Pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA) strains producing a cytotoxin known as Panton–Valentin leukocidin (PVL) is a severe and difficult to treat disease.1–4 Linezolid has excellent epithelial lining fluid (ELF) penetration rates but drug concentrations in critically ill patients are highly variable.5-8 We describe the clinical case of a patient with septic shock secondary to a community-acquired PVL-secreting MRSA pneumonia, in which linezolid plasma concentration within the therapeutic range were only reached with the use of higher than recommended doses (600mg every 8h) administered in continuous infusion.
A 56-year-old Caucasian man was admitted on February 2, 2015 with a 4-day history of dyspnea, mucopurulent expectoration, and fever (>38°C). His weight was 80kg (body mass index [BMI] 27.7kg/m2), smoked 2–3 cigarettes a day, and consumed approximately 7 standard drinks daily. Physical examination revealed sinus tachycardia (160beats/min), diaphoresis, hypertension (blood pressure [BP] 154/92mmHg), and marked respiratory distress as well as bilateral crackles and disperse rhonchi up to the apex on lung auscultation. This caused a hypoxemic respiratory insufficiency with an initial PaO2 of 55mmHg that did not improve despite an elevated inspired oxygen fraction (FiO2 0.5) and noninvasive mechanical ventilation, for which he needed orotracheal intubation and ICU admission. Blood urea nitrogen was 32mg/dL, serum creatinine 0.88mg/dL, and glomerular filtration rate (Cockcroft–Gault equation) 106mL/min, C-reactive protein 25.6mg/L, procalcitonin 24.17ng/mL, and lactic acid 5mmol/L. Other laboratory data were unrevealing. Chest radiography revealed bilateral alveolar and interstitial infiltrates and alveolar condensation in the right upper lobe. The patient was tentatively diagnosed with CAP and was treated empirically with intravenous ceftriaxone (2g every 24h), intravenous levofloxacin (0.5g every 12h) (positive urinary detection of pneumococcal antigen), and oseltamivir (positive PCR assay detection of influenza A and B viruses in oropharyngeal swabs). During the first 24h of ICU admission, the patient's condition deteriorated, with secondary septic shock and need of vasoactive drugs (norepinephrine 0.2 ¿g/kg/min). Microbiologically, a MRSA was isolated in cultures from bronchial aspirates and in four blood culture bottles (linezolid minimum inhibitory concentration [MIC]=2mg/L) on the day of admission. Each MRSA isolate carried the PVL genes. The MRSA strain was sent to the National Microbiology Center (Institute of Health Carlos III, Madrid). The initial empirical antibiotic treatment was discontinued and linezolid at a standard dose (600mg every 12h infused over 60min) was administered.
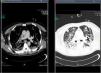
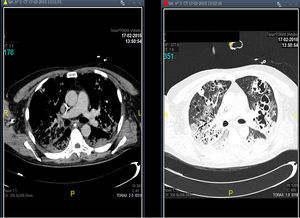
Treatment with linezolid was maintained and the remaining antimicrobials were discontinued. Steady state plasma concentrations of linezolid were determined from the third day of treatment using a high-performance liquid chromatography technique, with a minimum plasma concentration state (Cminss) (or pre-dose) of 0.8mg/L and maximum (Cmaxss) (at the end of infusion) of 4.8mg/L, which were considered subtherapeutic (therapeutic range Cminss>2mg/L). Although blood cultures became negative on the fifth day of treatment, the patient showed a protracted respiratory clinical course with worsening of radiographic images, and according to drug susceptibility testing, antimicrobial coverage was expanded with intravenous clindamycin (600mg every 8h) and ciprofloxacin (400mg every 8h). In addition, the dosing interval of linezolid as well as the duration of the time of infusion were modified on several occasions based on results of plasma drug concentrations in order to achieve an optimal pharmacokinetic (PK) and pharmacodynamic (PD) ratio (Table 1). Seventeen days after starting treatment with linezolid, therapeutic levels were reached with a regimen of 600mg every 8h administered in continuous infusion, which coincided with eradication of PVL producing MRSA in pulmonary samples. Thoracic computed tomographic (CT) scans performed over the course of treatment revealing extensive involvement of the pulmonary parenchyma with multiple cavities and important necrotic areas in the upper lobes compatible with pneumatoceles and bronchiectasis (Fig. 1). The patient required mechanical ventilation during 73 days (with tracheostomy) with procedural sedation, analgesia, and prolonged muscle relaxation. After 5 weeks of treatment with linezolid, the patient was successfully weaned. Linezolid was administered for a total of 35 days, clindamycin for 29 days and ciprofloxacin for 19 days. He was discharged after a 3-month hospitalization. At the present time, his oxygen saturation on room air is >95%.
Changes in doses, intervals between doses, forms of administration, and plasma concentrations of linezolid during the patient's stay in the ICU.
| Day of sampling | Dose (mg) | Dosing interval doses | Type and duration of infusion | GFR (mL/min) | Cminss (mg/L) | Platelet count (×109/L) |
|---|---|---|---|---|---|---|
| February 13, 2015 | 600 | 12h | Intermittent (60min) | 176 | 0.5 | 112 |
| February 17, 2015 | 600 | 12h | Intermittent (60min) | 239 | <0.5 | 85 |
| February 23, 2015 | 600 | 8h | Extended (2h) | 345 | <0.5 | 456 |
| February 24, 2015 | 600 | 6h | Extended (2h) | 267 | 1.4 | 531 |
| February 26, 2015 | 600 | 6h | Extended (2h) | 301 | 0.6 | 540 |
| Day of sampling | Dose (mg) | Dosing interval doses | Type and duration of infusion | GFR (mL/min) | Cminss (mg/L) | Platelet count (×109/L) |
|---|---|---|---|---|---|---|
| March 2, 2015 | 600 | 6h | Continuous (6h) | 301 | 8.5 | 544 |
| March 6, 2015 | 600 | 8h | Continuous (8h) | 275 | 2.7 | 527 |
| March 9, 2015 | 600 | 8h | Continuous (8h) | 333 | 4.6 | 467 |
GFR: glomerular filtration rate.
Mortality related to PVL-secreting MRSA pneumonia is around 70% despite the use of appropriate antimicrobials according to results of susceptibility testing.4 A combination of antimicrobials active against MRSA and with adequate penetration in the pulmonary parenchyma should be selected. Linezolid is a synthetic antibiotic belonging to the oxazolidinone family especially active against Gram-positive bacteria, including multiresistant pathogens, with 100% diffusion in the ELF.5
Although in the technical specifications of linezolid, a standard dose of 600mg every 12h administered in 60-min intermittent infusion is recommended, recent pharmacokinetic studies have shown that the administration of this fixed dose in some population groups did not allow to reach an optimal predictive pharmacokinetic/pharmacodynamic (pk/pd) ratio of clinical efficacy of the drug (Cminss≥2–4mg/L), whereas it has also been related to overexposure (Cminss≥10mg/L) and higher risk of hematologic toxicity.9 These circumstances are frequent in ICU patients with severe sepsis or septic shock, in whom most factors associated with underexposure or overexposure to linezolid may be present.9,10 Subtherapeutic linezolid concentrations have been related to glomerular filtration rates greater than 80mL/min,7 which is frequent in septic patients treated with vasoactive drugs as was in our patient.
Strategies for optimizing linezolid plasma levels include an increase in the total dose, by-reducing the dosing interval from 12h to every 8 or 6h, or extending the duration of infusion to 2–4h (extended infusion) or using a continuous infusion.9,10 Our patient in whom glomerular filtration rates >200mL/min were recorded, a Cminss≥4mg/L were only obtained with the administration of a linezolid dose of 600mg every 6–8h in continuous infusion, which coincided with negativization of pulmonary exudate cultures. This high dose, between 1.5 and 2-fold higher than the standard recommended dose was well tolerated, without signs of toxicity, particularly without a decrease in platelet count or hemoglobin levels.
In ICU patients with severe infections and increased renal clearance, linezolid should be administered at high doses and in continuous infusion with close monitorization of plasma drug levels.
Source of fundingNone declared.
Conflicts of interestNone declared.