Massive hemorrhage is the main cause of mortality and morbidity in trauma patients, and is one of the most important causes in any patient following major surgery. Conventional treatment consists of volume replacement, including the transfusion of blood products, so that tissue perfusion and oxygenation may be maintained. Associated hypothermia, acidosis and coagulopathy is a lethal triad.
This review focuses on the latest therapeutic management of massive hemorrhage. The authors advocate the use of crystalloids as per protocol (controlled volumes) in order to achieve a systolic blood pressure of 85mmHg. The administration of the three blood products (red cells, plasma, and platelets) should be on a 1:1:1 basis. Where possible, this in turn should be guided by thromboelastography performed at point of care near the patient. Coagulopathy can occur early and late. With the exception of tranexamic acid, the cost-benefit relationships of the hemostatic agents, such as fibrinogen, prothrombin complex, and recombinant F VII, are subject to discussion.
La hemorragia masiva es la principal causa de morbimortalidad en el paciente traumatizado, y una de las más importantes en el paciente sometido a cirugía mayor. El tratamiento convencional se basaba en la reposición inicial de la volemia con la infusión de grandes cantidades de fluidos y en la transfusión de hemoderivados, con objeto de asegurar la perfusión y oxigenación tisular. Hipotermia, acidosis y coagulopatía se considera triada letal.
En esta revisión los autores abordan un enfoque terapéutico actualizado del manejo de la hemorragia masiva. Se preconiza infundir cristaloides de forma pautada (no masiva) para lograr una presión arterial sistólica de 85mmHg. La administración de hemoderivados debe ser precoz y con ratio 1:1:1 (cantidades equiparables de concentrados de hematíes, plasma y plaquetas), y si es posible, guiada por tromboelastograma a la cabecera del paciente. La coagulopatía puede ser precoz y tardía. Salvo el ácido tranexámico, se discute la relación coste-beneficio de fármacos prohemostáticos, como fibrinógeno, complejo protrombínico, y FVII recombinante.
Each year over 100 million people suffer traumatism of some kind, and over five million−particularly young individuals–die as a result of violence or accidents. Massive bleeding and brain damage due to traumatic brain injury (TBI) are the main causes of death in severe trauma. Massive bleeding is also an important cause of morbidity–mortality in major surgery, including oncological, cardiac and solid organ transplant surgery. The estimated mortality associated to such bleeding varies from 30 to 70%.1
Massive bleeding is defined as hemorrhage requiring the transfusion of 10 or more red cell concentrate units in 24h. Other arbitrary definitions include 6 or more such units in 12h or over 50 blood product units in 24h–including red cells, platelet concentrates and fresh frozen plasma (FFP).
Classically, massive bleeding has been subjected to damage control surgery designed to arrest the source of bleeding, with fluid replacement therapy and the administration of blood products. Despite such measures, however, the morbidity–mortality associated to massive hemorrhage remains unacceptably high. As a result, new treatment strategies have been developed in the last decade with a view to improve patient survival. These measures include the early replacement of coagulation factors, platelets and red cells in equivalent proportions (i.e., so-called 1:1:1 ratio replacement), the use of prohemostatic drugs (prothrombin complex, activated factor VII and tranexamic acid), the introduction of tests at the patient bedside and the thromboelastogram for the individualized and effective management of massive bleeding.
Conventional management of massive hemorrhageThe three key elements in the treatment of massive bleeding are volume expansion or replacement with crystalloids and colloids, the optimization of tissue oxygenation with the transfusion of red cells, and the correction of coagulopathy. In general, the principles described below should be followed.
The main objective is to restore circulating volume and arrest the source of bleeding (damage control surgery). In this context, normovolemic anemia is better tolerated than hypovolemic anemia.2
The infusion of fluids should be guided by the blood losses, the rate of bleeding, and the hemodynamic condition of the patient. Hemoglobin initially may be normal, even in the presence of important hemorrhage.3
No ideal volume replacement fluid has been defined to date. In principle, crystalloids (Ringer lactate or saline solution) may be a good choice, and are recommended by the American College of Surgeons, depending on the magnitude of the losses (Table 1). However, the aggressive use of crystalloids dilutes the coagulation factors and platelets, favoring coagulopathy and multiorgan dysfunction.4,5 As a result, earlier administration of colloids is advised, especially in hemorrhagic shock. Nevertheless, the infusion of colloids such as hydroxyethyl starch or dextrans has been associated with alterations in platelet function, inhibition of fibrin polymerization, and the stimulation of fibrinolysis.6,7
Classification of acute hemorrhage (reference 2).
| Class I | Class II | Class III | Class IV | |
| Losses | ||||
| Percentage % | <15 | 15–30 | 30–40 | >40 |
| Volume (ml) | 750 | 800–1500 | 1500–2000 | >2000 |
| Systolic pressure | No change | Normal | Reduced | Very low |
| Heart rate | Slight tachycardia | 100–120 | 120 (filiform) | >120 |
| Capillary filling | Normal | Slow (>2s) | Slow (>2s) | Undetectable |
| Respiratory frequency | Normal | Normal | >20min–1 | >20min–1 |
| Diuresis/hour | >30 | 20–30 | 10–20 | 0–10 |
| Extremities | Normal | Pale | Pale | Pale, cold |
| Consciousness | Alert | Anxious | Anxious/sleepy | Sleepy/unconscious |
The objective or goal of resuscitation has not been clearly defined. Keeping normal blood pressure and hemoglobin values can lead to an increased use of fluids, favoring coagulopathy and exacerbating the bleeding. Possibly “permissive hypotension” (i.e., blood pressure values slightly below normal) may be a better objective,8 except in TBI, where higher pressure values may be needed in order to maintain brain perfusion. In monitored patients, maintenance of the cardiac index and of oxygen transport and consumption may constitute an adequate objective of resuscitation. In order to assess the blood losses and the success of resuscitation with fluids, venous oxygen saturation and acidemia are more sensitive measures than the traditional hemodynamic measurements.9
Massive transfusion protocol (MTP). 1:1:1 ratioValidated algorithms have been developed for predicting whether a patient is suffering from massive blood loss and requires inclusion in a massive transfusion protocol (MTP).10 If transfusion is urgent, we request 0 negative red cell concentrate and AB plasma, until cross-testing is performed.
The concept of MTP is recent,11 and such protocols are advised in patients with massive bleeding who present the lethal triad of acidosis, hypothermia and coagulopathy. The aim is to treat the patient early and aggressively with blood products, in order to avoid exsanguination and coagulopathy. MTP requires coordination with the blood bank, core laboratory and intensivist for requesting tests referred to coagulation, hemoglobin and platelets. In MTP, the patient is administered equivalent amounts of the three blood products (Table 2).
Management of patients with critical hemorrhage.
| Conventional management | Current management |
| Evaluation of hemodynamic profile (SBP, HR) to maintain normal SBP values | Permissive hypotension (PAS ∼95mmHg) |
| Arterial blood gases. Control of acidemia | Idem+Venous blood gases. Maintain SvO2>70% |
| Control of coagulopathy (late) based on conventional laboratory testing: aPTT, PT; fibrin and platelets | Control of coagulopathy (early and late) based on TEG and tests at patient bedside |
| Early infusion of high volume crystalloids, followed by administration of colloids | Protocolized infusion of crystalloids and earlier infusion of colloids |
| Transfusion of red cells and, depending on the coagulation study, of plasma and platelets | Transfusion of equivalent doses of red cells, plasma and platelets from the start |
Patients with central nervous system and/or spinal cord injuries may require more aggressive volume replacement and higher SBP to maintain adequate brain perfusion.
PCC: prothrombin complex concentrate; HR: heart rate; SBP: systolic blood pressure; rFVIIa: recombinant activated factor VII; SvO2: venous oxygen saturation, measured in venous blood gases.
Conventional resuscitation with large volumes of fluids can lead to dilutional coagulopathy–the latter in turn being worsened by the hypothermia and acidosis that accompany trauma. In order to deal with this problem, the United States Army's Institute of Surgical Research conference12 in 2005 proposed a transfusion strategy involving immediate administration of the three blood products in massive bleeding secondary to trauma (Table 3).
Pre-established massive transfusion protocol used in the Grady Memorial Hospital/Emory University Massive Transfusion Protocol.
| Hours | Red cells | Plasma | Platelets | Cryoprecipitatea |
| Initial | 6 | 6 | ||
| 1 | 6 | 6 | 1 pool | |
| 2 | 6 | 6 | 20u. | |
| 3 | 6 | 6 | 1 pool | |
| 4 | 6 | 6 | 10u. | |
| 5 | 6 | 6 | 1 pool | |
| 6 | 6 | 6 | 10u. |
Cryoprecipitate can be replaced by fibrinogen.
Since then, transfusion strategies have been introduced with higher plasma and platelet to red cell concentrate ratios, simulating whole blood. Although some non-randomized observational studies have found these ratios to improve survival among patients with massive bleeding,13,14 other recent studies have been unable to confirm these findings.15,16
Despite the disparity of published data and the increase in resource consumption associated with the use of such high ratios, MTP has been accepted in many centers in North America and Europe. In this sense, the Canadian Blood Services reported a 50% increase in the demand for AB plasma between the years 2007 and 2009.17
While acknowledging these data, the European massive transfusion guideline of 2007 gave no specific recommendations referred to the mentioned ratio, in the same way as the more recent guides of the European task force18 and the American Association of Blood Banks,19 which recommend early intervention with FFP, though without establishing ratios.
A recent Scandinavian study15 presented as main finding the observation that the change in transfusion strategy (in the context of a pre- and post-intervention retrospective study), referred to more aggressive and earlier administration of plasma and platelets, exerts no influence upon the survival of patients with bleeding due to trauma–this being confirmed by a multivariate analysis in patients subjected to massive transfusion, concluding that the devastating effects of trauma cannot be reverted by this change in strategy alone.
Another recent systematic review on this same subject compared high and low transfusion ratios, noting that most studies report favorable results with high ratios. However, the presence of screening error was evidenced, which could affect the results obtained. The authors concluded that there is not enough evidence supporting indication of the fixed 1:1:1 ratio.20
At present, a priority concern is the conduction of controlled and randomized clinical trials designed to document the risk-benefit ratio of the different transfusion ratios in massive bleeding9.
Coagulopathy of massive bleedingCoagulopathy is the most feared complication in massive bleeding. Patients with severe trauma and also those subjected to major surgery suffer alterations in the integrity of the vascular endothelium. These situations give rise to coagulopathy, of a multifactorial origin, and in which all the coagulation factors are deficient to one degree or other. When such coagulopathy is accompanied by hypothermia and acidosis, the patient prognosis is seriously worsened (lethal triad), though coagulopathy in itself constitutes an independent mortality factor.
Two phases are recognized in coagulopathy associated to massive bleeding: primary coagulopathy, which is of early onset, and secondary coagulopathy, which manifests in a later stage. Traditionally, coagulopathy was always believed to be a late event, though it is now known that coagulation disorders can manifest very early, from as soon as the time of patient admission to hospital. Primary coagulopathy is related to tissue factor exposure, thrombin generation, and the activation and consumption of protein C–leading to the very early development of disseminated intravascular coagulation and fibrinolysis. This situation is also referred to as trauma-induced early coagulopathy, and its presence implies a significant increase in mortality.21,9
In contrast, secondary coagulopathy is of later onset and is related to the loss of coagulation factors and the dilution of the existing factors. Both coagulopathy phases manifest as the presence of nonsurgical bleeding (non-massive bleeding from multiple locations, and with difficult control). The laboratory diagnosis includes the following parameters: prolongation of prothrombin time (PT) and of activated partial thromboplastin time (aPTT) to >1.5 times the control value, an international normalized ratio (INR) of >1.5, a platelet count of <50×109/l, and fibrinogen values of <0.5–1g/l. Fibrinolysis and platelet dysfunction manifest very early.22
The concept of primary coagulopathy is beginning to prevail over that of secondary coagulopathy. As a result, the current recommendations suggest immediate management of primary coagulopathy, with the early transfusion of large amounts of fresh plasma and platelets, avoiding hypothermia and acidosis. Both coagulopathy phases can coincide in one same patient. A recent study23 in patients with serious TBI confirmed a high prevalence of early and late coagulopathy, and both were independently correlated to a poor clinical outcome. These data suggest that coagulopathy must be monitored and corrected in its most incipient or early phase.
The principal efforts referred to the transfusion of blood products and the use of other hemostatic interventions in general begin when the patient is admitted to a third level hospital center. Consequently, the time elapsed from injury to admission is a critical factor, particularly in situations of hemorrhagic shock. Conventional transfusion is not usually performed at the site of the accident. The best strategy referred to coagulopathy secondary to massive bleeding remains the subject of debate.24
Transfusion of blood products in massive bleedingRed cell concentratesThe only accepted indication of red cell transfusion is for increasing tissue oxygenation in anemic patients with tissue oxygen deficiency.2 Prescription is made in the form of 220–400ml units of red cell concentrate depleted of leukocytes and stored in the blood bank for up to 42 days. One red cell concentrate unit increases hemoglobin by 1g/dl (or hematocrit by 3%).25 The optimum hematocrit for ensuring good oxygen transport and preventing dilutional coagulopathy in massive bleeding is not known, but is probably in the range of 35%. More stable young patients with a good cardiopulmonary reserve can tolerate normovolemic anemia to a hemoglobin concentration of 8g/dl. Older patients or individuals with a poorer cardiopulmonary reserve require hemoglobin 9–10g/dl.
Frequent measurement is required of hemoglobin and hematocrit. Initially, the hemoglobin values may be normal, despite important blood loss. Good communication with the laboratory and Department of Hematology is needed. It is particularly important to inform the laboratory of the urgency with which the blood is needed: immediately, in 20min, or in the next hour. If the need is immediate and the blood group is not known, the laboratory must supply group 0 negative red cell concentrates, without cross-testing. Women of child-bearing potential must receive group 0 negative Rh D negative units. In other cases group 0 Rh D positive blood is acceptable, if Rh D negative units are not available. Definitive blood grouping should take no more than 10min, after which isogroup blood transfusion is indicated.
Fresh frozen plasma (FFP)Fresh frozen plasma consists of 200–250ml units frozen within 8h after donation. At least 30min for thawing are required. These units afford coagulation factors and fibrinogen, with procoagulants, anticoagulants, albumin and immunoglobulins.22 Although classically the administration dose has been 10–15ml/kg, the current tendency in massive bleeding, as commented above, is to perform infusion in 1:1:1 proportion (red cells, plasma, platelets). Excluding viruses such as CMV and HTLV-II, plasma exposes the recipient to the same types of viruses as red cell concentrates, and is associated to the development of transfusion related acute lung injury (TRALI).25
After thawing, FFP can be refrigerated at 4°C for up to 24h, but cannot be frozen again. It is important to anticipate the thawing of FFP in order to avoid delays. The administration of prothrombin complex concentrate (PCC) is recommended as a more optimum alternative to FFP in patients with massive bleeding secondary to overdose of vitamin K antagonists.16
Platelet concentratesA pool of platelets contains approximately 200ml of platelet concentrate, obtained from 6 donors. These concentrates are stored in the blood bank at 22°C for up to 5 days. ABO group compatibility is not necessary. A platelet concentrate unit increases the platelet count by approximately 5–10×109/l. Although classically the threshold for deciding platelet transfusion has been 50×103/μl, the tendency in recent years has been to administer a platelet unit simultaneously with a red cell concentrate and plasma (1:1:1 ratio) in massive bleeding.14,15,20,25 In patients with platelet dysfunction (secondary to antiplatelet medication, aspirin and/or clopidogrel, for example), additional platelet transfusions may be needed, despite the presence of a normal platelet count.
Conventional transfusion versus guided transfusionThe administration of blood products ideally could be guided by the observed blood losses and coagulation disorders. However, the usual coagulation tests, which include prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen and platelet count are scantly sensitive in predicting bleeding. Moreover, the test results take time in becoming available, and therefore do not reflect the coagulation condition of the patient on a real-time basis.26
At present we can use the thromboelastogram (TEG®) or rotational thromboelastometer (ROTEM®) and tests at the patient bedside (Table 4). Both options allow dynamic online and global evaluation of the coagulation cascade, platelet function and the degree of fibrinolysis–thus ensuring more effective management of coagulopathy.25,26 The TEG complements rather than replaces the data obtained from standard laboratory testing.
Comparison of thromboelastography versus conventional tests.
| Thromboelastogram | Conventional coagulation tests |
| Rapid evaluation coagulation | Late evaluation of coagulation |
| Applicable at patient bedside | Laboratory dependent |
| Rapid diagnosis | Late diagnosis |
| Allows early start of treatment | Delay in start of treatment |
| Specialized personnel | Blood bank personnel |
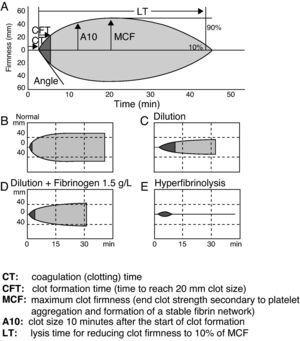
The TEG is a computerized device for the in vitro measurement of the viscoelastic properties of blood, documenting integration of the platelets within the coagulation cascade. The most common variables obtained include coagulation time, clot formation time, time to lysis (in seconds), amplitude 10, and the angle and maximum firmness of the clot (in mm)27 (Fig. 1). The measurements are made at the patient bedside (i.e., outside the core laboratory and in the physical environment of the patient).
The superiority of TEG over the conventional tests has been documented.28 It is known that its routine use implies fewer blood component transfusions than transfusion therapy based on conventional laboratory testing.29 In severe trauma, the TEG allows the early diagnosis and treatment of coagulopathy.24 Maximum clot firmness is well correlated to fibrinogen concentration.30 In primary coagulopathy, a clot amplitude after 10min of less than 5mm is a good predictor of fibrinogen <1.0g/l, with a sensitivity of 91% and a specificity of 85%.30
Prohemostatic drugsThis section considers those drugs which can be useful in reducing or controlling bleeding in patients with massive hemorrhage. However, their high cost and the lack of studies consistently documenting their efficacy limit the generalized clinical application of such substances.
Antifibrinolytic agentsAntifibrinolytic agents inhibit the plasminogen-plasmin fibrinolytic system, preventing blood clot lysis, which can lead to a decrease in bleeding. Their efficacy has been well documented in surgical patients, including patients subjected to heart surgery and liver transplantation.31 A recent randomized, double-blind, placebo-controlled trial32 has evaluated the use of tranexamic acid administered early in the first 8h after trauma in the form of an intravenous dose of 2g (1g in an initial bolus dose and 1g in perfusion during 8h). This treatment was seen to decrease both global mortality and mortality attributable to bleeding in polytraumatized patients. The mechanism by which tranexamic acid reduces mortality may be related to an antiinflammatory effect mediated by plasmin.
Since this is the first time a drug has been shown being able to reduce the mortality of traumatic hemorrhagic shock, and considering the large number of fatalities due to this reason, its compassionate use has been proposed until the above indication is included in the Summary of Product Characteristics.33,34
Recombinant activated factor VII (rFVIIa)Recombinant activated factor VII is being used off label (i.e., indicated outside the Summary of Product Characteristics) for the treatment of massive bleeding refractory to conventional therapy with blood products, in the absence of solid supporting scientific evidence.35 Before its administration, the patient acidosis and hypothermia must be corrected, attempting to maintain fibrinogen levels of ≥150mg/dl, a platelet count of >50,000mm–3 and a hematocrit of >24%.25
In polytraumatized patients with incoercible bleeding, a starting dose of 200μg/kg is advised, followed by two doses of 100μg/kg, 1–3h after the first dose–though the efficacy of such treatment has only been shown in closed traumatisms.36 In perioperative hemorrhage, the recommended dose is 90μg/kg, which can be repeated if the bleeding persists.
Recombinant activated factor VII has been used in application to incoercible bleeding related to trauma and in perioperative hemorrhage. However, rFVIIa can give rise to thromboembolic phenomena in over 6% of the cases.37 The main adverse effects are related to the presence of tissue factor abnormally exposed in the diseased endothelium (atheroma plaques)–with the consequent risk of arterial or venous thrombosis. Thrombotic complications are more frequent in arterial territories than in veins, and include non-hemorrhagic cerebrovascular stroke and acute coronary syndrome. The venous complications in turn include deep venous thrombosis and pulmonary thromboembolism.38
A recent metaanalysis39 including 5 observational studies and a controlled clinical trial40 evaluated the use of rFVIIa in refractory hemorrhage in heart surgery patients, and documented a statistically nonsignificant increase in the incidence of cerebrovascular events. More controlled clinical trials are needed to determine the risk-benefit ratio of the administration of rFVIIa in patients with massive bleeding. Its high cost, about 4000 euros (mean dose 90μg/kg), limits the use of this treatment.
FibrinogenFibrinogen is a soluble plasmatic glycoprotein with a molecular weight of 340kDa, produced in the liver, and with a plasma concentration of between 2 and 3.5g/l. The half-life is between 72 and 120h. As coagulation factor I, fibrinogen is the precursor of fibrin and the physiological substrate of three enzymes: thrombin, FXIII and plasmin. Although the minimum levels needed to reduce bleeding due to coagulopathy are not known, the European guideline18 recommends the maintenance of levels of 1.5–2g/l.
Fibrinogen is also the coagulation factor that most quickly reaches critical levels in massive bleeding. When its levels drop to under 1g/l, fresh plasma, fibrinogen concentrate or cryoprecipitate must be administered. Early correction to optimum levels (2–3g/l) can reduce mortality among trauma patients.28,41
The transfusion of FFP may be insufficient to increase the plasma fibrinogen levels (30ml/kg of plasma increase fibrinogen by 1g/l; a patient weighing 80kg would need 2400ml of FFP). In contrast, the administration of fibrinogen concentrate is more effective in increasing the plasma levels. A high fibrinogen/red cell concentrate ratio has been associated with a decrease in mortality in war wound patients. Levels of over 3g/l can even compensate a low platelet count.28
There is growing scientific evidence that the early use of fibrinogen concentrate reduces bleeding and the transfusion of blood products after a major surgery, without an associated increase in thrombotic complications.42–44 Although restoring the levels of this factor seems to be advantageous for the control of massive bleeding secondary to major surgery or trauma, the choice among FFP, cryoprecipitate or fibrinogen concentrate remains the subject of debate.25
Prothrombin complex concentrate (PCC)Prothrombin complex concentrate contains variable amounts of coagulation factors II, VII, IX and X, obtained from a plasma pool of at least 1000 donors. Depending on its factor VII concentration, PCC is divided into 3-factor PCC (low concentrations of factor VII) or 4-factor PCC (high concentrations of factor VII). PCC contains a 1000-fold higher concentration of coagulation factors than FFP. In fact, one FFP unit (about 250ml) contains only 0.5–1U/ml of all the plasma factors.25
PCC is only indicated in patients with massive bleeding involving a prolongation of the coagulation times (INR>1.5) and overdosed with vitamin K antagonists.26 However, observational studies have been published including polytransfused patients, patients with coagulopathy, incoercible bleeding, and patients not administered vitamin K antagonists.24,45
PCC has been used in perioperative bleeding unrelated to the administration of vitamin K antagonists. In 38 patients with perioperative bleeding, the administration of 2000IU of PCC decreased INR from 1.7 to 1.4, arresting bleeding in 96% of the patients with diffuse microvascular hemorrhage (due to coagulopathy) and in 36% of the patients with surgical bleeding.34 The administration of 1500IU of PCC reduced the transfusion rate in 16 patients subjected to heart surgery, with incoercible bleeding not previously treated with vitamin K antagonists.35 This suggests that the administration of PCC may be useful for the treatment of perioperative bleeding unrelated to the administration of vitamin K antagonists.
Lastly, a single observational study explored the efficacy of PCC in improving coagulation, and in reverting or preventing bleeding in 22 patients with severe liver failure. The INR value normalized 10minutes after infusion, and the clinical response (prevention, reduction or cessation of bleeding) was considered very good or good in 100% of the patients.46 PCC could prove useful in application to bleeding or in preventing bleeding in patients with a deficiency of liver-dependent factors secondary to acute liver failure (Table 5).
Advantages of prothrombin complex concentrate (PCC) versus fresh frozen plasma (FFP).
| 1. Affords a greater amount of coagulation factors in a lesser volume, can be infused quickly and requires no prior thawing |
| 2. Blood group compatibility not required |
| 4. Small administration volume |
| 5. Less than 30min to correct INR |
| 6. Lesser risk of infection and transfusion related acute lung injury (TRALI) |
In experimental animal models in which coagulopathy was induced by exsanguination and polytransfusion, the administration of PCC decreased hemorrhage and the transfusion needs.47
Although the initial results are promising, the studies documenting the efficacy of PCC in controlling coagulopathy unrelated to the administration of vitamin K antagonists are few and of an observational nature. As a result, the generalized use of PCC cannot be recommended, outside its established indication for reverting the antagonistic effect of vitamin K.
The main adverse effect of PCC is venous or arterial thrombosis, particularly in patients with liver disease, newborn infants, or individuals requiring very high or repeated PCC doses. Nevertheless, the described adverse effect rates are very low, and moreover no clear cause-effect relation has been established.
The mean cost per vial of 4-factors PCC is about 1000 euros.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fernández-Hinojosa E, et al. Alternativas terapéuticas de la hemorragia masiva. Med Intensiva. 2012;36:496–503.