In patients on mechanical ventilation, the ventilator cycle of the respirator can induce inspiratory efforts. This mode of interaction has been called trigger reverse (TR). This is a not very well-known asynchrony but potentially more common than expected, possibly due to difficulties when conducting the bedside monitoring of inspiratory muscle effort.
We hereby present two cases of critically ill patients admitted to the ICU who showed this asynchrony right after the ventilator withdrawal. The reason for admission was pneumonia and severe acute pancreatitis. Volume assist-control ventilation (ACV) due to heart failure and acute respiratory distress syndrome in the adult patient was used. After withdrawing sedation, the patients showed one complex interaction, with inspiratory efforts that did not precede the ventilator cycle (Fig. 1). We monitored the pressures (airway, esophageal) and the flow, and confirmed that the ventilator cycle induces the inspiratory cycle—an asynchrony known as trigger reverse. The ventilation mode was changed to pressure support ventilation (PSV), thus improving the interaction with the ventilator in one of them (Fig. 2) and requiring ACV in the second case. This mode of interaction is difficult to identify on the ventilator screen, and it is useful to use ACV with constant flow and inspiratory pause. The beginning of inspiration is usually passive, without initial deflection, but it is hard to say that in the pressure-flow curves no effort triggers the ventilator.1 This makes us think that the effort follows the ventilator cycle not only when the beginning of inspiration seems passive but also when the plateau pressure changes cycles and there are oscillations in the peak of expiratory flow. Based on the programmed respiratory frequency, this sequence alternates between synchronized cycles through which the patient triggers the ventilator, and other cycles with double trigger. In the modes controlled by pressure, the effort that follows the ventilator cycle increases inspiratory flow after the initial peak and when it is intense enough, it reduces the pressure curve based on the capacity of response of the ventilator (Fig. 2).
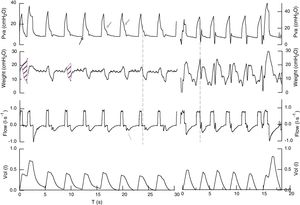
Registry of airway pressure, esophageal pressure, flow, and volume in volume assist-control ventilation (ACV) in 2 patients (left and right). Airway pressure the beginning of breathing is passive, there is no pressure reduction indicative of breathing effort (←). During the phase of inspiratory pause, morphology and amplitude change from one cycle to the next (←). Inspiratory flow is constant, expiratory flow has oscillations at the beginning of mechanical expiration that usually shows inspiratory efforts (←). Esophageal pressure shows increased intrathoracic pressure at the beginning of mechanical inspiration followed by reduced intrathoracic pressure, all indicative of the patient's effort (dotted line). The ratio between the mechanical cycle and the effort is 1/1 in one case and variable in the other (1/1 in 90% of the total registry, coexisting with 1/3 and 1/2). The mistmatch was 60 and 47ms or 21 and 14° (θ=Tin−Tim/Ttotm×360). Inspiratory time of muscle effort is 0.8–0.9s and when it causes double trigger it goes up to 1.4s. Occlusion pressure during the first 100ms (P01) was 3.9 and 8.2cmH2O in cycles with double trigger in one case, and 4.5 and 8.5cmH2O, respectively in the second case. Parameters associated with inspiratory effort: delta esophageal pressure (Δpes) vales of 11cmH2O, and pressure time integral (PTI) of 1.6±0.3 and 2.5cmH2Os, the first patient showed undrained pleural effusion, which is why we measured the chest wall compliance during controlled mechanical ventilation (160ml/cmH2O) and estimated the pressure-time product9 (darkened area). Considering the chest wall elastic recoil pressure, in this case the estimated respiratory effort is 5.6cmH2Os (96.2cmH2Os/m) and double trigger efforts 12.5cmH2Os. In the second case: Δpes 16cmH2O, PTI 2.5cmH2Os (54cmH2Os/m) and double trigger cycles: 17cmH2Os (370cmH2Os/m).
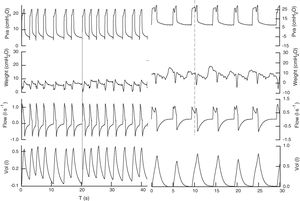
Registry of the signals (Pva, Pes, flow, vol) in pressure support from both patients. In the first patient, pressure support is set at 18 and PEEP is set at 5cmH2O, and by improving the interaction with the ventilator, the effort precedes the mechanical cycle (continuous line) with a respiratory frequency of 22bmp to achieve a tidal volume of 0.450l, with no signs of excessive respiratory effort and delta esophageal pressure (Δpes) values <5cmH2O. In the second case, pressure support is set at 25 and PEEP set at 6 keeping heart rate at 12bmp and tidal volume at 0.783l. In this second case, the patient's effort follows the beginning of the mechanical cycle and, possibly, the ventilator trigger is the change of pressure (or flow) caused by the heartbeat. The patient's effort (dotted line) reduces the phase of airway plateau pressure originating a new increase of inspiratory flow.
Even though the possible implication of RT in mechanical ventilation was recognized years ago,2 there is little information published on trigger reverse in patients with mechanical ventilation and most data come from anesthetized animal experiments3 and anesthetized healthy individuals with non-invasive4 and invasive ventilation.2 Recently it has been described after the retrospective analysis of registries conducted on patients with ARDS, being this type of interaction not taken into consideration during the management of patients.5 It has also been reported in situations of brain death after cardiac arrest6 and in transplanted patients.4
The mechanism responsible is still not clear and it occurs in the absence of central ventilatory drive.4 The Hering–Breuer reflex is important, since its frequency is reduced with vagotomy, yet it is not essential.
Whenever there is synchronization or regularity between the ventilator and the neural cycles we call it «entrainment or specific phase». In the cases presented here the relation between the ventilator-induced respiratory frequency and programmed frequency was the same in one case and variable in the other. This has already been described with different frequencies of stimulation (1:1, 1:2, 1:3), even with variable and irregular frequencies.2,3
Registries of pressures, flows, and volumes similar to ours have already been published in a patient with ARDS.7
The clinical implication of RT depends on the degree of mismatch between the ventilator cycle and the muscle effort. If it is significant then it can cause double trigger with increased tidal volume and alveolar distention pressure.5 It has been described as one of the causes of double trigger,1 possibly the second cause of asynchrony with the ventilator.1 In modes controlled by pressure, the muscle effort will be compensated by an increased volume in every cycle of the ventilator. The inspiratory work of breathing increases, in our case it is within the limits considered appropriate in mechanical ventilation,7,8 but it is a lost effort, without performance and excessive at times as it occurs during double trigger. For the management of this asynchrony it has been suggested to use pressure support and modes controlled by pressure with programmed low frequencies.1
The incidence and meaning of RT in critically ill patients on mechanical ventilation is still being studied. We still do not know whether it is due to the adequate management of patients, sedation, ventilator parameters, or an adaptative physiological response to mechanical ventilation.
Please cite this article as: Ruiz Ferrón F, Serrano Simón JM. Identificación del trigger inverso en UCI. Med Intensiva. 2018;42:391–393.