To assess incidence, risk factors and impact of acute kidney injury(AKI) within 48 h of intensive care unit(ICU) admission on ICU mortality in patients with SARS-CoV-2 pneumonia. To assess ICU mortality and risk factors for continuous renal replacement therapy (CRRT) in AKI I and II patients.
DesignRetrospective observational study.
SettingSixty-seven ICU from Spain, Andorra, Ireland.
Patients5399 patients March 2020 to April 2022.
Main variables of interestDemographic variables, comorbidities, laboratory data (worst values) during the first two days of ICU admission to generate a logistic regression model describing independent risk factors for AKI and ICU mortality. AKI was defined according to current international guidelines (kidney disease improving global outcomes, KDIGO).
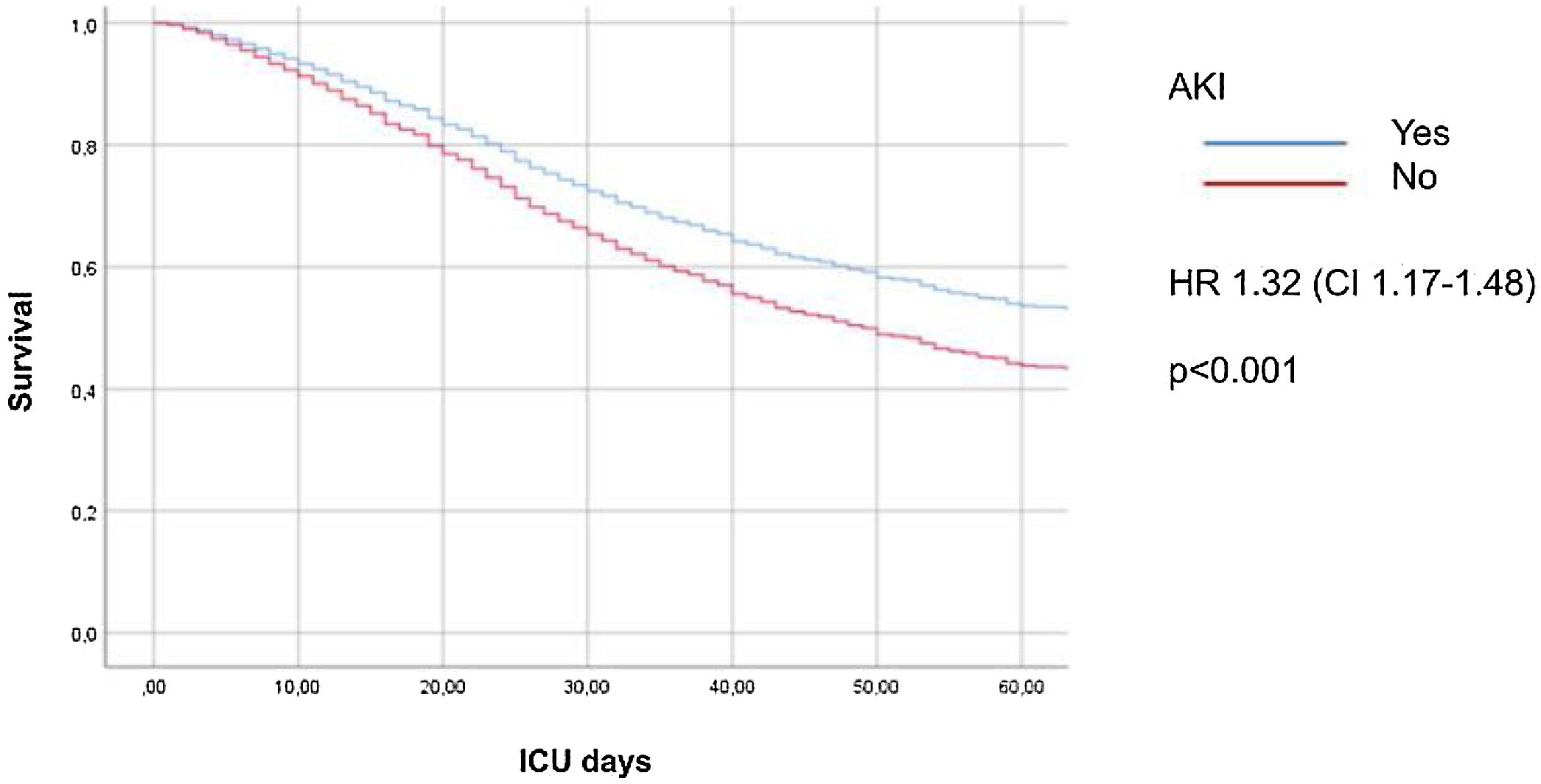
ResultsOf 5399 patients included 1879 (34.8%) developed AKI. These patients had higher ICU mortality and AKI was independently associated with a higher ICU mortality (HR 1.32 CI 1.17–1.48; p < 0.001).
Male gender, hypertension, diabetes, obesity, chronic heart failure, myocardial dysfunction, higher severity scores, and procalcitonine were independently associated with the development of AKI.
In AKI I and II patients the need for CRRT was 12.6% (217/1710). In these patients, APACHE II, need for mechanical ventilation in the first 24 h after ICU admission and myocardial dysfunction were associated with risk of needing CRRT. AKI I and II patients had a high ICU mortality (38.5%), especially if CRRT were required (64.1% vs. 34,8%; p < 0.001).
ConclusionsCritically ill patients with SARS-CoV-2 pneumonia and AKI have a high ICU mortality. Even AKI I and II stages are associated with high risk of needing CRRT and ICU mortality.
Describir incidencia, factores de riesgo e impacto de insuficiencia renal aguda (IRA) en primeras 48 horas de ingreso en unidad de cuidados intensivos(UCI) sobre la mortalidad en UCI en pacientes con neumonía por SARS-CoV-2. Evaluar mortalidad en UCI y factores de riesgo para técnicas continuas de reemplazo renal (TCRR) en pacientes con IRA I y II.
DiseñoEstudio descriptivo retrospectivo.
ÁmbitoUCI España, Andorra, Irlanda.
PacientesPacientes marzo 2020 abril 2022.
Variables de interés principalsVariables demográficas, comorbilidades, datos de laboratorio (valores peores) durante los dos primeros días de ingreso en UCI para generar modelo de regresión logística que describa factores de riesgo de IRA y mortalidad en UCI. Se definió IRA según guías Internacionales actuales (Kidney disease improving global outcomes, KDIGO).
ResultadosDe 5.399 pacientes incluidos 1879 (34,8%) desarrollaron IRA. Estos pacientes presentaron mayor mortalidad en UCI; la IRA se asoció con mayor riesgo de mortalidad en UCI (HR 1,32 IC 1,17−1,48; p < 0,001).
El sexo masculino, hipertensión, diabetis, obesidad, insuficiencia cardíaca crónica, disfunción miocárdica, scores de gravedad mayores, y procalcitonine se asociaron con desarrollo de IRA.
El 12.6% de pacientes con IRA I y II precisaron TCRR. En éstos el APACHE II, necesidad de VM en primeras 24 horas de ingreso en UCI y la disfunción miocárdica se asociaron al riesgo de necesitar TCRR. Estos pacientes tuvieron una elevada mortalidad en UCI (38.5%) sobre todo si precisaron TCRR (64.1% vs. 34,8%; p < 0.001).
ConclusionesLos pacientes críticos con neumonía por SARS-CoV-2 e IRA tienen elevada mortalidad. Incluso IRA I y II se asocia a mayor necesidad de TCRR y mortalidad en UCI.
In March 2020, the World Health Organization, declared the SARS-CoV-2 virus infection as an emerging pandemic. Initially considered a respiratory disease, COVID-19 is now recognised as affecting multiple organ systems. Renal involvement is common in these patients and can progress from asymptomatic proteinuria to acute kidney injury (AKI) in advanced stages.1,2
The pathogenesis of AKI in patients with COVID-19 may be multifactorial, including haemodynamic, pro-inflammatory and pro-apoptotic effects of pulmonary inflammation, cytokine release syndrome and hypercoagulable state affecting renal function.3,4 In addition, autopsy findings indicate that SARS-CoV-2 virus can directly infect the renal tubular epithelium and podocytes through an angiotensin converting enzyme 2 (ACE2) pathway.5,6
AKI is a common clinical problem in critically ill patients, with an incidence of 30–50%, and a need for continuous renal replacement therapies (CRRT) of 14–25% in most studies.7–9 An even higher incidence has been observed in mechanically ventilated patients.10 Mortality in hospitalised patients with COVID-19 is particularly hight if they develop AKI.
Some AKI and COVID-19 studies have assessed the development of AKI from the first week of admission to the intensive care unit (ICU).8,11 However, early knowledge of patients who develop AKI could perhaps improve prognosis in these patients. On the other hand, when the need of CRRT is discussed it is usually done globally and not by stage of AKI.8,11 Moreover, the need for CRRT is not usually assessed in early stages of AKI (AKI I and II).
For this reason, the aim of our study is to assess incidence, risk factors and impact of AKI within 48 h of ICU admission on ICU crude mortality. Finally, we aimed to assess in the subgroup of AKI I and II patients the risk factors for requiring CRRT and their mortality in ICU.
Patients and methodsStudy populationThis is a retrospective, descriptive, multicentre study. Sixty-seven ICUs from Spain, Andorra and Ireland participated in the study. Consecutive adult critically ill patients with confirmed SARS-CoV-2 pneumonia admitted to the ICU between March 2020 and April 2022 were included. Diagnosis was confirmed by nasopharyngeal swab and real-time reverse transcription polymerase chain reaction. Patients with chronic kidney disease (CKD) were excluded.
ObjectivesThe primary objective was to assess incidence, risk factors and impact of AKI within 48 h of ICU admission on ICU crude mortality.
The secondary objectives were:
- 1)
To describe patients’ characteristics by AKI stages.
- 2)
To describe the risk factors for overall mortality.
- 3)
To assess the outcome in patients with “early” AKI stages (AKI I and II); therefore, we want to describe in this subgroup of patients:
- a
The need for CRRT during ICU stay, and identify risk factors for CRRT.
- b
ICU Mortality and risk factors for ICU mortality.
- a
Demographic and epidemiological variables, comorbidities, laboratory data (peak values) during the first two days of ICU admission, severity scores through Acute Physiology And Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scoring, clinical status at ICU admission, need for mechanical ventilation (MV) and CRRT during ICU stay, ICU mortality, ICU length of stay (LOS), hospital LOS, MV days and hospital mortality were recorded.
AKI was defined as a creatinine rise of 1.5 times the baseline value according to Kidney Disease Improving Global Outcomes (KDIGO) consensus criteria.12 Baseline creatinine was obtained from ambulatory data. Indication for initiation CRRT was at the discretion of the treating physicians according to KDIGO guidelines. Myocardial dysfunction was defined as a left ventricular ejection fraction less than 50%. Shock was defined hypotension (systolic arterial pressure <90 mmHg or mean arterial pressure <70 mmHg or decrease ≥40 mmHg from baseline) associated with some sign of tissue hypoperfusion (poor peripheral perfusion, oliguria, altered mental status, lactate >2 mEq/L) or need for vasomotor support. Immunosuppression was defined as active cancer, chemotherapy, haematological disease, immunosuppressive treatment and chronic corticosteroid treatment (>20 milligrams/day of prednisone-equivalent for >3 weeks).
Statistical analysisData are presented as number (percentage) for categorical variables, median (interquartile range (IQR) for non-parametric variables, and mean and standard deviation for parametric variables. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov-test. Chi-square test (χ2) was used for comparison of categorical variables. Student’s t test was applied for normally distributed continuous variables; otherwise, Mann–Whitney U test was used.
Logistic regression analysis was used to identify risk factors associated with the development of AKI and the need for CRRT. The association between AKI and mortality was assessed by logistic and Cox hazard regression analysis. Variables with significant results in the univariate analysis were included in the adjusted models. Statistical significance was defined as a p-value <0.05. All study variables were analysed using SPSS statistical software (IBM, USA, version 25).
Ethical considerationsPatients were included in the COVID-19 SEMICYUC database (created by the Spanish Society of Intensive Care Medicine and Coronary Units; NCT04948242). The study was approved by the Institutional Ethics Committee board of the Hospital Universitari Joan XXIII (IRB#CEIM/066/2020). All data were anonymised, allowing the requirement for informed consent to be waived.
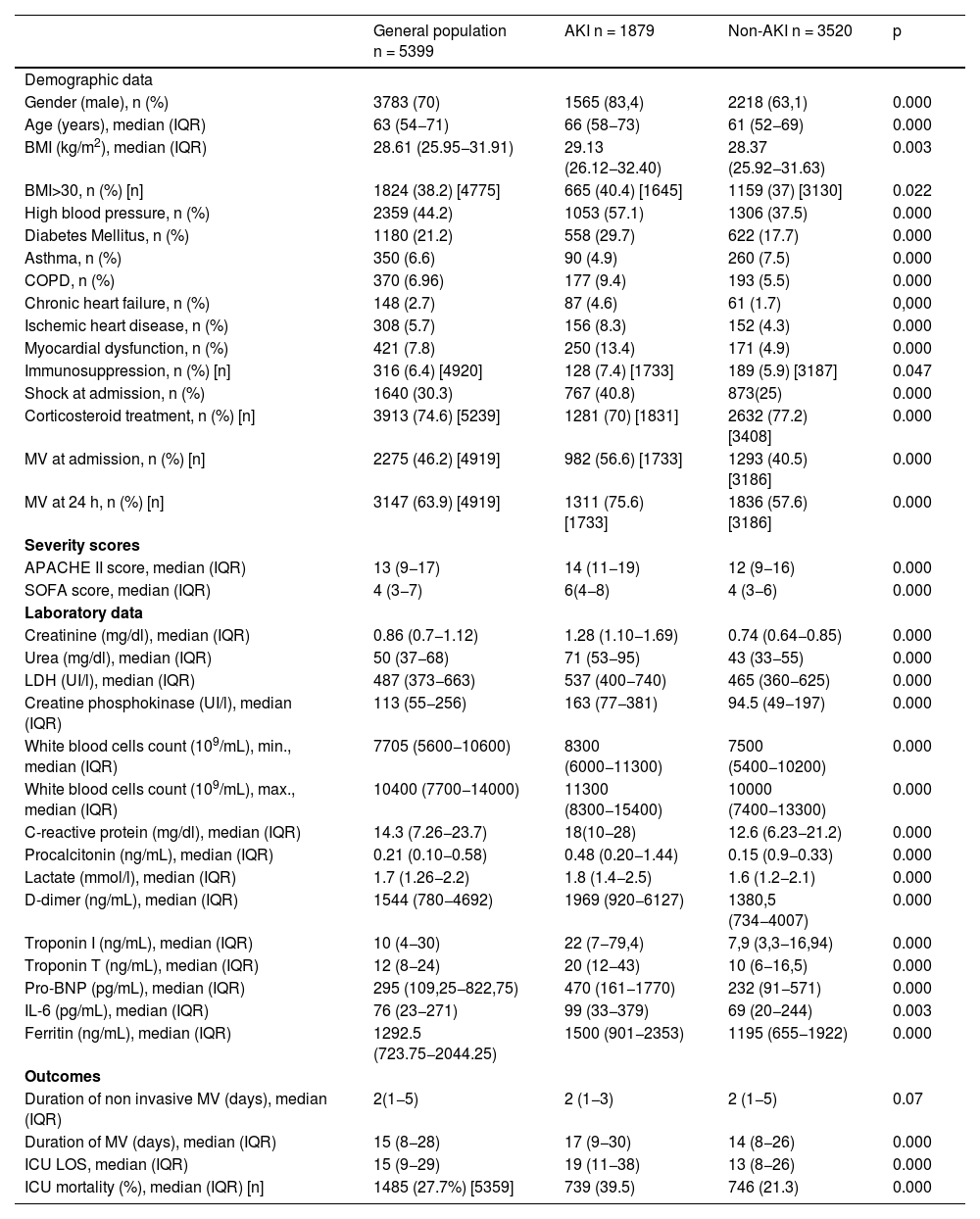
ResultsStudy population and patient characteristicsDuring the study period, 5726 patients were admitted to the ICU; 327 (5.7%) were excluded because of CKD. Thus, 5399 patients were included in the final analysis (Fig. S1 in the Supplementary Appendix). The median age was 63 years and 70% of patients were male. The most common comorbidities were hypertension, obesity and diabetes. APACHE II score was 13 and SOFA score was 4. Median ICU LOS was 15 days, ICU mortality was 27.6% and hospital mortality was 3.3%. Baseline clinical characteristics are summarised in Table 1.
Baseline characteristics of patients with and without AKI.
| General population n = 5399 | AKI n = 1879 | Non-AKI n = 3520 | p | |
|---|---|---|---|---|
| Demographic data | ||||
| Gender (male), n (%) | 3783 (70) | 1565 (83,4) | 2218 (63,1) | 0.000 |
| Age (years), median (IQR) | 63 (54−71) | 66 (58−73) | 61 (52−69) | 0.000 |
| BMI (kg/m2), median (IQR) | 28.61 (25.95−31.91) | 29.13 (26.12−32.40) | 28.37 (25.92−31.63) | 0.003 |
| BMI>30, n (%) [n] | 1824 (38.2) [4775] | 665 (40.4) [1645] | 1159 (37) [3130] | 0.022 |
| High blood pressure, n (%) | 2359 (44.2) | 1053 (57.1) | 1306 (37.5) | 0.000 |
| Diabetes Mellitus, n (%) | 1180 (21.2) | 558 (29.7) | 622 (17.7) | 0.000 |
| Asthma, n (%) | 350 (6.6) | 90 (4.9) | 260 (7.5) | 0.000 |
| COPD, n (%) | 370 (6.96) | 177 (9.4) | 193 (5.5) | 0.000 |
| Chronic heart failure, n (%) | 148 (2.7) | 87 (4.6) | 61 (1.7) | 0,000 |
| Ischemic heart disease, n (%) | 308 (5.7) | 156 (8.3) | 152 (4.3) | 0.000 |
| Myocardial dysfunction, n (%) | 421 (7.8) | 250 (13.4) | 171 (4.9) | 0.000 |
| Immunosuppression, n (%) [n] | 316 (6.4) [4920] | 128 (7.4) [1733] | 189 (5.9) [3187] | 0.047 |
| Shock at admission, n (%) | 1640 (30.3) | 767 (40.8) | 873(25) | 0.000 |
| Corticosteroid treatment, n (%) [n] | 3913 (74.6) [5239] | 1281 (70) [1831] | 2632 (77.2) [3408] | 0.000 |
| MV at admission, n (%) [n] | 2275 (46.2) [4919] | 982 (56.6) [1733] | 1293 (40.5) [3186] | 0.000 |
| MV at 24 h, n (%) [n] | 3147 (63.9) [4919] | 1311 (75.6) [1733] | 1836 (57.6) [3186] | 0.000 |
| Severity scores | ||||
| APACHE II score, median (IQR) | 13 (9−17) | 14 (11−19) | 12 (9−16) | 0.000 |
| SOFA score, median (IQR) | 4 (3−7) | 6(4−8) | 4 (3−6) | 0.000 |
| Laboratory data | ||||
| Creatinine (mg/dl), median (IQR) | 0.86 (0.7−1.12) | 1.28 (1.10−1.69) | 0.74 (0.64−0.85) | 0.000 |
| Urea (mg/dl), median (IQR) | 50 (37−68) | 71 (53−95) | 43 (33−55) | 0.000 |
| LDH (UI/l), median (IQR) | 487 (373−663) | 537 (400−740) | 465 (360−625) | 0.000 |
| Creatine phosphokinase (UI/l), median (IQR) | 113 (55−256) | 163 (77−381) | 94.5 (49−197) | 0.000 |
| White blood cells count (109/mL), min., median (IQR) | 7705 (5600−10600) | 8300 (6000−11300) | 7500 (5400−10200) | 0.000 |
| White blood cells count (109/mL), max., median (IQR) | 10400 (7700−14000) | 11300 (8300−15400) | 10000 (7400−13300) | 0.000 |
| C-reactive protein (mg/dl), median (IQR) | 14.3 (7.26−23.7) | 18(10−28) | 12.6 (6.23−21.2) | 0.000 |
| Procalcitonin (ng/mL), median (IQR) | 0.21 (0.10−0.58) | 0.48 (0.20−1.44) | 0.15 (0.9−0.33) | 0.000 |
| Lactate (mmol/l), median (IQR) | 1.7 (1.26−2.2) | 1.8 (1.4−2.5) | 1.6 (1.2−2.1) | 0.000 |
| D-dimer (ng/mL), median (IQR) | 1544 (780−4692) | 1969 (920−6127) | 1380,5 (734−4007) | 0.000 |
| Troponin I (ng/mL), median (IQR) | 10 (4−30) | 22 (7−79,4) | 7,9 (3,3−16,94) | 0.000 |
| Troponin T (ng/mL), median (IQR) | 12 (8−24) | 20 (12−43) | 10 (6−16,5) | 0.000 |
| Pro-BNP (pg/mL), median (IQR) | 295 (109,25−822,75) | 470 (161−1770) | 232 (91−571) | 0.000 |
| IL-6 (pg/mL), median (IQR) | 76 (23−271) | 99 (33−379) | 69 (20−244) | 0.003 |
| Ferritin (ng/mL), median (IQR) | 1292.5 (723.75−2044.25) | 1500 (901−2353) | 1195 (655−1922) | 0.000 |
| Outcomes | ||||
| Duration of non invasive MV (days), median (IQR) | 2(1−5) | 2 (1−3) | 2 (1−5) | 0.07 |
| Duration of MV (days), median (IQR) | 15 (8−28) | 17 (9−30) | 14 (8−26) | 0.000 |
| ICU LOS, median (IQR) | 15 (9−29) | 19 (11−38) | 13 (8−26) | 0.000 |
| ICU mortality (%), median (IQR) [n] | 1485 (27.7%) [5359] | 739 (39.5) | 746 (21.3) | 0.000 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Assessment Failure; MV, mechanical ventilation. ICU, Intensive Care Unit; LOS, length of stay; AKI, acute kidney injury; Pro-BNP, Pro Brain natriuretic peptide; IL, interleukin. [n] corresponds if the number of subjects of the variable does not match the total population.
The incidence of AKI was 34.8% (1879/5399). Of these patients, 15.3% (288/1879) required CRRT. AKI patients were significantly older, had a significantly higher prevalence of comorbidities, more inflammatory parameters and higher severity scores. In addition, AKI patients had significantly higher ICU mortality, longer ICU LOS and more MV days (Table 1).
Male gender (OR = 3.88), hypertension (OR = 1.52), diabetes (OR = 1.45), obesity (OR = 1.48), CHF (OR = 2.32), myocardial dysfunction (OR = 2.23), higher APACHE score (OR = 1.03), SOFA score (OR = 1.17) and PCT (OR = 1.17) were independently associated with the development of AKI (Fig. S2 Supplementary Appendix).
Characteristics and outcomes by stage of renal failureOf the 1879 patients with AKI, 1556 (82.8%) had AKI I, 196 (10.4%) had AKI II and the remaining 127 (6.8) had AKI III. According to AKI stage, a significant difference in comorbidities, inflammatory parameters, severity scores, need for CRRT, ICU LOS, hospital LOS and MV days was observed (Table S1 in the Supplementary Appendix).
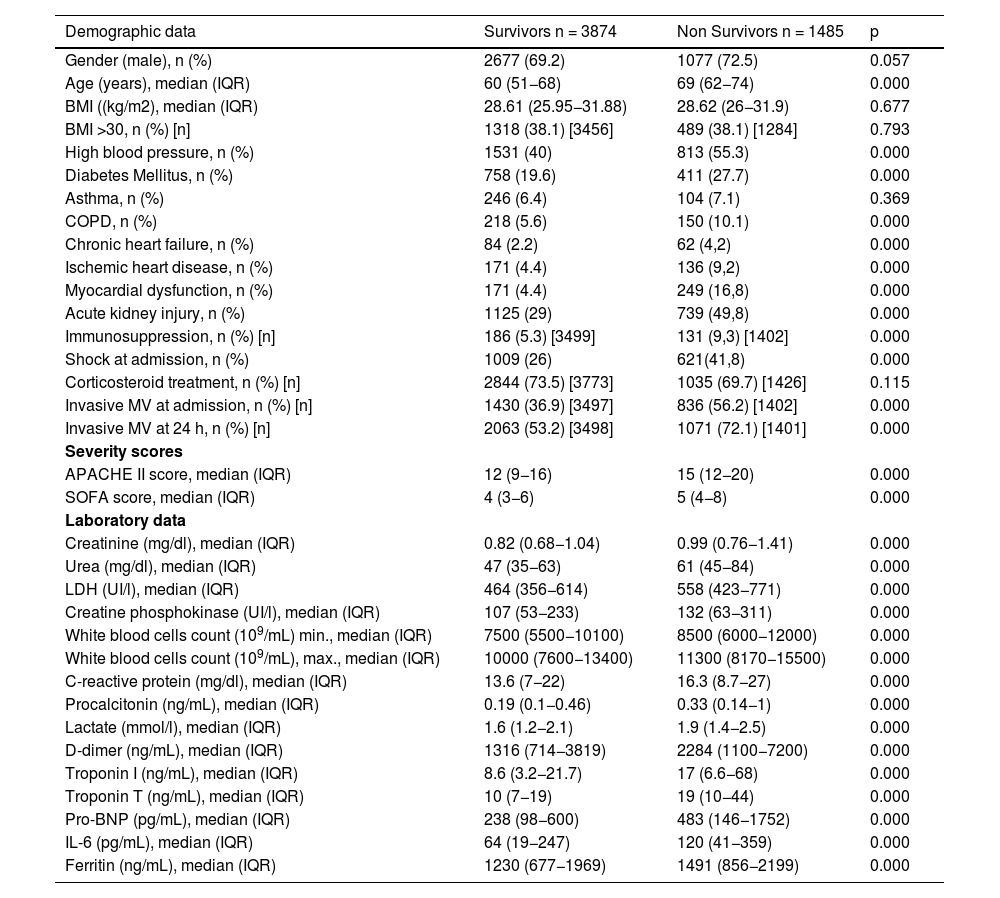
Overall ICU mortalityAt the time of data analysis, 40 patients were still hospitalised. Overall ICU mortality was 27.7% (1485/5359 patients) and was higher in patients with AKI (39.5% vs 21.3%, p < 0.001) (Table 1).
Non-survivors had a significantly higher prevalence of comorbidities, inflammatory parameters and severity scores (Table 2).
Risk factors associated with ICU mortality (for all AKI categories).
| Demographic data | Survivors n = 3874 | Non Survivors n = 1485 | p |
|---|---|---|---|
| Gender (male), n (%) | 2677 (69.2) | 1077 (72.5) | 0.057 |
| Age (years), median (IQR) | 60 (51−68) | 69 (62−74) | 0.000 |
| BMI ((kg/m2), median (IQR) | 28.61 (25.95−31.88) | 28.62 (26−31.9) | 0.677 |
| BMI >30, n (%) [n] | 1318 (38.1) [3456] | 489 (38.1) [1284] | 0.793 |
| High blood pressure, n (%) | 1531 (40) | 813 (55.3) | 0.000 |
| Diabetes Mellitus, n (%) | 758 (19.6) | 411 (27.7) | 0.000 |
| Asthma, n (%) | 246 (6.4) | 104 (7.1) | 0.369 |
| COPD, n (%) | 218 (5.6) | 150 (10.1) | 0.000 |
| Chronic heart failure, n (%) | 84 (2.2) | 62 (4,2) | 0.000 |
| Ischemic heart disease, n (%) | 171 (4.4) | 136 (9,2) | 0.000 |
| Myocardial dysfunction, n (%) | 171 (4.4) | 249 (16,8) | 0.000 |
| Acute kidney injury, n (%) | 1125 (29) | 739 (49,8) | 0.000 |
| Immunosuppression, n (%) [n] | 186 (5.3) [3499] | 131 (9,3) [1402] | 0.000 |
| Shock at admission, n (%) | 1009 (26) | 621(41,8) | 0.000 |
| Corticosteroid treatment, n (%) [n] | 2844 (73.5) [3773] | 1035 (69.7) [1426] | 0.115 |
| Invasive MV at admission, n (%) [n] | 1430 (36.9) [3497] | 836 (56.2) [1402] | 0.000 |
| Invasive MV at 24 h, n (%) [n] | 2063 (53.2) [3498] | 1071 (72.1) [1401] | 0.000 |
| Severity scores | |||
| APACHE II score, median (IQR) | 12 (9−16) | 15 (12−20) | 0.000 |
| SOFA score, median (IQR) | 4 (3−6) | 5 (4−8) | 0.000 |
| Laboratory data | |||
| Creatinine (mg/dl), median (IQR) | 0.82 (0.68−1.04) | 0.99 (0.76−1.41) | 0.000 |
| Urea (mg/dl), median (IQR) | 47 (35−63) | 61 (45−84) | 0.000 |
| LDH (UI/l), median (IQR) | 464 (356−614) | 558 (423−771) | 0.000 |
| Creatine phosphokinase (UI/l), median (IQR) | 107 (53−233) | 132 (63−311) | 0.000 |
| White blood cells count (109/mL) min., median (IQR) | 7500 (5500−10100) | 8500 (6000−12000) | 0.000 |
| White blood cells count (109/mL), max., median (IQR) | 10000 (7600−13400) | 11300 (8170−15500) | 0.000 |
| C-reactive protein (mg/dl), median (IQR) | 13.6 (7−22) | 16.3 (8.7−27) | 0.000 |
| Procalcitonin (ng/mL), median (IQR) | 0.19 (0.1−0.46) | 0.33 (0.14−1) | 0.000 |
| Lactate (mmol/l), median (IQR) | 1.6 (1.2−2.1) | 1.9 (1.4−2.5) | 0.000 |
| D-dimer (ng/mL), median (IQR) | 1316 (714−3819) | 2284 (1100−7200) | 0.000 |
| Troponin I (ng/mL), median (IQR) | 8.6 (3.2−21.7) | 17 (6.6−68) | 0.000 |
| Troponin T (ng/mL), median (IQR) | 10 (7−19) | 19 (10−44) | 0.000 |
| Pro-BNP (pg/mL), median (IQR) | 238 (98−600) | 483 (146−1752) | 0.000 |
| IL-6 (pg/mL), median (IQR) | 64 (19−247) | 120 (41−359) | 0.000 |
| Ferritin (ng/mL), median (IQR) | 1230 (677−1969) | 1491 (856−2199) | 0.000 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Assessment Failure; ICU, intensive care unit; LOS, length of stay; MV, mechanical ventilation; IL, interleukin. [n] corresponds if the number of subjects of the variable does not match the total population.
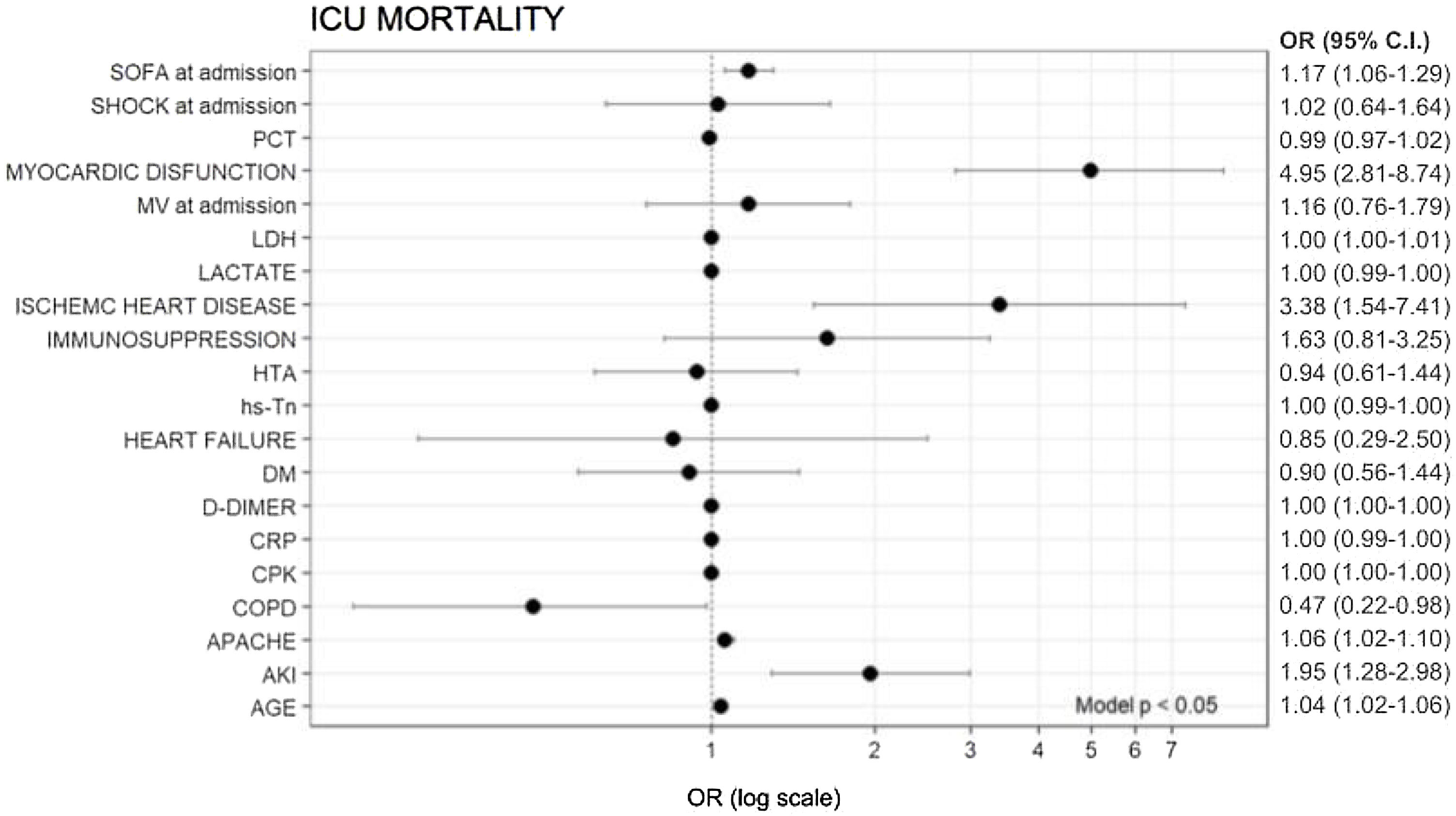
In multivariate analysis, age (OR = 1.04), myocardial dysfunction (OR = 4.95), ischaemic heart disease (IHD) (OR = 3.38), APACHE II (OR = 1.06), SOFA (OR = 1.17) and AKI (OR = 1.95) were independently associated with ICU mortality (Fig. 1).
Variables associated with overall ICU mortality (logistic regression).
SOFA, sequential organ failure assessment; APACHE, Acute Physiology and Chronic Health Evaluation; DM, diabetes mellitus; OR, odds ratio; PCT, procalcitonin; CRP, C reactive protein; HTA, hypertension; hs-Tn, troponin; COPD, chronic obstructive pulmonary disease; BMI, body mass index; AKI, acute kidney injury; LDH, lactate dehydrogenase.
AKI (HR = 1.32, 95% CI 1.17–1.48, Fig. 2) and AKI stages (Fig. S3 in the Supplementary Appendix) were independently associated with a higher risk of ICU mortality in Cox hazard regression analysis.
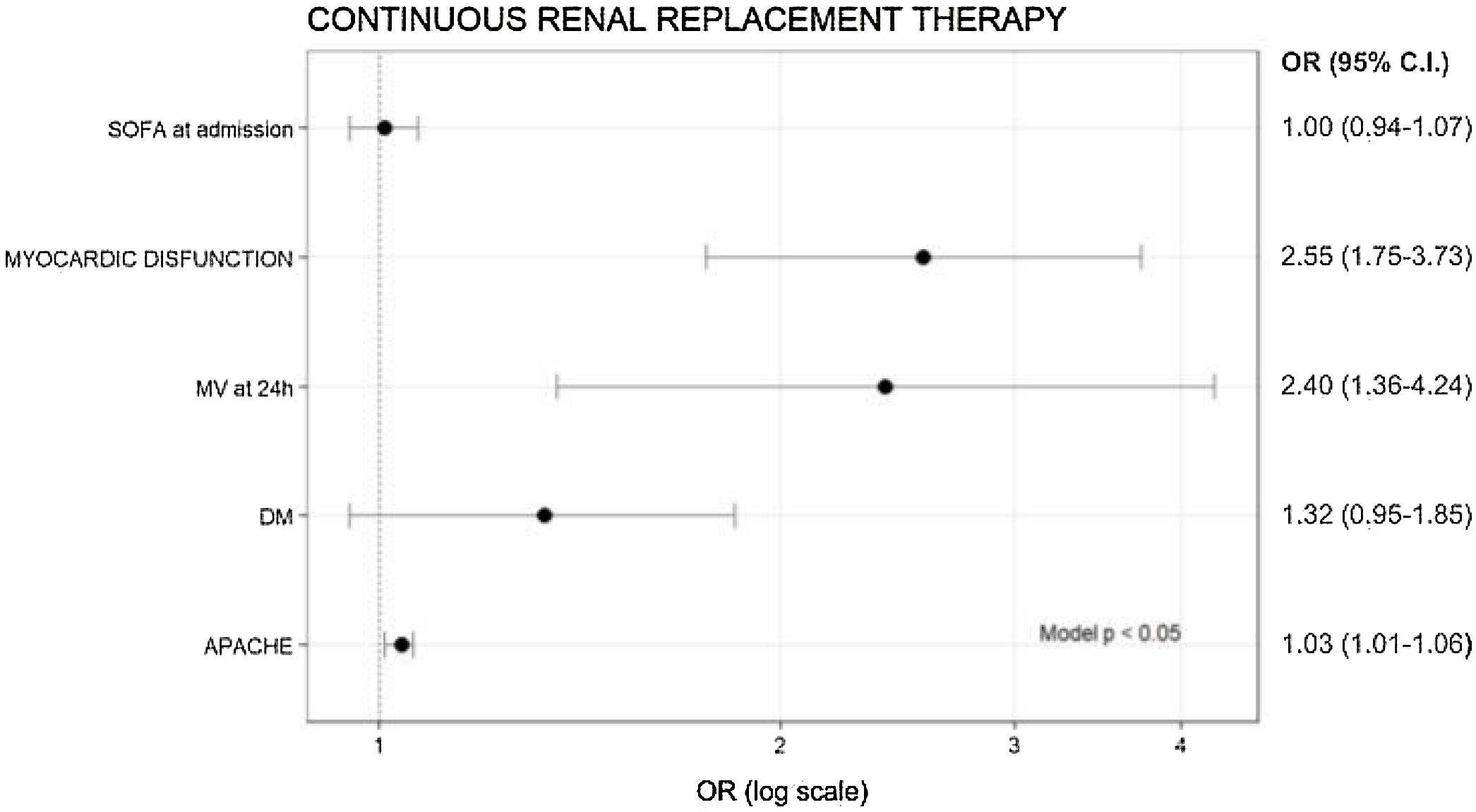
AKI I/II and risk factors for CRRTIn the subgroup of patients with AKI I and AKI II (n = 1752), the need for CRRT was recorded in 1710 patients. In these patients, the need for CRRT was 12.6% (217/1710). Diabetes, myocardial dysfunction, APACHE II, SOFA, C reactive protein (CRP), procalcitonin (PCT), troponin T, need for MV in the first 24 h, days of MV, ICU and hospital LOS were higher in patients with CRRT. In addition, ICU mortality in patients requiring CRRT was almost double (64%) compared to those not receiving CRRT (34.8%, p < 0.001). Table S2 in the Supplementary Appendix.
Only APACHE II (OR = 1.03), need for MV in the first 24 h (OR = 2.4) and myocardial dysfunction (OR = 2.55) were variables associated with the risk of needing CRRT (Fig. 3).
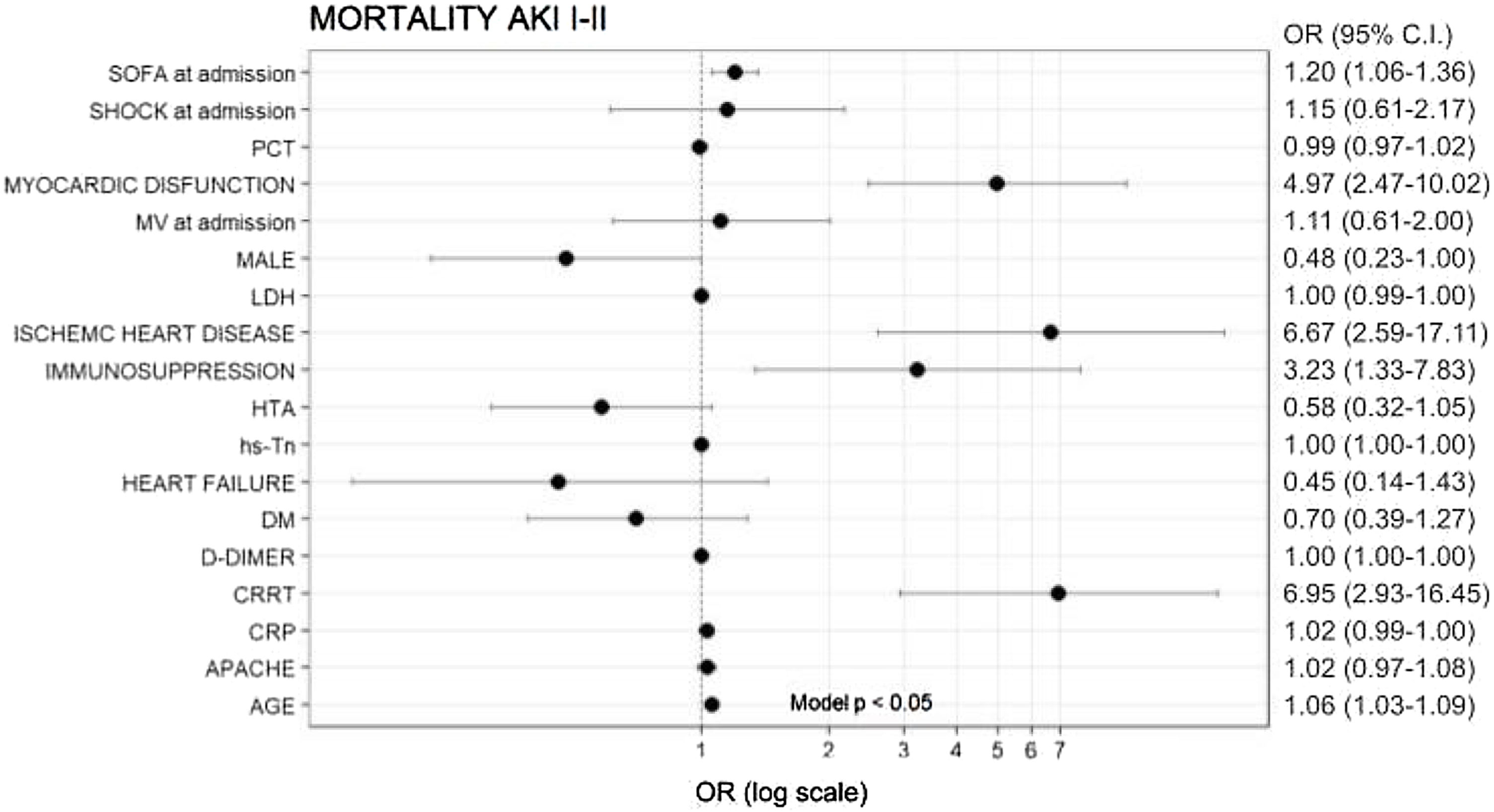
AKI I/II and ICU mortalityMortality in this subgroup of patients was 38.5% (670/1737 patients). Non-survivors were significantly older and had a higher prevalence of comorbidities, inflammatory parameters and severity scores (Table S3 in the Supplementary Appendix).
In multivariate analysis, age (OR = 1.06), SOFA (OR = 1.2), myocardial dysfunction (OR = 4.97), IHD (OR = 6.67), immunosuppression (OR = 3.23) and need for CRRT (OR = 6.95) were independently associated with ICU mortality (Fig. 4).
DiscussionIn this study the incidence of AKI was high. Different cardiovascular risk factors and more severe patients were more at risk for developing AKI. Even patients with AKI I and II had a higher risk of needing CRRT as well as a worse prognosis.
In our study, the incidence of AKI was 34.8%. Several studies have shown similar results. In a multicentre study with more than 9000 patients admitted to hospitals in New York, Ng JH et al.13 showed an AKI incidence of 39.9%. Gupta et al.14 observed an incidence rate of 42.8% (limited to stage II and III) in 2215 patients with COVID-19 admitted to ICU. Finally, a multicentre study in critically ill patients in Spain described an AKI incidence of 40%.15
In terms of AKI stage, the incidence was 82.8% for AKI I, 10.4% for AKI II and 6.7% for AKI III. This incidence varies between studies.13,15–17 This could be due to differences in how AKI was diagnosed, mainly whether baseline creatinine was obtained and whether oliguria was assessed for AKI diagnosis. Some trials used ambulatory data.15,16 Others used ambulatory data and, if unavailable, they used creatinine at hospital admission and Modification of Diet in Renal Disease (MDRD).17 Ng JH et al.13 used a pre-built internal algorithm. Finally, only the Belgian group used diuresis for diagnosis8.
Here we describe that hypertension, diabetes and obesity are risk factors for the development of AKI, as observed in previous studies.8,13,15 Cardiovascular disease is another risk factor often reported in the literature.13,16–18 This is consistent with our results, as our patients had a high incidence of CHF and myocardial dysfunction.
In our study, AKI was independently associated with ICU mortality (OR 1.64, CI 1.19–2.25). Similar results were found in a meta-analysis of 5166 patients.19 These results are consistent with those of other studies in critically ill COVID-19 patients.20
AKI stage was also significantly associated with ICU mortality. Some studies have shown a higher mortality with increasing AKI severity.8,21 On the other hand, other authors did not show significant differences.22,23 In contrast to these studies, we observed a higher mortality in AKI II patients than in AKI III patients (55.6% vs 54.3%, p < 0.001). If our data would had been collected after the first 48 h of admission to the ICU, some patients diagnosed with AKI II would probably have progressed to the AKI III group; thus, the mortality of this group would possibly have been higher. Nevertheless, our objective was to assess the impact of early AKI on the patients and so we made the cut-off at 48 h after admission to the ICU. Furthermore, the difference in mortality observed between AKI II and II, although statistically significant, is not clinically relevant.
In AKI I-II patients, the presence of myocardial dysfunction, the need for MV in the first 24 h after ICU admission and higher APACHE scores were independently associated with the need for CRRT during ICU stay. In addition, SOFA scores, myocardial dysfunction, IHD, immunosuppression, age and need for CRRT were independently associated with ICU mortality.
In this study, we evaluated the need for CRRT in AKI I and II patients and the impact that CRRT had on the mortality of these patients. To our knowledge, this relationship has not been evaluated in previous studies, especially in this subgroup of patients. In these patients, the need for CRRT was 12.6%. Our incidence differs from previous reports. According to Schaubroeck et al.,8 in a study of more than 1000 patients, 5.7 % of them required CRRT. According to Estella et al.,24 11.8% of AKI patients received renal replacement therapy (RRT). In the study by Gupta et al.11 the need for RRT was 20.1%. Cummings et al.10 analysed 257 critically ill patients with COVID-19 and reported 31% on RRT. Salgueira et al.16 reported 21% of AKI patients on CRRT. However, these studies included all AKI stages,8 all types of RTT (CRRT and intermittent haemodialysis)11,14,24 and patients with chronic kidney disease,8,10,14 which may explain the difference with our results.
Myocardial dysfunction and the need for MV in the first 24 h were independently associated with the need for CRRT in our study. Since myocardial dysfunction is an independent risk factor for AKI, it is logical to assume that it is also an independent risk factor for CRRT. Regarding MV, Salgueira et al.16 also described VM as a risk factor (OR 20 [6.5–62.7]) for need for CRRT. In the study by Gupta et al.,11 patients with VM and a PaO2:FiO2 ratio <200 mmHg had a higher risk of requiring CRRT. Fominskiy et al.25 studied patients with COVID-19 on MV and showed that 17.7% of patients required dialysis. Several studies suggest that MV may induce or worsen AKI26,27 and several mechanisms have been proposed to explain this association.28,29 Therefore, CRRT seems to be common in critically ill patients with COVID-19, especially in those with MV.
Our AKI I and II patients on CRRT had a high mortality compared to those who did not require CRRT (64.1% vs. 34.8%, respectively), which is consistent with what has been observed in other studies.14–16,21,30 In addition, CRRT was independently associated with ICU mortality in this subgroup of patients. Similar results have been reported in previous studies.16,30 Although we did not record the time of initiation of CRRT, most studies collecting this data report that it was during the first week of ICU admission.8,14,16 This suggests that even early stages of AKI in the first days of ICU admission would have a relatively high risk of requiring CRRT, as well as a higher risk of ICU mortality. Although these data must be interpreted with caution, we recommend close monitoring to prevent progression of AKI in these patients, as this leads to a worse outcome.
This study has several limitations. First, it is observational and retrospective in design. Second, the diagnosis of AKI was mainly based on creatinine variation, without taking into account variations in urine output. Third, data on AKI were only collected for the first 2 days after ICU admission. Therefore, we may have underestimated the true incidence of AKI in critically ill patients with COVID-19. Fourthly, there was no uniform protocol for the management of COVID-19 between hospitals, neither for the prevention and management of AKI nor for the use of CRRT. Fifth, in our database we do not know when CRRT was started, so we cannot assume that CRRT was necessary for the first AKI due to COVID-19. Sixth, the results of our study cannot be extrapolated to other non-COVID populations. Finally, the study describes what happened to our cohort of patients from the beginning of the pandemic until April 2022 (including wave 6). During this time, some aspects such as corticosteroid treatment and patient profile changed,31 which may have influenced the results obtained.
In conclusion, AKI is a common feature in critically ill patients with SARS-CoV-2 pneumonia and it is associated with an increased ICU mortality. Even AKI stages I and II are associated with a high risk of needing CRRT and ICU mortality.
Author contributionsIban Oliva and Cristina Ferré worked in the writing of the manuscript.
Xavier Daniel, Marc Cartanyà, Christian Villavicencio, Melina Salgado, Loreto Vidaur, Elisabeth Papiol, Francisco Javier González, María Bodí, Manuel Herrera and Alejandro Rodríguez worked on data collection and analysis.
FundingThere was no funding for this project.
Conflict of interestThe authors declare there is no conflict of interest
The authors would like to thank all the members of the COVID-19 Spanish Society of Intensive Care Medicine and Coronary Units (SEMICYUC) for their efforts in collecting patient data during the challenging situation of the pandemic.