Critically ill patients with COVID-19 require high sedative drugs doses under invasive mechanical ventilation (MV).1 Moreover, most of them need long MV periods because of prolonged course of respiratory failure.2 The Food and Drug Administration recently issued a midazolam shortage and reported a more than 50% consumption increase for other common sedative drugs such as propofol due to high demand for patients with COVID-19.3
Thiopental is commonly used in refractory status epilepticus as burst suppression therapy or in traumatic brain injury to limit increase in cranial pressure.4,5 For these patients, barbiturate therapy induce deep coma with bispectral index score lower than 20 and, as a result, prolonged MV.4 To our knowledge, no study has reported use of thiopental in critically ill patients besides acute neurological conditions.
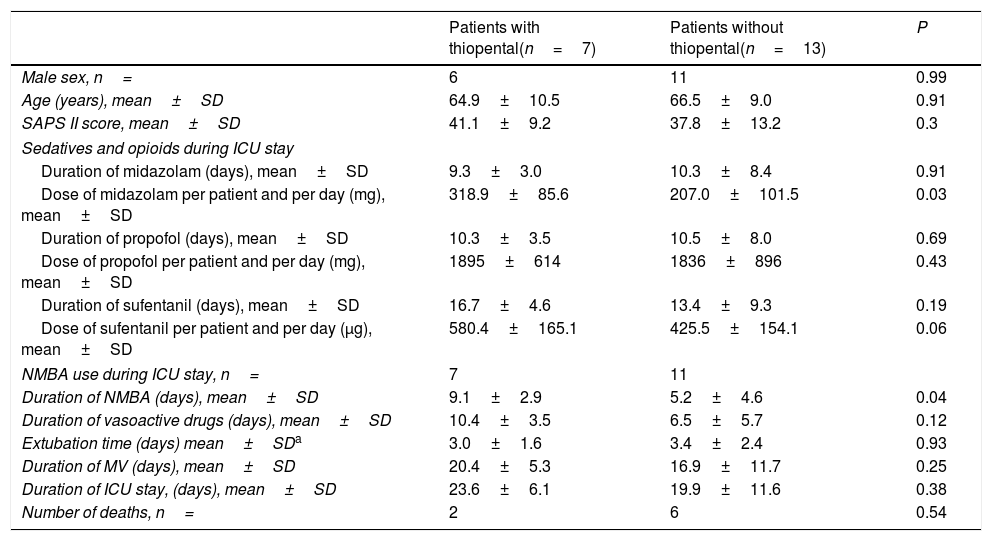
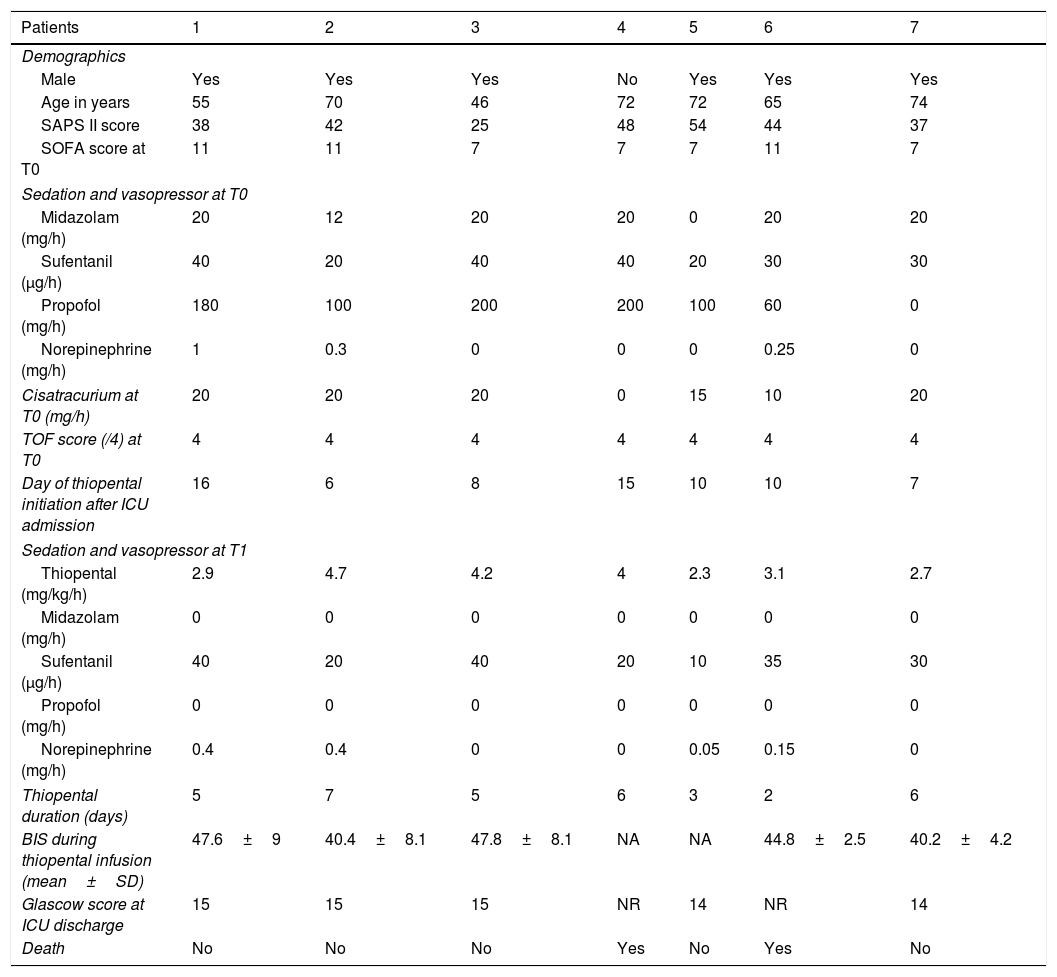
We retrospectively analyzed our experience with thiopental in our intensive care unit (ICU) patients with COVID-19 pneumonia during a shortage of midazolam and propofol. Twenty-two patients with COVID-19 pneumonia required MV between March 15 and April 15, 2020. During their ICU stay, 2 patients were transferred to an extra corporeal membrane oxygenation referral center for worsening respiratory failure. Of the remaining 20 patients, 7 have received thiopental as a result of major agitation and/or patient-ventilator asynchrony recurrence despite delivery of usual recommended doses of sedatives and analgesics. For all studied patients, baseline data on ICU admission and sedatives treatment during ICU stay are reported in Table 1. Data were analyzed with the chi-squared test or Fischer exact test for categorical parameters and Mann–Whitney test for continuous variables. Most patients (n=20) have received a combination of midazolam, propofol and sufentanil. In the thiopental group, patients have received more midazolam, opioids and have required a longer duration of neuromuscular blockade agents (NMBA). Time to extubation after sedation cessation, duration of MV and outcome were not different between the two groups. Sedation, analgesia and barbiturates management for each patient of the thiopental group are reported in Table 2. Thiopental was delivered 10.3±3.9 days after ICU admission. A bolus infusion of thiopental at a dose of 3mg/kg was administered before starting continuous infusion. Bispectral index (BIS) monitoring was used to determine adequate thiopental dosage. A BIS level between 40 and 60 and the discontinuation of patient-ventilator asynchrony were the goal to be achieved. Highest mean dose of thiopental was 3.4±0.8mg/kg/h. There was no more patient-ventilator asynchrony. Thiopental was progressively reduced by half every 12h according to clinical status and was delivered overall during 4.8±1.8 days. Just before thiopental initiation, mean doses of midazolam and propofol were respectively 16±7.6 and 120±76.6mg/h. Midazolam and propofol were completely stopped one hour after the thiopental loading dose in all patients. Only one patient needed initiation of small doses of norepinephrine. Cisatracurium could be stopped 1.3±1.2 days following thiopental initiation. Mean level of bispectral index monitoring during thiopental infusion was between 40 and 50. Dexmedetomedine, commonly used in our unit during sedative drugs withdrawal, was administered to 6 patients. A Richmond Agitation Sedation Scale (RASS) score at 0 was obtained 3.8±1.1 days following thiopental cessation. Five of seven patients survived the ICU stay. All surviving patients were discharged from the ICU with a Glasgow score ≥14.5
Baseline data, treatment characteristics and clinical outcome in the two studied groups.
| Patients with thiopental(n=7) | Patients without thiopental(n=13) | P | |
|---|---|---|---|
| Male sex, n= | 6 | 11 | 0.99 |
| Age (years), mean±SD | 64.9±10.5 | 66.5±9.0 | 0.91 |
| SAPS II score, mean±SD | 41.1±9.2 | 37.8±13.2 | 0.3 |
| Sedatives and opioids during ICU stay | |||
| Duration of midazolam (days), mean±SD | 9.3±3.0 | 10.3±8.4 | 0.91 |
| Dose of midazolam per patient and per day (mg), mean±SD | 318.9±85.6 | 207.0±101.5 | 0.03 |
| Duration of propofol (days), mean±SD | 10.3±3.5 | 10.5±8.0 | 0.69 |
| Dose of propofol per patient and per day (mg), mean±SD | 1895±614 | 1836±896 | 0.43 |
| Duration of sufentanil (days), mean±SD | 16.7±4.6 | 13.4±9.3 | 0.19 |
| Dose of sufentanil per patient and per day (μg), mean±SD | 580.4±165.1 | 425.5±154.1 | 0.06 |
| NMBA use during ICU stay, n= | 7 | 11 | |
| Duration of NMBA (days), mean±SD | 9.1±2.9 | 5.2±4.6 | 0.04 |
| Duration of vasoactive drugs (days), mean±SD | 10.4±3.5 | 6.5±5.7 | 0.12 |
| Extubation time (days) mean±SDa | 3.0±1.6 | 3.4±2.4 | 0.93 |
| Duration of MV (days), mean±SD | 20.4±5.3 | 16.9±11.7 | 0.25 |
| Duration of ICU stay, (days), mean±SD | 23.6±6.1 | 19.9±11.6 | 0.38 |
| Number of deaths, n= | 2 | 6 | 0.54 |
Baseline data and characteristics of sedation, analgesia and NMBA delivery just before and following thiopental initiation.
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Male | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Age in years | 55 | 70 | 46 | 72 | 72 | 65 | 74 |
| SAPS II score | 38 | 42 | 25 | 48 | 54 | 44 | 37 |
| SOFA score at T0 | 11 | 11 | 7 | 7 | 7 | 11 | 7 |
| Sedation and vasopressor at T0 | |||||||
| Midazolam (mg/h) | 20 | 12 | 20 | 20 | 0 | 20 | 20 |
| Sufentanil (μg/h) | 40 | 20 | 40 | 40 | 20 | 30 | 30 |
| Propofol (mg/h) | 180 | 100 | 200 | 200 | 100 | 60 | 0 |
| Norepinephrine (mg/h) | 1 | 0.3 | 0 | 0 | 0 | 0.25 | 0 |
| Cisatracurium at T0 (mg/h) | 20 | 20 | 20 | 0 | 15 | 10 | 20 |
| TOF score (/4) at T0 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Day of thiopental initiation after ICU admission | 16 | 6 | 8 | 15 | 10 | 10 | 7 |
| Sedation and vasopressor at T1 | |||||||
| Thiopental (mg/kg/h) | 2.9 | 4.7 | 4.2 | 4 | 2.3 | 3.1 | 2.7 |
| Midazolam (mg/h) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sufentanil (μg/h) | 40 | 20 | 40 | 20 | 10 | 35 | 30 |
| Propofol (mg/h) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Norepinephrine (mg/h) | 0.4 | 0.4 | 0 | 0 | 0.05 | 0.15 | 0 |
| Thiopental duration (days) | 5 | 7 | 5 | 6 | 3 | 2 | 6 |
| BIS during thiopental infusion (mean±SD) | 47.6±9 | 40.4±8.1 | 47.8±8.1 | NA | NA | 44.8±2.5 | 40.2±4.2 |
| Glascow score at ICU discharge | 15 | 15 | 15 | NR | 14 | NR | 14 |
| Death | No | No | No | Yes | No | Yes | No |
T0: Time just before thiopental initiation. T1: Time at 24h following thiopental initiation.
SAPS II: Simplified Acute Physiology Score II; SOFA: Sepsis-related Organ Failure Assessment; TOF: Train-of-four; NR: not relevant; NA: not available.
More severely ill patients with COVID-19 require deep sedation with combination of multiple agents during 2–3 weeks. The recommended drug strategy to maintain a RASS score of −4 or −5 is the combination of midazolam with an opiate and propofol.6 However, prolonged and high requirements of sedatives and opioids can lead to drug tolerance, accumulation, withdrawal and/or propofol syndrome. Government agencies and medical organizations have reported major shortage of benzodiazepines and propofol during the COVID-19 pandemic and providing sedation with less commonly used agents as barbiturates has been suggested.1 Major side effects of thiopental are essentially hypotension and prolonged ICU stay. In our unit, we have used thiopental in patients presenting with severe ventilator asynchrony despite delivery of usual recommended doses of sedatives, opioids and NMBA.7 Low dose of vasopressor was started in only one patient and norepinephrine was decreased in three patients 24h following thiopental initiation. BIS monitoring was used to determine adequate dosage of thiopental. Low BIS level (<40) has been associated with long term mortality (≥1 year).8 In our study, a level ranged from 40 to 50 prevented deep sedation and large accumulation of barbiturates. Midazolam and propofol could be totally discontinued allowing a significant sparing of these drugs and extubation time was similar between the two groups. Dexmedetomedine was used in the two groups of patients during withdrawal of sedative drugs to prevent occurrence of delirium as recommended by a recent guideline.6 Another drug option for sedation of critically ill patients with COVID-19 could be inhaled volatile anaesthetics.9,10 Some studies have reported that inhaled agents such as isoflurane and sevoflurane shorten awakening and extubation times in mechanically ventilated patients compared to benzodiazepines or propofol. Another benefit of volatile anaesthetics could be pulmonary anti-inflammatory effects and dose-dependent bronchodilatation. However, most of these studies included patients with a mean sedation duration less than 4 days and the potential effects of volatile anaesthetics in mechanically ventilated COVID-19 patients remains to be studied.
Thiopental seems to be an acceptable substitute to sedative drugs in this period of high midazolam and propofol demand for ICU patients with COVID-19. When barbiturate therapy can be discontinued, awakening occurs relatively quickly if bispectral index scores were kept between 40 and 50.