Total or partial cooling of human beings has been used sporadically in a variety of diseases of the central nervous system for centuries. Intuitively it is believed that hypothermia may preserve neuronal viability and increase the chances of survival and functional recovery.
However, it was not until the last decade that its use has been generalized in a particular situation–recovered cardiac arrests (RCA) after the publication, in the year 2002, of Holzer's study (HACA group) and Bernard et al.’s study1,2 that found out that by inducing mild hypothermia (32–34°C) mortality was lower and the chances of neurological recovery after an out-of-hospital RCA of presumed cardiac origin due to defibrillation rhythms were higher. The recommendation to use hypothermia came fast and it reached out to two groups that had not been evaluated in the clinical trials: non-defibrillation rhythms, and hospital cardiac arrests. As it has happened in other occasions, the desire to find effective therapies in a situation that leads to such higher mortality rates as RCAs is accompanied by an enthusiastic adherence and a not very critical evaluation of the apparently favourable results, so it is not surprising that such studies are usually referred to as “large sample” and “high quality” studies, when they are really small studies with important methodological limitations (Table 1).
Risk of bias in Holzer (HACA), Bernard, and Nielsen's studies on therapeutic hypothermia after recovered cardiac arrests.
| Risk of bias | |||
|---|---|---|---|
| Holzer | Bernard | Nielsen | |
| 1. Randomization sequence | B | A | B |
| 2. Blinded randomization | B | A | B |
| 3. Blinded participants | A | A | A |
| 4. Blinded assessors | B | B | B |
| 5. Balanced groups | A | A | B |
| 6. Estimation of sample size | A | B | B |
| 7. Intermediate analyses | B | A | B |
| 8. Intention-to-treat analysis | B | D | B |
| 9. Losses to follow-up | B | B | B |
| 10. Selective assessment | B | B | B |
| 11. Trial prospective registry | A | A | B |
| 12. Publication of the protocol | A | A | B |
| 13. Funding | B | D | B |
| 14. Protocol of predefined limitation of therapeutic effort | A | A | B |
| 15. Unplanned completion | A | A | B |
| Final grade | A | A | B |
| Risk of bias score (0–30) | 16 | 22 | 2 |
| Other aspects | |||
|---|---|---|---|
| Number of patients included | 275 | 77 | 939 |
| Relative weight per sample size | Low | Low | High |
| Optimal management of the control group | No | No | Yes |
| Contemporaneity of the study | Medium | Medium | High |
After the support given by the scientific societies to therapeutical hypothermia (although with a less-than-expected adherence in the clinical practice) and the favourable sanction by the Cochrane collaboration in a questionable practice where it is the researchers of the clinical trials who make the assessments through systematic reviews and meta-analyses, some independent voices have talked about the low quality of evidence on which the recommendation of hypothermia is based and highlighted the need for conducting a more rigorous clinical trial. Nielsen et al.’s clinical trial,3 published in 2013, is three times as big, in size, as all the other former trials and, in a transparent way, prospectively shows the study protocol, while applying a strict treatment to the control group, and clearly specifying what criteria should be followed in order to limit the therapeutic effort – something that was not done in the other trials yet despite the fact that it was a common practice after suffering a RCA, since it may clearly bias the results while favouring the group sought after by the researchers. Its results show that mild hypothermia (32–34°C) does not improve mortality or neurological recovery compared to the control of temperature in the range of monothermia (36°C), or perhaps it should be referred to as “minimal hypothermia”. Table 2 shows the main characteristics of the three main clinical trials on hypothermia in the management of RCA1–3 focusing on its methodological quality, and showing why we should give more credit to Nielsen's study than to the other two studies that support the recommendations coming from the scientific societies.
The largest and most recent clinical trials on induced hypothermia in neurocritical patients.
| Trial | Number of patients | Result | |
|---|---|---|---|
| Cardiac arrest | Nielsen et al., 20133 | 939 patients with RCA of cardiac origin | Negative |
| Cranioencephalic traumatism | NABIS-II, 20116 | 108 patients with nonpenetrating serious CET | Prematurely interrupted due to futility |
| Cranioencephalic traumatism | Andrews et al., 20157 | 387 patients with serious CET and intracranial hypertension | Prematurely interrupted due to worse results in the hypothermia group |
| Meningitis | Mourvillier et al., 20138 | 98 comatose patients with severe bacterial meningitis | Prematurely interrupted due to higher mortality in the hypothermia group |
| Ischaemic stroke | Llanos Méndez and Prieto Uceda, 20149 | Systematic review and meta-analysis | More adverse events with hypothermia; tendency to higher mortality with hypothermia |
RCA: recovered cardiac arrest; CET: cranioencephalic traumatism.
Yet despite the publication of Nielsen's clinical trial and observational studies with results clearly negative on hypothermia in the management of intrahospital cardiac arrests,4 the scientific societies have underestimated the evidence against the effectiveness of hypothermia when the evidence in its favour is quantitative and qualitatively inferior. After taking the most recent studies into consideration, and in a decision difficult to understand, ILCOR has “tempered” its recommendations leaving the clinicians the option to choose and maintain, at least during 24h, a target temperature between the wide range of 32 and 36°C,5 but without initiating the cooling process before hospital arrival, since this therapy that would be beneficial a few minutes later may be detrimental if administered earlier.10
The theoretical mechanisms through which hypothermia may have neuroprotective effects in the clinical practice are highly varied. This means that it may be effective in different diseases of the central nervous system, and this is why hypothermia has been tested in a variety of severe neurological conditions among which the severe cranioencephalic traumatism,6,7 the acute bacterial meningitis,8 and the acute ischaemic stroke9 are the main conditions due to their frequency and impact on healthcare. In all of them without exception, the hypothermia has given negative or detrimental results in recent significant clinical trials (Table 2). The absence of effectiveness of hypothermia in such different clinical circumstances seriously questions the biological plausibility of this therapy in neurocritical patients, and the excessive amount of adverse events and increased mortality in some patients7–9 makes us think about implementing a moratorium in its use.
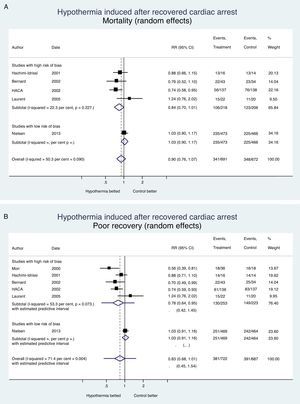
It is always bad news when a therapy is ineffective or detrimental; at least, the lessons learned from the clinical trials should avoid making more mistakes, spending more money, or causing iatrogenia. Fig. 1 shows the evidence from clinical trials conducted to assess the effects of induced hypothermia on mortality and neurological recovery in the management of recovered cardiac arrests; this effect was assessed by authors using one random-effects meta-analysis with the STATA v 13.1 statistics package. We may conclude that hypothermia has not proven its effectiveness in the management of cardiac arrests, or other diseases of the central nervous system (Table 2); today there is enough information to withdraw the recommendation to use hypothermia and if the scientific societies involved think that there is enough uncertainty (“equipoise”), they may promote the use of a new clinical trial to provide us with definitive information on what temperature should we used in the management of patients who suffer RCA. Meanwhile, the existing evidence claims that hypothermia should be avoided, and that the active control of temperature should occur in the range of normotherapy, or minimal hypothermia (36°C)–not lower.
Hypothermia induced in recovered cardiac arrest. Mortality and neurological recovery (random-effects meta-analysis modela). (A) Effect of hypothermia on mortality. (B) Effect of hypothermia on neurological recovery. The confidence intervals, and the estimated prediction intervals are includeda. The random-effects meta-analysis model penalizes studies of large samples and low risk of bias such as Nielsen's compared to studies of small samples and high risk of bias.
The authors declare that while conducting this paper there were no conflicts of interests linked whatsoever.
Please cite this article as: Palencia Herrejón E, Díaz Díaz D. Hipotermia terapéutica: tiempo para una moratoria. Med Intensiva. 2017;41:425–428.