultos que ingresaron en la UCI en los que se confirmó el diagnóstico de neumonía por influenza A (H1N1)pdm09 y que recibieron ventilación mecánica.
VariablesEdad, sexo, puntuaciones de gravedad, administración de corticoides, oseltamivir dentro de las 72h de la sintomatología, días de sintomatología previos al ingreso, cuadrantes afectados, fracaso hemodinámico, renal y datos analíticos al ingreso, mortalidad y estancia en UCI y hospitalaria.
ResultadosIngresaron 54 pacientes y 49 fueron ventilados. Sexo femenino, 29 (59,2%) y una edad media±desviación estándar de 66,77±14,77 años. Fueron ventilados con VNI 43 (87,75%), de los que fracasaron 18 (41,9%). Los pacientes que fracasaron presentaron menor edad (63 vs. 74 años; p=0,04), mayor puntuación SOFA (7 vs. 4; p=0,01) y mayor fracaso hemodinámico (61,1 vs. 8%; p=0,01). Además, presentaron estancias más largas tanto en UCI (26,28 vs. 6,88 días; p=0,01) como hospitalarias (32,78 vs. 18,8 días; p=0,01), y mayor mortalidad en UCI (38,9 vs. 0%; p=0,02). Se identificaron como factores de riesgo de fracaso a VNI recibir corticoides (OR 7,08; IC 95% 1,23-40,50) y el fallo hemodinámico precoz (OR 14,77; IC 95% 2,34-92,97).
ConclusionesEl tratamiento con corticoides y el fracaso hemodinámico precoz se asociaron con el fracaso de la VNI en pacientes con neumonía primaria por virus influenza A (H1N1)pdm09. Estos tienen una mortalidad superior.
The appearance of the influenza A (H1N1)pdm09 pandemic in 2009 represented a change in world health and a challenge for the healthcare services.1 The Intensive Care Units (ICUs) received a large proportion of affected patients, accounting for up to 54.8% of all infectious disease cases admitted to these Units.2
The use of noninvasive ventilation (NIV) has become widespread over the last two decades. The benefits of the technique have been demonstrated, and its use therefore has been recommended in patients with exacerbated chronic obstructive pulmonary disease (COPD), cardiogenic lung edema and respiratory failure in immunocompromised patients.3 However, the usefulness of NIV in respiratory failure due to pneumonia caused by influenza A (H1N1)pdm09 virus remains subject to controversy. Although ventilation failure rates of 75% were recorded in the first years of the pandemic,4 these figures have gradually decreased to 35.3% in the latest series.5 The mortality rate among the failed cases varies between 25.6–58%.4,6,7 Delays in orotracheal intubation in patients where NIV fails increases the mortality rate; early detection of those patients at risk of failure is therefore important, and may be associated to a decrease in mortality.
The main objective of the present study was to establish the risk factors for NIV failure in patients admitted to the ICU due to severe influenza A (H1N1)pdm09 pneumonia. In addition, the study aimed to demonstrate that patients with failed NIV have greater mortality and longer ICU and in-hospital stays.
Patients and methodsThe study included those patients admitted to the ICU of Dr. Peset University Hospital (Valencia, Spain) in the period between June 2010 and December 2018 due to respiratory failure, with a confirmed diagnosis at discharge of influenza A (H1N1)pdm09 pneumonia requiring mechanical ventilation. The study was approved by the Ethics Committee of the hospital. A confirmed case of influenza A (H1N1)pdm09 pneumonia was defined as that in which molecular nucleic acid amplification testing (RT-PCR) proved positive. For this purpose we obtained nasopharyngeal exudate samples in all patients, and respiratory secretion samples through bronchial aspiration in intubated patients. Community-acquired pneumonia was defined as the presence of consistent clinical manifestations (cough, expectoration, fever >38°C, leukocytosis, and C-reactive protein and procalcitonin [PCT] elevation), with the identification of alveolar infiltrates on chest X-rays. Respiratory failure in turn was defined by blood gas criteria (PaO2 <60mmHg and/or PaCO2 >45mmHg).
We excluded patients under 18 years of age and those in which limitation of life support measures was decided in clinical session upon patient admission or during the subsequent clinical course.
The NIV systems used were BiPAP Vision® (Respironics Inc., PA, USA) and V60® (Respironics Inc., PA, USA), in both BiPAP and CPAP mode, connected to full facial or oronasal interfaces (Respironics Inc., Pennsylvania, USA) and an active humidification system (Fisher and Paykel Healthcare, Auckland, New Zealand).
Noninvasive ventilation failure was defined as the need to switch to orotracheal intubation and invasive mechanical ventilation (IMV). The decision to convert was based on clinical criteria according to the local protocol, considering not only blood gas criteria (impossibility of maintaining SatO2 >90% with FiO2 >0.60) but also the persistence of signs of increased respiratory exertion, the presence of abundant secretions, metabolic acidosis, multiorgan failure, encephalopathy or agitation with the rejection of NIV.
Demographic data were collected, together with information referred to previous diseases, patient origin, laboratory test data upon admission to our Unit, ventilation support received upon arrival, and severity scores such as the Sequential Organ Failure Assessment (SOFA) upon admission and the Acute Physiology and Chronic Health Evaluation II (APACHE II) after 24h. A history of chronic respiratory disease was also evaluated (asthma, COPD and pulmonary fibrosis). Hemodynamic failure was recorded, defined as the need to start vasoactive drug treatment before or during NIV, along with the need for renal replacement therapy during the clinical course. In turn, we documented corticosteroid use before or during NIV, and the prescription of oseltamivir within the first 72h after symptoms onset, as well as mortality in the ICU, after 30 days and in hospital, and the duration of ICU and hospital stay.
The statistical analysis of the data obtained was performed using the SPSS® version 17.0 statistical package (Chicago, IL, USA). The normal distribution of quantitative variables was assessed with the Kolmogorov–Smirnov test. Quantitative parameters were reported as the mean±standard deviation (SD) or median (and interquartile range [IQR]), while qualitative parameters were reported as frequencies and percentages. Quantitative variables were compared using the Student t-test or Mann–Whitney U-test, while qualitative variables were compared using the chi-squared test or Fisher exact test. The risk factors were assessed by logistic regression analysis, with calculation of the corresponding odds ratios (ORs) (and 95% confidence interval [95%CI]). Those variables yielding p<0.20 in the univariate analysis in turn were entered in the multivariate analysis. Statistical significance was considered for p<0.05.
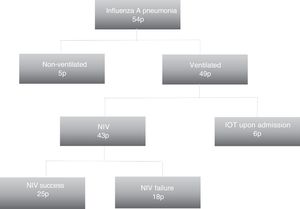
ResultsA total of 54 patients were admitted to our Unit with a confirmed diagnosis, and of these, 49 were subjected to ventilation. The patients mainly came from the hospital emergency service (26 patients; 53.1%). The mean age was 66.77±14.77 years, and 29 (59.2%) were women. With regard to disease history, 28 (57.1%) were hypertensive, 19 (38.8%) had diabetes mellitus, 17 (34.7%) were smokers, and 19 (38.8%) presented some chronic respiratory disease, including COPD in 11 subjects (22.4%). The SOFA score upon admission was 5.6±3.5, with an APACHE II score of 16 (range 11–22). The prior symptoms had been present for four days (range 2–7). Upon admission, 21 patients (42.9%) had not received mechanical ventilatory support; in 22 cases (40.7%) NIV was started, and 6 (11.1%) were directly intubated. Globally, in the course of stay, 43 patients (87.75%) underwent NIV, with a duration of 48h (±40); of these, failure was recorded in 18 (41.9%), with the need for IMV. Fig. 1 shows the distribution of the patients according to the ventilatory support received.
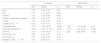
The patients in which NIV failed were found to have higher SOFA scores upon admission (7 vs. 4; p=0.01), were younger (63 vs. 74 years; p=0.04), had received more corticosteroids (55.6 vs. 12%; p=0.002), developed more early hemodynamic failure (61.1 vs. 8%; p=0.01) and were more often in need of renal replacement therapy (27.8 vs. 4%; p=0.02) (Table 1). There were no significant differences between the patients with COPD (16.7 vs. 32%; p=0.31). The failure of NIV was associated to longer ICU (26.28 vs. 6.88 days; p=0.01) and hospital stay (32.78 vs. 18.8 days; p=0.01), as well as to greater ICU mortality (38.9 vs. 0%; p=0.02), mortality after 30 days and in-hospital mortality (44.4 vs. 0%; p=0.001) (Table 2).
Baseline characteristics of the patients subjected to mechanical ventilation. Comparison of patients with NIV failure versus success.
| IMVn=6 | NIV successn=25 | NIV failuren=18 | p | |
|---|---|---|---|---|
| Age (years) | 71 (54.25–77.25) | 74 (56.5–81) | 63 (45.25–75.25) | 0.04 |
| Female gender | 1 (17) | 15 (60) | 9 (50) | 0.51 |
| APACHE II | 28 (23–33) | 15 (11–17) | 15 (11–23) | 0.52 |
| SOFA | 10±2 | 4±2 | 7±4 | 0.01 |
| Creatinine (mg/dl) | 2.92±2.4 | 1.55±1.17 | 1.48±1.02 | 0.84 |
| Urea (mg/dl) | 120.33±108.92 | 56±44 | 78±66 | 0.24 |
| Albumin (g/dl) | 2.55 (2.15–3.02) | 3.2 (2.8–3.5) | 2.7 (2.3–3.5) | 0.06 |
| Sodium (mEq/l) | 133 (128–143) | 137 (134–139) | 137 (129–141) | 0.99 |
| Potassium (mEq/l) | 4.3 (4–5.1) | 4.2 (3.9–4.7) | 4.4 (3.6–4.7) | 0.69 |
| Lactic acid (mmol/l) | 2.96±3.07 | 2.08±1.58 | 2.08±1.3 | 0.99 |
| Hemoglobin (g/dl) | 11.5±1.66 | 17.3±3.5 | 11.6±2.4 | 0.29 |
| Leukocytes ×109/l | 16.05 (11.40–26.95) | 8.5 (6.9–12.6) | 9.2 (4–15.4) | 0.81 |
| Eosinophils | 0.01 (0.04) | 0.04 (0.11) | 0.06 (0.23) | 0.66 |
| Platelets ×109/l | 198 (102–259) | 145 (118–307) | 167 (102–242) | 0.87 |
| COPD | 2 (33.3) | 8 (32) | 3 (16.7) | 0.31 |
| Oseltamivir first 72h | 4 (67) | 14 (56) | 8 (44) | 0.45 |
| Corticosteroidsa | 3 (50) | 3 (12) | 10 (55.6) | 0.002 |
| Early hemodynamic failureb | 6 (100) | 2 (8) | 11 (61.1) | 0.01 |
| Renal replacement therapy | 4 (66.7) | 1 (4) | 5 (27.8) | 0.02 |
| Hours of NIV | - | 48 (27–88) | 24 (12–54) | 0.029 |
APACHE II: Acute Physiology and Chronic Health Evaluation II; COPD: chronic obstructive pulmonary disease; SOFA: Sequential Organ Failure Assessment score upon admission to the ICU; NIV: noninvasive ventilation; IMV: invasive mechanical ventilation.
Data reported as the mean±standard deviation (SD), median (interquartile range [IQR] P25-P75) or n (percentage).
The analytical data correspond to those upon admission to the ICU.
Stay and mortality among the patients with NIV failure, NIV success and IMV. Comparison of the patients with NIV failure versus those receiving IMV as first support measure.
| GlobalN=49 | NIV successn=25 | NIV failuren=18 | IMVn=6 | p | |
|---|---|---|---|---|---|
| ICU stay (days) | 14.64±16.36 | 6.88±7 | 26.28±20 | 19.67±14.43 | 0.47 |
| Hospital stay (days) | 23.83±18.72 | 18.8±15 | 32.78±22 | 31±29.13 | 0.87 |
| Mortality in ICU | 9 (18.4) | 0 (0) | 7 (38.9) | 3 (50) | 0.66 |
| Mortality after 30 days | 11 (22.4) | 0 (0) | 8 (44.4) | 4 (66.7) | 0.64 |
| Mortality in hospital | 11 (22.4) | 0 (0) | 8 (44.4) | 4 (66.7) | 0.64 |
IMV: invasive mechanical ventilation; NIV: noninvasive ventilation.
Data reported as the mean±standard deviation (SD) or n (percentage).
The identified NIV failure risk factors were early corticosteroid use (OR 7.08; 95%CI 1.23–40.50) and early hemodynamic failure (OR 14.77; 95%CI 2.34–92.97) (Table 3).
Univariate and multivariate analysis of comorbidities and parameters associated to NIV failure.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | |
| AHT | 0.70 | 0.20–2.42 | 0.57 | |||
| DM | 0.75 | 0.21–2.65 | 0.65 | |||
| Smoker | 1.63 | 0.45–5.93 | 0.45 | |||
| History of respiratory disease | 0.63 | 0.18–2.24 | 0.48 | |||
| COPD | 0.37 | 0.47–2.99 | 0.60 | |||
| Oseltamivir 72h | 0.62 | 0.18–2.12 | 0.45 | |||
| Corticosteroids | 9.16 | 1.99–42.03 | 0.004 | 7.08 | 1.23–40.50 | 0.02 |
| Hemodynamic failure | 18.07 | 3.21–101.72 | 0.001 | 14.77 | 2.34–92.97 | 0.004 |
| SOFA >7 | 3.18 | 0.76–13.32 | 0.11 | 2.62 | 0.63–3.80 | 0.105 |
| APACHE II >15 | 0.62 | 0.17–2.20 | 0.46 | |||
| Quadrants >2 | 0.71 | 0.20–2.44 | 0.59 | |||
| Platelets > 150×109 | 1.57 | 0.45–5.43 | 0.47 | |||
APACHE II: Acute Physiology and Chronic Health Evaluation II; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; AHT: arterial hypertension; 95%CI: 95% confidence interval; OR: odds ratio; SOFA: Sequential Organ Failure Assessment score upon admission to the ICU; RRT: renal replacement therapy; IMV: invasive mechanical ventilation; NIV: noninvasive ventilation.
There currently are doubts regarding the usefulness of NIV in patients with hypoxemic respiratory failure due to influenza A. Nevertheless, and in contrast to the practice reported in other studies, NIV is often used in our Unit as an initial support measure in patients with acute respiratory failure.4,8 One of the classical arguments for not using NIV – the risk of contagion on the part of the healthcare staff9,10 – was not seen to apply in our cohort, since no cases of contagion were observed. The European Society of Intensive Care Medicine considers the procedure to involve a high risk of contagion.11 In our Unit we place an antibacterial/antiviral filter at the ventilator outlet.12
The NIV failure rate in our patient cohort was 41.9%, which is lower than in other published studies.13–15 Our results are in line with those reported by Marín-Corral et al.,5 with a failure rate of 35.3%. This figure is lower than in our study, though their recorded mortality rate in the ICU was comparatively higher (18.4% versus 24.2%). In contrast, Rodríguez et al.13 recorded a higher failure rate (56.8%), though their mortality data were similar to our own. This is an interesting finding, considering that the patients were of similar severity, and that NIV failure has been associated to greater mortality and a poorer prognosis.6 In our study there were significant differences in mortality on comparing the patients with NIV failure versus those without.
The variables found to be associated to increased NIV failure were corticosteroid use and early hemodynamic failure. Taking this into account, the lesser mortality recorded in our cohort may have been due to more limited corticosteroid use compared with the study published by Masclans et al.6 (32.65% versus 39.7%). The administration of corticosteroids has been related to increased mortality,16,17 as evidenced by a meta-analysis.18 The data of another more recent meta-analysis of the use of corticosteroids in patients with pneumonia also speak against the administration of such drugs – though these findings must be interpreted with caution in view of the small proportion of patients with influenza A (H1N1)pdm09 infection included in the analysis.19 Nevertheless, there is agreement that corticosteroid use should be avoided in patients with influenza A pneumonia – this principle being an important reference in our Unit in the management of such patients. The recommendations of 2012 did not include such treatment as part of patient management.20 A pending issue refers to the discrepancy of corticosteroid use in patients with associated shock, in which the start of corticosteroid replacement therapy would be assessed as part of the integral management of shock. In our study, the patients with early hemodynamic failure received more corticosteroids (54.5% versus 18.8%; p<0.05). This observation could support the suspicion that corticosteroid use in these patients is largely due to the appearance of shock.
With regard to the benefits of NIV support in patients with COPD,3 we observed no differences in NIV success in such patients, and COPD was not found to be independently correlated to NIV failure. This observation is consistent with the data published by Rodríguez et al.,13 who found the beneficial effects of NIV in these patients to be lost in the presence of a SOFA score ≥5.
One of the important aspects that can also produce controversy is the evolution of those patients subjected to IMV, and the question of whether there are differences between those individuals that are intubated upon admission and those who require intubation once NIV has failed. In our series, with severity scores higher than those in the study of Rello et al.,14 the incidence of IMV upon admission was lower. However, in the series published by Rodríguez et al.13 the percentage of patients that initially received IMV support was higher (57–5% versus 12.24%), and mortality in the ICU was lower (31.3% versus 50%), despite the few patients involved. Of note is the great severity of these patients in our cohort. The comparison between those patients subjected to IMV upon admission and those that received IMV following NIV failure showed no differences in terms of the duration of stay or mortality in the ICU and in hospital.
With regard to the duration of NIV support and its association to failure of the technique, a number of studies have found delays in intubation to increase mortality in patients with hypoxemic respiratory failure.21 However, in our series the patients with NIV failure required earlier intubation, and these individuals consequently received fewer hours of NIV. This may be because the mentioned patients were in more serious condition and moreover presented more hemodynamic failure – both of these circumstances probably contributing to decide earlier introduction of MIV. Furthermore, the patients with NIV failure required more renal replacement therapy over time, despite the absence of differences in renal function parameters upon admission. Based on these findings, we could consider maintaining ventilation support with NIV in the case no second organ failure occurs (especially hemodynamic failure), contemplating and limiting it only for those patients who present respiratory failure.
The main limitation of our study is referred to its retrospective observational design. It is probable that patients with the same diagnosis and a need for NIV support were not admitted to our Unit on the grounds that admission was discarded. Such patients were obviously not included in the study analysis. On the other hand, it was not possible to compile important information such as all the blood gas data. Their inclusion would have allowed classification of the type of acute respiratory failure (for example PaO2, SatO2, PaO2/FiO2) and analysis of its role as a possible prognostic factor in NIV. The selection bias implied by the inclusion of critical patients admitted to the IC likewise means that the results cannot be extrapolated to other patient populations.
In conclusion, we may consider the use of NIV in patients with influenza A (H1N1)pdm09 infection as a first respiratory support measure, particularly in younger individuals with less severe disease and who have not received corticosteroids or developed early hemodynamic failure. The presence of either of the latter two circumstances would be associated to an increased probability of NIV failure.
AuthorshipH. Hernández, A. Navarro and L. Lizama participated in data compilation and generation of the database. In turn, H. Hernández performed the data analysis, as well as drafting and correction of the manuscript, while R. Zaragoza supervised the statistical analysis and reviewed the paper for intellectual content. All authors approved the final version of the article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Hernández Garcés H, Navarro Lacalle A, Lizama López L, Zaragoza Crespo R. Factores de riesgo de fracaso de ventilación no invasiva en neumonía primaria por influenza A en pacientes críticos. Med Intensiva. 2021;45:347–353.