A series of 7 cases of infection, likely related to contamination of propofol used in GI endoscopy procedures, is described.
The first case involved a 46-year-old man who was admitted to the ICU due to septic shock of unknown etiology after undergoing an upper GI endoscopy at a private center earlier that morning. He reported fever, chills, and vomiting that began just a few minutes after the procedure. He presented with fever, PCT > 100 ng/mL, and hemodynamic, renal, and hepatic dysfunction. The abdominal CT ruled out GI complications, and empirical antibiotic therapy with meropenem and daptomycin was initiated. The remaining 6 patients who had undergone endoscopic studies on that same day at that same center presented to our hospital within the next 24 h. All were outpatients without relevant comorbidities and showed the same clinical signs, with varying degrees of severity (Table 1).
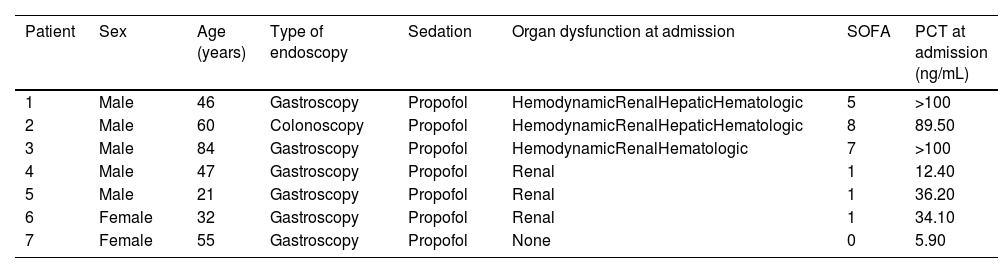
Characteristics of the patients from the series.
| Patient | Sex | Age (years) | Type of endoscopy | Sedation | Organ dysfunction at admission | SOFA | PCT at admission (ng/mL) |
|---|---|---|---|---|---|---|---|
| 1 | Male | 46 | Gastroscopy | Propofol | HemodynamicRenalHepaticHematologic | 5 | >100 |
| 2 | Male | 60 | Colonoscopy | Propofol | HemodynamicRenalHepaticHematologic | 8 | 89.50 |
| 3 | Male | 84 | Gastroscopy | Propofol | HemodynamicRenalHematologic | 7 | >100 |
| 4 | Male | 47 | Gastroscopy | Propofol | Renal | 1 | 12.40 |
| 5 | Male | 21 | Gastroscopy | Propofol | Renal | 1 | 36.20 |
| 6 | Female | 32 | Gastroscopy | Propofol | Renal | 1 | 34.10 |
| 7 | Female | 55 | Gastroscopy | Propofol | None | 0 | 5.90 |
SOFA: Sepsis-related Organ Failure Assessment.
Upon reviewing the cases, we found that propofol had been the common element among them. Samples from the remaining vial contents were sent, and Pantoea agglomerans was isolated. The suspicion was reported to the Spanish pharmacovigilance system.
Several propofol-related epidemic outbreaks have been described, as it is susceptible to microbial growth due to its lipid-based composition. In 2016, a systematic review was published analyzing 20 outbreaks since its approval for use.1 Most occurred in operating rooms and ICUs, followed by endoscopic procedures. Extrinsic contamination (after opening the vial) was the most common. The main contaminants were Gram-positive bacteria (27%), Gram-negative bacteria (20%), Candida albicans (21%), hepatitis C virus (18%), and B (4%). Mortality rate was 9.3% (range, 0% up to 50%).
Pantoea agglomerans, formerly known as Enterobacter agglomerans, is a facultative aerobic Gram-negative bacterium widely distributed in nature. In humans, it causes soft tissue and bone infections following injuries from plant material2,3 and health care-related infections, particularly in immunocompromised patients, most of which are mild.3
The largest epidemic outbreak caused by P. agglomerans and other enterobacteria occurred in the United States in 1970, where 378 cases of septicemia were recorded due to contamination of IV fluid stoppers.4 Another group reported 19 cases of bacteremia from parenteral nutrition contamination.5 In another instance, 12 cases of bacteremia caused by P. agglomerans were diagnosed in cancer patients, originating from exposure of IV drug to a contaminated pharmacy sink.6 Another outbreak of 17 cases was associated with the infection of tunneled hemodialysis catheters.7 Eight cases of bacteremia were also reported from contamination of the anticoagulant solution used in plasmapheresis and hemodialysis systems,8 and an outbreak of 3 cases was due to contamination of water system drains in a hemodialysis center.9
In our series, blood cultures from all patients tested negative. However, causality is presumed; the administration of propofol was the common factor, the immediate temporal relationship is clear, and there is also biological plausibility. Furthermore, no other source of infection was detected, and symptoms were highly suggestive of bacteremia, which was likely transient due to the low bacterial inoculum and disappearance of the source of infection once the infusion was stopped. In 2021, another series of 6 cases of sepsis following propofol sedation in urological procedures was published, where P. agglomerans was also isolated in the used vial, and although causality appeared highly probable, there was no growth in blood cultures either.10
The 7 patients from our series had a favorable progression, with short ICU and hospital stays, achieved complete recovery, and were discharged on ciprofloxacin treatment.
Our experience highlights the importance of strict safety measures against the possibility of contamination of health care products and emphasizes the need to maintain a high index of suspicion for infections caused by less common microorganisms, which can still lead to severe septic conditions.
FundingNone declared.