Edited by: Ana Ochagavía - Hospital Universitario de Bellvitge. L'Hospitalet de Llobregat. Barcelona. Spain.
Last update: March 2024
More infoThe use of point-of-care ultrasonography (POCUS) is not limited to the diagnosis and/or monitoring of critically ill patients. Further, ultrasound guidance is of paramount relevance to aid in successfully and safely performing several procedures in the intensive care unit (ICU). In this article, we review the role of POCUS as a procedural guidance in the ICU. Core procedures include, but are not limited to, vascular cannulation, pericardiocentesis, thoracentesis, paracentesis, aspiration of soft-tissue collections/arthrocentesis and lumbar puncture. With time, the procedures performed by intensivists may extend beyond the core competencies depicted in this review. Ultrasound guidance should be part of the intensivist’s competencies, for which appropriate training should be achieved.
El uso de la ecografía no se limita al diagnóstico y/o monitorización del paciente crítico. Además, la guía ecográfica es de gran importancia para la ejecución exitosa y segura de diversos procedimientos en la Unidad de Cuidados Intensivos (UCI). En este artículo, revisamos el papel de la ecografía como guía intervencionista en la UCI. Los procedimientos ecodirigidos claves son, aunque no se limitan a estos, la canulación vascular, pericardiocentesis, toracocentesis, paracentesis, aspiración de colecciones de partes blandas/artrocentesis y la punción lumbar. Con el tiempo, los procedimientos realizados por los intensivistas se extenderán más allá de los descriptos en esta revisión. La guía ecográfica debe formar parte de las competencias del intensivista, para lo cual se requiere un entrenamiento adecuado.
Point-of-care ultrasonography (POCUS) has revolutionized the care of critically ill patients around the world.1 In addition to its well-known diagnostic applications, POCUS is gaining ground as a guide for the majority of interventional procedures performed in the Intensive Care Unit (ICU).2–4
The benefits of POCUS include its fastness, absence of ionizing radiation, low cost, and bedside use without the need to move the patient to the radiology department or the operating room. The main disadvantages include operator dependency (i.e., lack of expertise) and patient dependency (i.e., inadequate ultrasound views).
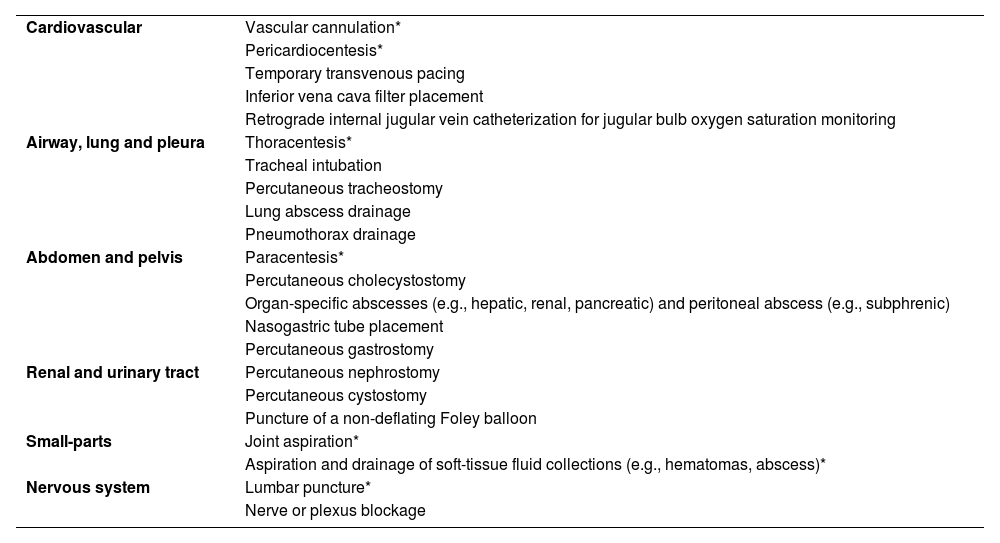
In this article, we review the role of POCUS as a procedural guidance in the ICU. A list of ultrasound-guided procedural applications is provided in Table 1. The core competencies of intensivists include, but are not limited to, vascular cannulation, pericardiocentesis, thoracentesis, paracentesis, soft-tissue collection aspiration/arthrocentesis, and lumbar puncture. These are described here in detail, while suggested readings are provided in Supplementary Material, Appendix A, for less frequent, evolving interventions or those performed by the interventional radiologist. The general aspects of ultrasound-guided procedures are detailed in Supplementary Material,Appendix B.
Ultrasound-guided procedural applications in critical care patients.
| Cardiovascular | Vascular cannulation* |
| Pericardiocentesis* | |
| Temporary transvenous pacing | |
| Inferior vena cava filter placement | |
| Retrograde internal jugular vein catheterization for jugular bulb oxygen saturation monitoring | |
| Airway, lung and pleura | Thoracentesis* |
| Tracheal intubation | |
| Percutaneous tracheostomy | |
| Lung abscess drainage | |
| Pneumothorax drainage | |
| Abdomen and pelvis | Paracentesis* |
| Percutaneous cholecystostomy | |
| Organ-specific abscesses (e.g., hepatic, renal, pancreatic) and peritoneal abscess (e.g., subphrenic) | |
| Nasogastric tube placement | |
| Percutaneous gastrostomy | |
| Renal and urinary tract | Percutaneous nephrostomy |
| Percutaneous cystostomy | |
| Puncture of a non-deflating Foley balloon | |
| Small-parts | Joint aspiration* |
| Aspiration and drainage of soft-tissue fluid collections (e.g., hematomas, abscess)* | |
| Nervous system | Lumbar puncture* |
| Nerve or plexus blockage |
Asterisks indicates the core procedures for intensivists.
Central venous catheters (CVC) placement is the most common procedure performed under ultrasound guidance in the ICU.5 However, data show that the use of POCUS is suboptimal, since as many as 50% of CVC are still placed using anatomical landmarks.5 Compared with the landmark insertion technique, ultrasound guidance showed a higher first-time and overall success rate, lower procedural times, while reducing complications such as arterial puncture, hematomas and pleural puncture.3,6–13
For peripherally inserted central catheters (PICC), ultrasound guidance outperforms also over the landmark technique, with data showing a higher success rate, lower rate of thrombosis and lower costs.14–18
Ultrasonography-guided peripheral vein cannulation has particularly gained interest among ICU nurses, given that up to one-third of patients have difficult intravenous access resulting from edema, hypovolemia, obesity or previous cannulations.19 Compared with landmarks, ultrasound guidance has been shown to improve the cannulation rate from 25% to 90%, with fewer attempts, shorter time to access,20–22 decreased physician intervention,23 improved patient satisfaction,24 and reduced the need for a CVC.25,26 In veins >12 mm from the skin, using long peripheral venous catheters and midline catheters (MC) improve catheter survival, avoid multiple peripheral vein cannulations and also reduce the need for a CVC.27–29 Research has shown that MC may perform similarly to CVC, with a lower rate of catheter-related bloodstream infections.30–32
For arterial cannulation, ultrasound-guided radial artery cannulation showed an increased first-time success rate, overall success rate and lower failure rate, with no differences in the time spent for cannulation or risk of hematoma compared with palpation alone.33 Ultrasound-guided femoral artery catheterization achieved a 49% reduction in overall complications, including hematoma and accidental venipuncture compared to the landmark technique; it was also associated with a 42% improvement in the likelihood of first-attempt success.34 Other options to radial or femoral artery cannulation, although with less supporting body of evidence, include ultrasound-guided cannulation of the ulnar, axillary, brachial, posterior tibial or dorsalis pedis artery.35–38
An intraosseous access is typically indicated in cardiac arrest patients when obtaining an intravenous line is unsuccessful or nonfeasible.39 Options include the proximal tibia, distal tibia, proximal humerus (greater tuberosity), and sternum. Although the needle is often inserted after identification by palpation of the tibial or sternal bones, recognizing the greater tuberosity of the humerus may be challenging. For this indication, ultrasound has demonstrated to be reliable and accurate.40
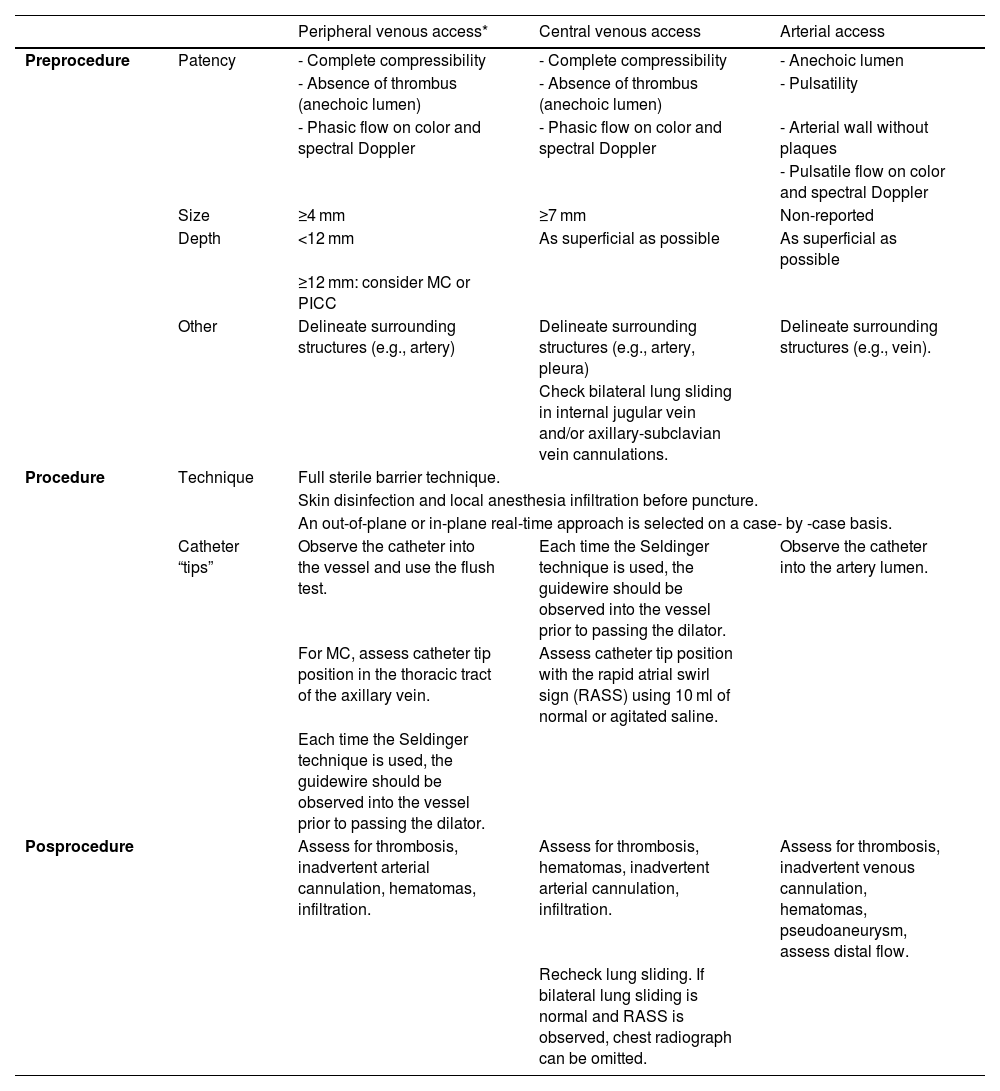
PreprocedureA high-frequency linear probe is mandatory for vascular cannulation, although a convex probe may be needed in patients with morbid obesity, particularly for femoral vessel targets.41 A venous or arterial preset is chosen based on the target vessel, although the preset often requires manual adjustments on a case-by-case basis.
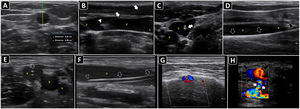
The vessels should be evaluated along both short and long axes. The target vessel should be selected based on patency (e.g., absence of thrombus, complete compressibility and phasic flow in veins; pulsatility, absence of plaques/stenosis and pulsatile flow in arteries), best depth (measuring the distance from the anterior wall of the vessel to the skin, in short axis) and size (measuring the anteroposterior vessel diameter) (Fig. 1A), course (straight better than tortuous) and the safest needle path assessing the relationship of the target vessel with pivotal structures such as the vein, artery or the pleura.42 As a rule of thumb, when selecting the catheter size, the anteroposterior diameter of the vessel corresponds to the maximal catheter size (e.g., 5 mm corresponds to a catheter up to 5F). In addition, at least 50% of the catheter length should lie in the vessel; therefore, longer catheters are needed for deeper vessels, and vice versa.
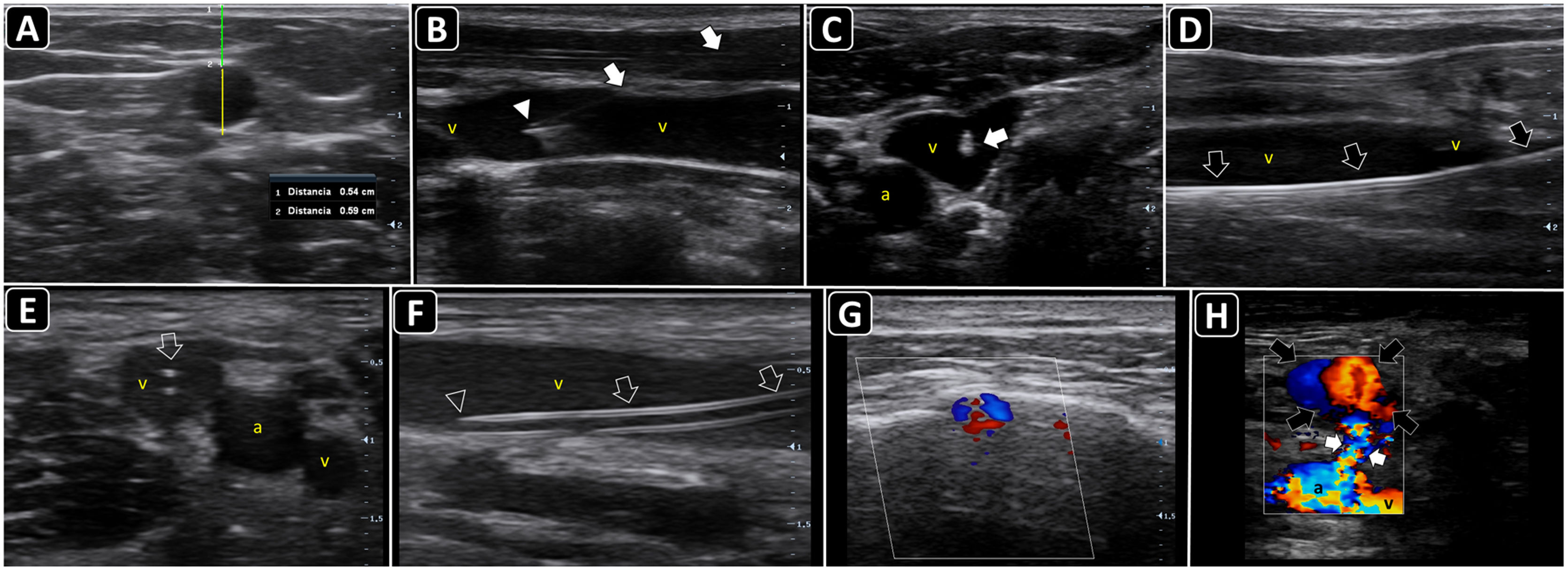
Ultrasound-guided vascular cannulation. A) Preprocedural assessment of a peripheral vein for ultrasound-guided cannulation. The anteroposterior diameter of the vein (continuous yellow line, Distancia 2) and distance from the vein to the skin (continuous green line, Distancia 1) are measured. B) In-plane cannulation of the internal jugular vein (v); arrows, needle shaft; arrowhead, needle tip. C) Out-of-plane cannulation of the internal jugular vein (v); a, common carotid artery; arrow, needle. D) The guidewire is observed in the vein lumen (arrows) before placement of a long catheter in the basilic vein (e.g., midline catheter); v, vein. E) A peripheral venous catheter (arrow) is observed in the lumen of a deep vein of the arm (short axis); v, brachial veins; a, brachial artery. F) A peripheral catheter (arrows) is depicted in the lumen of a superficial vein in the long axis; v, vein. G) Subperiostial flow is observed on color Doppler after intraosseous needle insertion. H) Pseudoaneurysm as a complication of femoral arterial catheterization; black arrows, pseudoaneurysm cavity; white arrows, pseudoaneurysm neck; a, common femoral artery; v, common femoral vein.
For internal jugular or subclavian vein cannulation, the Trendelenburg position is recommended to enlarge the target veins and minimize the risk of air embolism. However, it may not be safe in some patients, such as those with increased intracranial pressure. In such cases, ultrasound-guided cannulation of the internal jugular vein at a 30° head-up position was demonstrated to be feasible and safe.43 Bilateral lung sliding should also be assessed. To cannulate the femoral vein, the frog-legged position with reverse Trendelenburg aids in increasing the femoral vein size.
Palmar arch sufficiency should be assessed when radial artery cannulation is considered.42
For intraosseous cannulation, as stated previously, the site of needle insertion is often identified by palpation, such as the proximal and distal tibia. However, for insertion into the humerus, ultrasound has demonstrated better performance than palpation in identifying the target at the greater tuberosity.40
ProcedureReal-time cannulation technique is preferred. For simplicity, short-axis cannulation is synonymous with the out-of-plane technique, whereas long-axis cannulation is analogous to the in-plane technique.41 However, practitioners should bear in mind that the out-of-plane and the in-plane terms denote the relationship between the needle and the ultrasound beam, while the short axis and long axis denote the relationship between the ultrasound beam and the vessel. Therefore, it is possible to perform an out-of-plane cannulation in the long-axis or an in-plane cannulation in the short-axis. Either approach is selected on a case-by-case basis.
While a fully sterile technique may be omitted for peripheral venous cannulations, it is mandatory for MC, PICC, arterial, and CVC placements.44
After disinfecting the skin and applying a local anesthetic under real-time ultrasound guidance, the needle is inserted in-plane or out-of-plane (Fig. 1B and C), and the catheter is accommodated using the trocar or Seldinger technique. When using the latter, the guidewire should be confirmed in the vessel before progressing the dilator (Fig. 1D).
To assess the CVC tip position, one operator injects agitated saline or normal saline through the distal port of the CVC, while a second operator performs simultaneously a subcostal 4 chamber view or an apical 4 chamber view. The immediate (within 2 s) appearance of turbulent flow in the right atrium is known as the rapid atrial swirl sign (RASS) and predicts with excellent sensitivity and specificity for a correct catheter tip positioning45 (Video 1).
The intravenous position of a peripheral catheter is assessed by direct visualization in the short and/or long axis (Fig. 1E and F) and the flush test (Video 2).
For MC, the catheter is advanced or retroceded until the tip is observed lying in the thoracic tract of the axillary vein.
For arterial cannulation, transient ulnar artery compression may enlarge the radial artery and makes the cannulation easier.46 The intra-arterial position of the catheter is assessed by direct visualization in the short and/or long axis.
Intraosseous cannulation is not performed under real-time ultrasound guidance; however, its correct position may be evaluated using color Doppler when subperiosteal flow is observed after flushing the line (Fig. 1G).47
PosprocedurePOCUS is performed to assess complications, such as hematoma, pneumothorax (rechecking the lung sliding), infiltration, and catheter-related thrombosis. The distal flow should also be assessed after arterial cannulations, while a pseudoaneurysm should be ruled out (Fig. 1H), when the catheter is removed.
In patients in whom RASS is confirmed and lung sliding is normal, chest radiography can be safely omitted, reducing time, costs, and ionizing radiation exposure.48
Table 2 sumarizes the steps involved in ultrasound-guided vascular cannulation.
Steps for performing ultrasound-guided vascular cannulation.8,13,19,27–32,41,42,45
| Peripheral venous access* | Central venous access | Arterial access | ||
|---|---|---|---|---|
| Preprocedure | Patency | - Complete compressibility | - Complete compressibility | - Anechoic lumen |
| - Absence of thrombus (anechoic lumen) | - Absence of thrombus (anechoic lumen) | - Pulsatility | ||
| - Phasic flow on color and spectral Doppler | - Phasic flow on color and spectral Doppler | - Arterial wall without plaques | ||
| - Pulsatile flow on color and spectral Doppler | ||||
| Size | ≥4 mm | ≥7 mm | Non-reported | |
| Depth | <12 mm | As superficial as possible | As superficial as possible | |
| ≥12 mm: consider MC or PICC | ||||
| Other | Delineate surrounding structures (e.g., artery) | Delineate surrounding structures (e.g., artery, pleura) | Delineate surrounding structures (e.g., vein). | |
| Check bilateral lung sliding in internal jugular vein and/or axillary-subclavian vein cannulations. | ||||
| Procedure | Technique | Full sterile barrier technique. | ||
| Skin disinfection and local anesthesia infiltration before puncture. | ||||
| An out-of-plane or in-plane real-time approach is selected on a case- by -case basis. | ||||
| Catheter “tips” | Observe the catheter into the vessel and use the flush test. | Each time the Seldinger technique is used, the guidewire should be observed into the vessel prior to passing the dilator. | Observe the catheter into the artery lumen. | |
| For MC, assess catheter tip position in the thoracic tract of the axillary vein. | Assess catheter tip position with the rapid atrial swirl sign (RASS) using 10 ml of normal or agitated saline. | |||
| Each time the Seldinger technique is used, the guidewire should be observed into the vessel prior to passing the dilator. | ||||
| Posprocedure | Assess for thrombosis, inadvertent arterial cannulation, hematomas, infiltration. | Assess for thrombosis, hematomas, inadvertent arterial cannulation, infiltration. | Assess for thrombosis, inadvertent venous cannulation, hematomas, pseudoaneurysm, assess distal flow. | |
| Recheck lung sliding. If bilateral lung sliding is normal and RASS is observed, chest radiograph can be omitted. | ||||
The technique and corresponding ultrasound images of the most commonly cannulated vessels/intraosseous access are provided in the Supplementary Material, Appendix C.
PericardiocentesisPericardiocentesis is undoubtedly indicated in tamponade. In patients without hemodynamic compromise, it is indicated for symptomatic moderate-to-large effusion non-responsive to medical therapy, when tuberculous, bacterial, or neoplastic pericarditis is suspected, or in cases of chronic (lasting more than 3 months) large pericardial effusions (>20 mm on echocardiography in diastole).49
Blind pericardiocentesis carries morbidity and mortality risks of 20% and 6%, respectively. In contrast, ultrasound-guided pericardiocentesis has demonstrated a success rate of 97%, with a very low rate of complications, and is therefore recommended before, during, and after pericardiocentesis.49
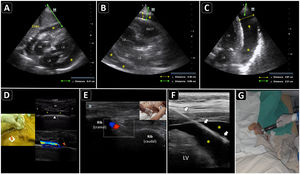
PreprocedureA convex or a phased-array probe is used to assess the view where the effusion accumulation is maximal and closest to the transducer. The thickness of the effusion is measured in diastole in each window, while the distance from the skin to the parietal pericardium and to the myocardium (visceral pericardium) should be obtained to estimate the needle depth of insertion and needle length (Fig. 2A–C). Qualitative assessment should also be performed. While anechoic fluid can be either a transudate or an exudate, the presence of debris or septations points towards the latter. Quantitative and qualitative assessments aid in decision making regarding whether pericardiocentesis, another procedure, or no procedure should be performed. Using a linear probe, the pleura, internal thoracic vessels, and intercostal vessels are delineated to exclude these structures from the needle trajectory (Fig. 2D, E.50
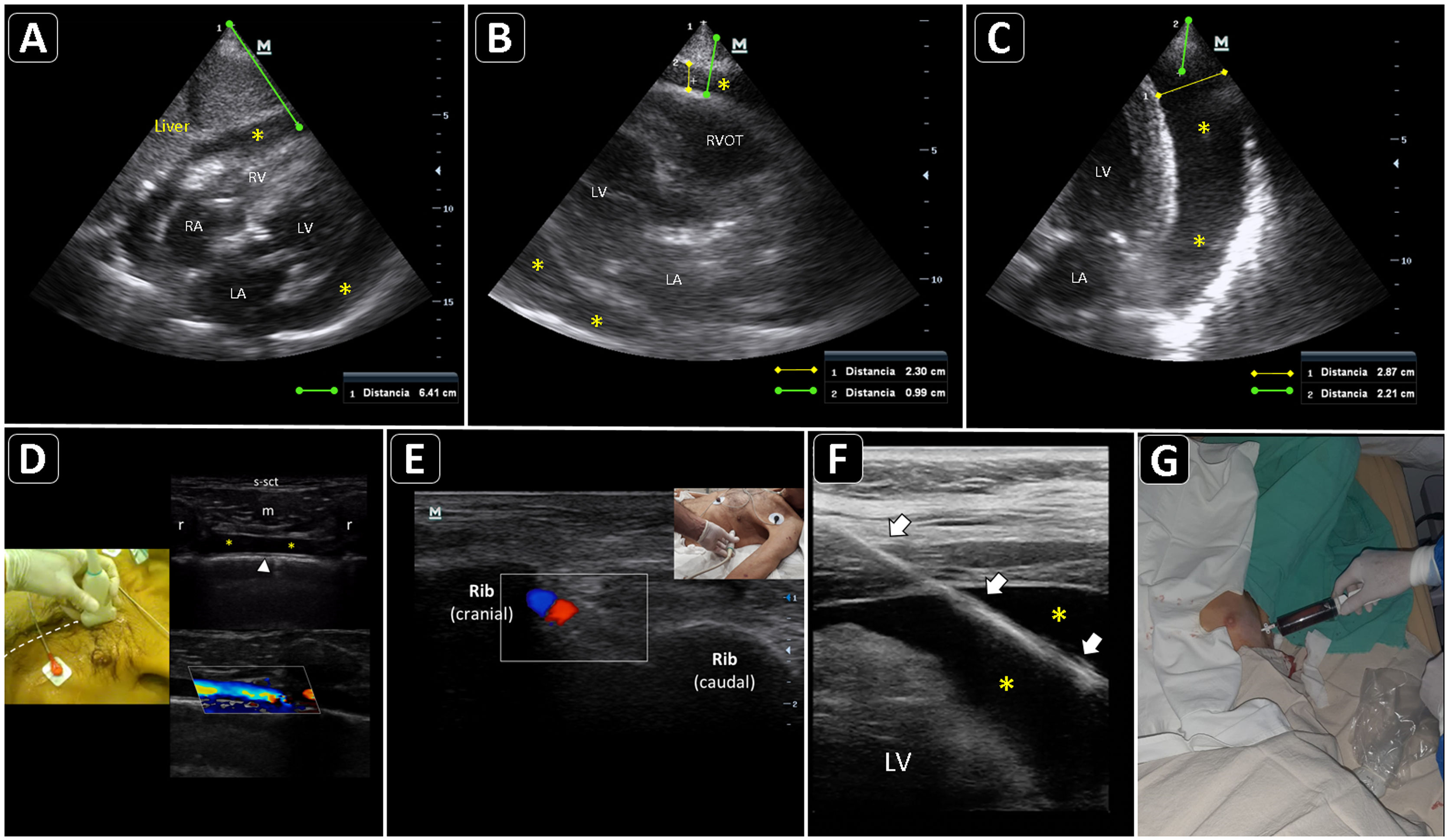
Ultrasound-guided pericardiocentesis. A) Subcostal 4 chamber view. B) Parasternal long-axis view. C) Apical view. As noted, the optimal window to insert the needle is the apical, given the shorter distance to reach the pericardial space (continuous green line) and higher pericardial fluid thickness (continuous yellow line). RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle; RVOT, right ventricular outflow tract. Asterisks indicate pericardial effusion. Adapted from Blanco P, Figueroa L, Menéndez MF, Berrueta B. Pericardiocentesis: ultrasound guidance is essential. Ultrasound J. 2022;14(1):9. https://theultrasoundjournal.springeropen.com/articles/10.1186/s13089-022-00259-5. (CC-BY-4.0). D) Recognition of the left internal thoracic vessels along the left parasternal line (dotted white line) with a linear probe on two-dimensional and color Doppler imaging; s-sct, skin-subcutaneous tissue; m, intercostal muscle; r, rib; arrowhead, pleura; asterisks, internal thoracic vessels. E) Recognition of the intercostal vessels with a linear probe on color Doppler imaging. Real-time in-plane ultrasound-guided pericardiocentesis via intercostal approach (apical view) using a linear probe. The needle (arrows) is entirely observed in the pericardial space (asterisks); LV: left ventricle. G) Hemorrhagic fluid is freely evacuated from the pericardial space after catheter placement.
There are two broad approaches to perform ultrasound-guided pericardiocentesis, which is a real-time procedure: intercostal (apical and left parasternal) and subcostal. The former is the preferred route, given that there is often a shorter distance for the needle to reach the pericardial cavity compared with the subcostal approach. However, it largely depends on the site of maximal fluid accumulation, which is usually non-uniform.49
A phased-array probe or convex probe is chosen to guide the procedure. Wherever possible, a linear probe aids in the best observation of the needle, particularly in intercostal pericardiocentesis (Fig. 2F). The entire intervention is performed under full sterile barrier precautions.
Pericardiocentesis is performed using a specific kit, or, eventually, in austere settings, a central venous cannulation kit.
After disinfecting and infiltrating the target site with local anesthetics, the needle is inserted in-plane into the pericardial cavity; after aspiration a few milliliters of pericardial fluid, 5 ml of agitated saline are injected through the needle. The immediate appearance of echo contrast in the pericardial space confirms that the needle is in the pericardial cavity and rules out a cardiac chamber perforation51(Video 3). The guidewire is then passed and observed in the pericardial space, and, after dilation, the catheter is finally accommodated (Fig. 2G) and connected to a three-way stopcock and a drainage system.
PosprocedureRechecking lung sliding is mandatory when using an intercostal approach. If catheter drainage function is optimal, serial ultrasound assessments will show a reduction in the amount of pericardial fluid. When using the subcostal approach, the liver should be observed for a hematoma, and the presence of free peritoneal fluid should also be assessed.
Table 3 summarizes the steps involved in ultrasound-guided pericardiocentesis.
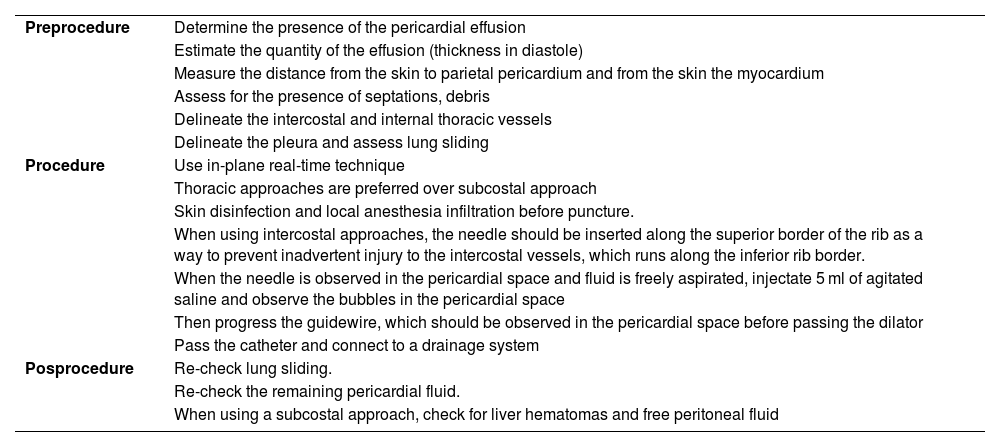
Steps for performing ultrasound-guided pericardiocentesis.49–51
| Preprocedure | Determine the presence of the pericardial effusion |
| Estimate the quantity of the effusion (thickness in diastole) | |
| Measure the distance from the skin to parietal pericardium and from the skin the myocardium | |
| Assess for the presence of septations, debris | |
| Delineate the intercostal and internal thoracic vessels | |
| Delineate the pleura and assess lung sliding | |
| Procedure | Use in-plane real-time technique |
| Thoracic approaches are preferred over subcostal approach | |
| Skin disinfection and local anesthesia infiltration before puncture. | |
| When using intercostal approaches, the needle should be inserted along the superior border of the rib as a way to prevent inadvertent injury to the intercostal vessels, which runs along the inferior rib border. | |
| When the needle is observed in the pericardial space and fluid is freely aspirated, injectate 5 ml of agitated saline and observe the bubbles in the pericardial space | |
| Then progress the guidewire, which should be observed in the pericardial space before passing the dilator | |
| Pass the catheter and connect to a drainage system | |
| Posprocedure | Re-check lung sliding. |
| Re-check the remaining pericardial fluid. | |
| When using a subcostal approach, check for liver hematomas and free peritoneal fluid |
Pleural effusions are common in critically ill patients. Although most effusions are of limited clinical significance, aggressive management is required in some cases. These are broadly classified as transudates or exudates. The causes of transudative effusions include volume overload, hypoalbuminemia, and regions of altered pleural pressure attributable to atelectasis and mechanical ventilation. Exudates may be caused by pulmonary or pleural infections, pulmonary embolism, postsurgical complications, and malignancy. Increases in pleural fluid volume are accommodated principally by chest wall expansion and, to a lesser degree, by lung collapse.52 When indicated, diagnostic thoracocentesis is key to distinguishing transudative effusions from exudative effusions. In addition, when large, pleural effusions may compromise respiratory function and patients may benefit from drainage.
A large body of evidence shows that when compared with the landmark technique, ultrasound-guided thoracentesis improves the success rate, decreases costs, reduces the rate of pneumothorax by 19%, and minimizes the occurrence of hemothorax.53,54
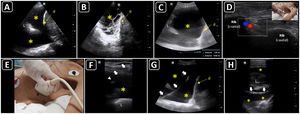
PreprocedureThe patient should lie supine in a slight semi-recumbent position. With a convex or phased-array probe, it is of paramount relevance to estimate the size of the pleural effusion to define whether thoracentesis is needed or safe, another procedure, or no procedure should be performed. While often eyeballed, a drainable pleural effusion may be defined when the distance between the visceral and parietal pleura is ≥10 mm.55 In addition, the ultrasonographic characteristics of the effusion should also be assessed based on the presence of septations, swirling debris (i.e., plankton sign) or pleural thickening. While anechoic effusions can be either transudative or exudative, those showing one or more of the aforementioned signs are often exudative and should prompt pleural fluid sampling (Fig. 3A and B).53 The distance from the skin to the parietal and visceral pleura should be measured using ultrasound to aid in the selection of an adequate needle length and estimate the depth of insertion (Fig. 3C). The diaphragm must also be delineated. Chest wall vessels should be excluded from the needle trajectory, which is assessed with a linear probe using color Doppler (Fig. 3D).56 Bilateral lung sliding should also be determined prior to the procedure.
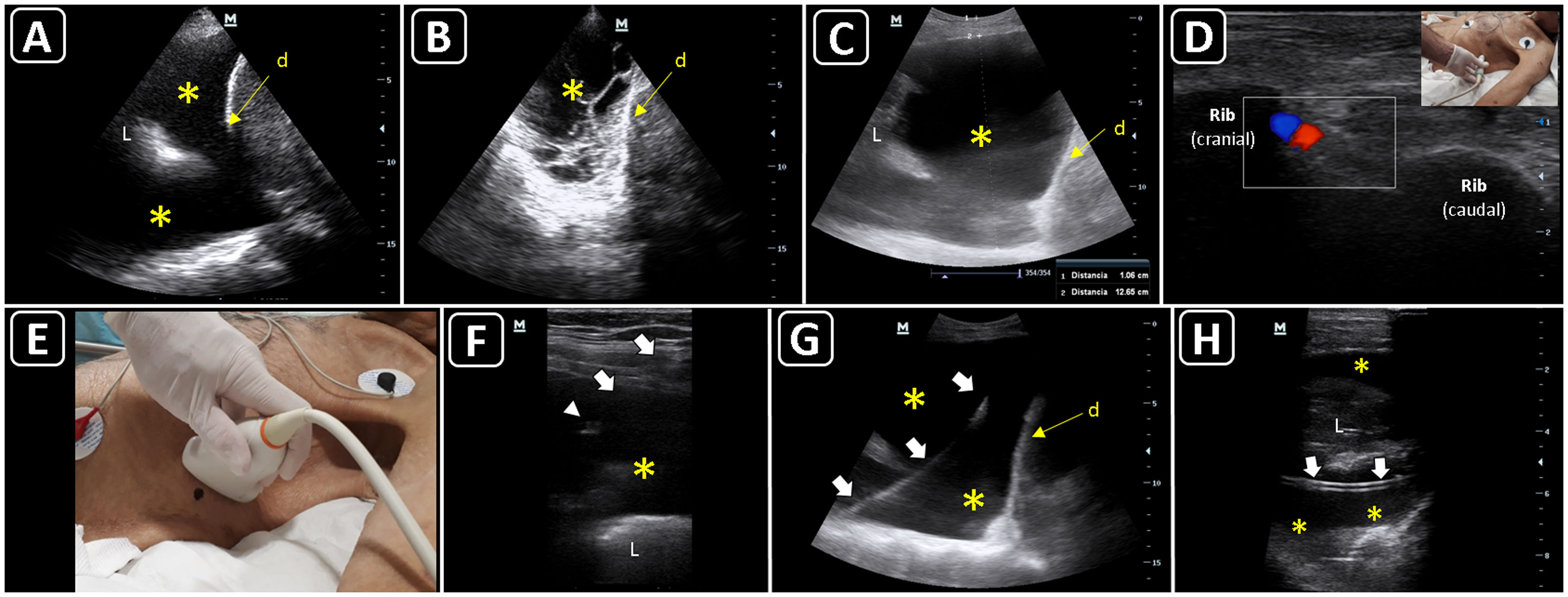
Ultrasound-guided thoracentesis. A) A simple pleural effusion (asterisks), diaphragm (d) and lung (L) are delineated using ultrasound. B) A complex pleural effusion (asterisk) is observed; d, diaphragm. C) The best fluid pocket for thoracentesis is selected by measuring the distance from the skin to the pleural effusion (asterisk) and the effusion depth; L, lung; d, diaphragm. D) The intercostal vessels are delineated before cannulation using a linear transducer and color Doppler. E) The insertion site is marked on the skin. F) Dynamic ultrasound guidance for thoracentesis. Arrows, needle shaft; arrowhead, needle tip; asterisk, pleural effusion; L, lung. G) The guidewire (arrows) is observed within the pleural effusion (asterisks); d, diaphram. H). A central catheter (arrows) is observed within the pleural effusion (asterisks); L, lung.
The procedure can be performed under static or real-time ultrasound guidance. Although both techniques have proven to be useful, the static technique is preferable. The added effort and complexity of the real-time technique is rarely justified, as moderate or large effusions are easy to access, provided the pre-procedure scan is made correctly.56 Real-time guidance is best reserved for small or loculated effusions, where strict control of needle trajectory and depth is mandatory.56
With the static technique, and with the patient lying in the same position as the preprocedural scan, the best insertion site is selected with ultrasound and is marked with a pen on the skin (Fig. 3E). After disinfecting and infiltrating the target site with local anesthetics, the needle is advanced until pleural fluid is obtained. The needle should be inserted along the superior border of the rib to prevent inadvertent injury to the intercostal vessels that run along the inferior rib border.
In the dynamic technique, the needle is inserted in-plane and observed in real-time, entering the pleural space when pleural fluid is aspirated (Fig. 3F). A fully sterile technique is required to do so.
When evacuation of the effusion is needed, a pigtail catheter is typically inserted.57 In patients with non-complicated pleural effusions, pleural drainage using central venous catheters has proven useful and safe.58 Each time the Seldinger technique is used, the guidewire should be observed in the pleural space before passing the dilator (Fig. 3G). Then, the catheter is advanced and the guidewire is removed. Intrapleural placement of the catheter can also be confirmed by ultrasonography, following its course from the superficial soft tissues to the pleural cavity (Fig. 3H). Finally, the catheter is connected to a drainage system, which could be a water-seal chest drainage or a urinary bag collection system,58 interposing a Heimlich valve.
PosprocedureLung ultrasound is performed to rule out pneumothorax. A comparison with preprocedural scanning is helpful. Among the several well know ultrasonographic signs of pneumothorax, the hydro-point sign should also be actively investigated.59 The hydro-point defines the presence of hydropneumothorax and is best assessed with a convex or phased-array probe in basal lateral or posterolateral views of the thorax.60,61 This sign is depicted as contact between the pleural fluid and the artifacts of the intrapleural air, which does not show lung sliding61(Video 4). Chest radiography may be safely omitted in patients in whom a successful thoracentesis is performed and no ultrasonographic signs of pneumothorax are detected.
Table 4 summarizes the steps involved in ultrasound-guided thoracentesis.
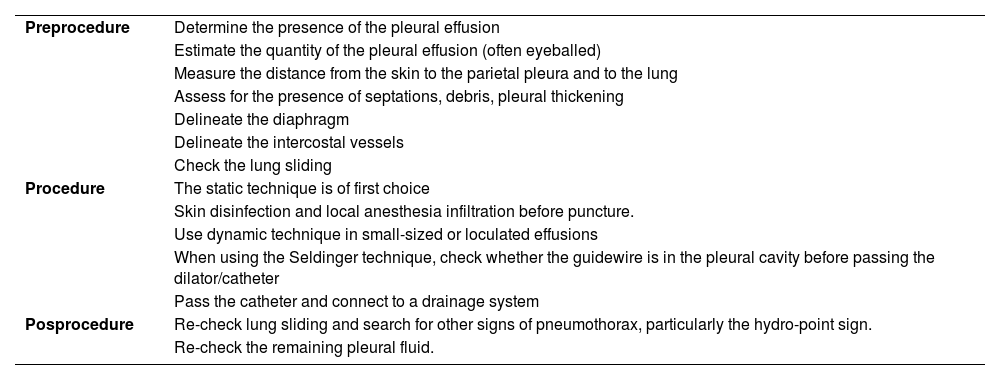
Steps for performing ultrasound-guided thoracentesis.53,55,56,57–61
| Preprocedure | Determine the presence of the pleural effusion |
| Estimate the quantity of the pleural effusion (often eyeballed) | |
| Measure the distance from the skin to the parietal pleura and to the lung | |
| Assess for the presence of septations, debris, pleural thickening | |
| Delineate the diaphragm | |
| Delineate the intercostal vessels | |
| Check the lung sliding | |
| Procedure | The static technique is of first choice |
| Skin disinfection and local anesthesia infiltration before puncture. | |
| Use dynamic technique in small-sized or loculated effusions | |
| When using the Seldinger technique, check whether the guidewire is in the pleural cavity before passing the dilator/catheter | |
| Pass the catheter and connect to a drainage system | |
| Posprocedure | Re-check lung sliding and search for other signs of pneumothorax, particularly the hydro-point sign. |
| Re-check the remaining pleural fluid. |
Free peritoneal fluid (i.e., ascites) is frequent in critical care patients, with etiologies ranging from fluid overload, cardiac or hepatic failure, peritonitis, etc. Several times, there is a need for fluid investigation (transudate vs. exudate) and also fluid evacuation to improve respiratory function and reduce intra-abdominal pressure. The most common serious complication of paracentesis is bleeding (abdominal wall hematoma and hemoperitoneum) through laceration of the abdominal wall vessels (e.g., inferior epigastric arteries or its branches), although puncture of the bowel and other abdominal organs has also been observed. Compared with the landmark technique, ultrasound guidance reduced the risk of bleeding by 68%,54 while improving the success rate to 95%–100%, as opposed to 65% for the landmark technique.62
PreprocedureUsing a convex or phased-array probe, POCUS aids in detecting the presence, volume, and characteristics of the peritoneal free fluid (anechoic fluid can be either transudative or exudative, while the presence of septations or debris indicates an exudative effusion),63 and determines whether a diagnostic or therapeutic paracentesis is needed or safe, another procedure, or no procedure should be performed.
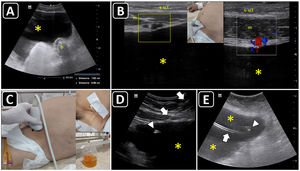
The maximal site of fluid accumulation is often eyeballed by ultrasonography (although the smallest fluid depth can be used to quantify the effusion), and the distance from the skin to the parietal peritoneum and visceral peritoneum is measured to aid in the selection of the needle length and estimate the depth of insertion (Fig. 4A).63
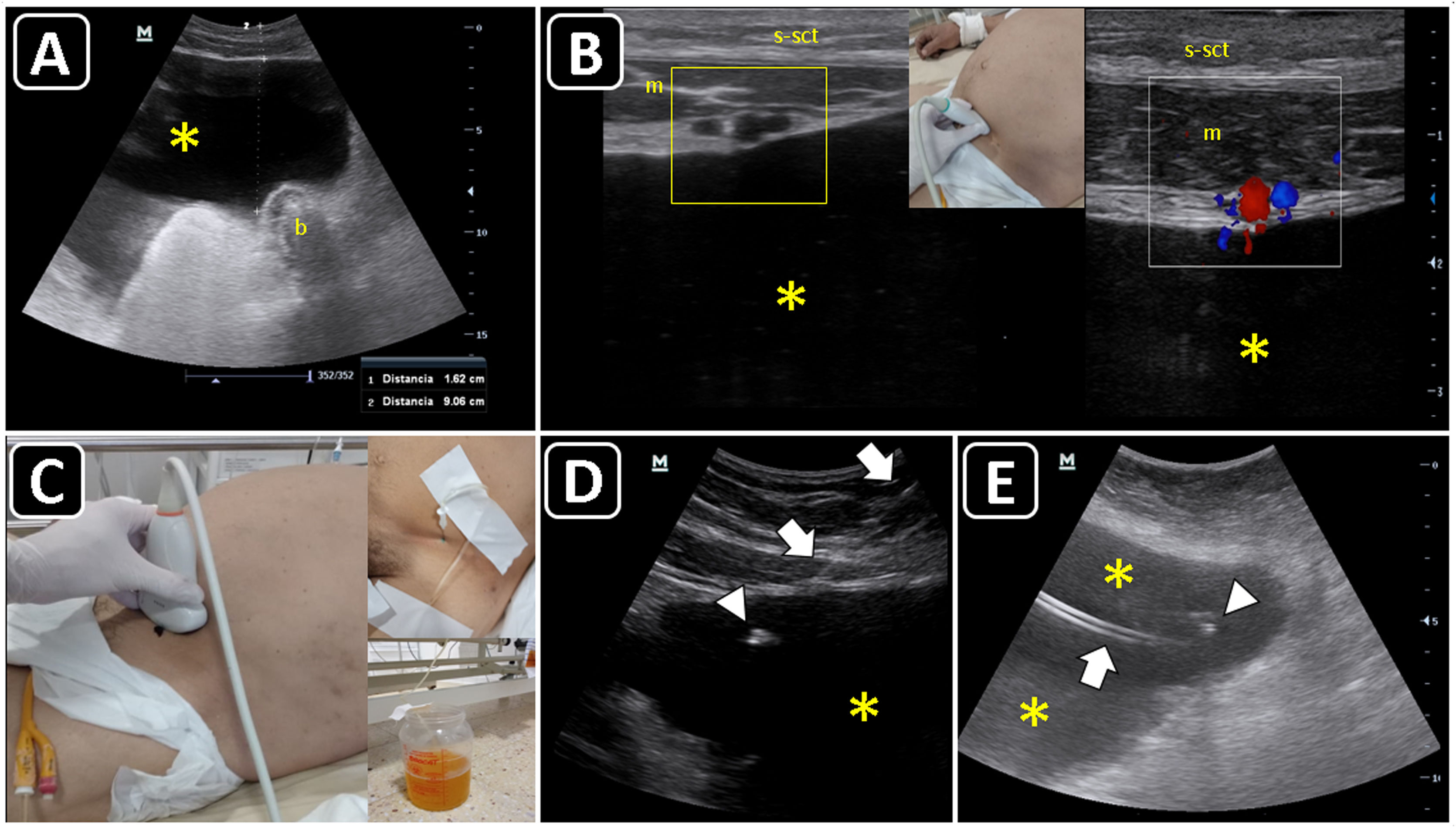
Ultrasound-guided paracentesis. A) Ascites is observed (asterisk), and the distance from the skin to the effusion and to a bowel loop (b) is obtained. B) The inferior epigastric vessels (boxes) are delineated into the abdominis rectus sheath using a linear probe on two-dimensional and color Doppler imaging; s, skin; sct, subcutaneous tissue; m, rectus abdominis muscle; asterisk, ascites. C) The insertion site is marked on the skin, and puncture is performed under static guidance. D) Real-time ultrasound-guided paracentesis; arrows, needle shaft; arrowhead, needle tip; asterisk, ascites. E) A locking pigtail catheter is observed within the ascites (asterisks). The arrow indicates the body, whereas the arrowhead indicates the tip of the pigtail.
Using a linear probe, the abdominal wall vessels should also be identified and excluded from the needle trajectory (Fig. 4B).
The patient should lie in a slightly semi-recumbent position.
ProcedureEither static or real-time technique can be used. Similar to ultrasonound-guided thoracentesis, the static technique is preferred. The insertion site should be marked immediately before performing the procedure (Fig. 4C), and the patient should remain in the same position between marking the site and performing the procedure, given that free-flowing peritoneal fluid and abdominal organs, especially loops of the small bowel, can easily shift when a patient changes position or takes a deep breath.62
Real-time ultrasound-guided paracentesis (in-plane or out-of-plane) (Fig. 4D) is best suited for obese patients, for patients with small fluid collections, or when performing the procedure near critical structures, such as loops of the small bowel, liver, or spleen.62
When there is a need to evacuate fluid, a pigtail catheter is preferred, although a central catheter can be used in selected cases.64 The catheter position could also be confirmed using ultrasound (Fig. 4E).
PosprocedureThe amount of remaining peritoneal fluid should be assessed. The presence of an abdominal wall hematoma can also be delineated using ultrasound.
Table 5 summarizes the steps involved in ultrasound-guided paracentesis.
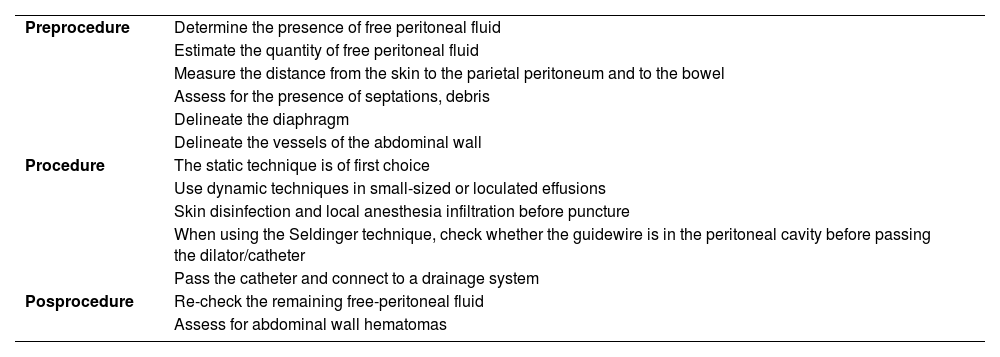
Steps for performing ultrasound-guided paracentesis.62,63
| Preprocedure | Determine the presence of free peritoneal fluid |
| Estimate the quantity of free peritoneal fluid | |
| Measure the distance from the skin to the parietal peritoneum and to the bowel | |
| Assess for the presence of septations, debris | |
| Delineate the diaphragm | |
| Delineate the vessels of the abdominal wall | |
| Procedure | The static technique is of first choice |
| Use dynamic techniques in small-sized or loculated effusions | |
| Skin disinfection and local anesthesia infiltration before puncture | |
| When using the Seldinger technique, check whether the guidewire is in the peritoneal cavity before passing the dilator/catheter | |
| Pass the catheter and connect to a drainage system | |
| Posprocedure | Re-check the remaining free-peritoneal fluid |
| Assess for abdominal wall hematomas |
Ultrasound has demonstrated high accuracy in differentiating superficial abscesses from cellulitis, with a sensitivity of 96.2%, specificity of 82.9%, positive likelihood ratio of 5.63, and negative likelihood ratio of 0.05.65 Cellulitis is observed as a cobblestoning pattern of the subcutaneous tissue, whereas an abscess is a heterogeneous fluid collection.66 Differentiating an abscess from other fluid collections (e.g., hematoma) is challenging; ultrasound-guided diagnostic needle aspiration may be useful in such cases.66 Furthermore, aspiration of small abscesses (<4 cm) may avoid incision and drainage (I&D) in some cases.67 However, aspiration of abscess with septations or satellite lesions may not be feasible despite the use of ultrasound guidance, as reported for Methicillin-resistant Staphylococcus aureus infections.68 In other cases, ultrasound aids in the best definition of the site for I&D compared with physical examination alone.69
Arthrocentesis is mainly indicated when septic arthritis (SA) is highly suspected. The incidence of SA is 4–21 per 100,000 person/years.70 Large and small joints can be involved; however, the knee is most frequently affected (50% of cases). Monoarticular involvement is the most common presentation; however, up to 20% of cases may have concurrent infection in several joints.70 Ultrasound-guided knee arthrocentesis provides greater accuracy and clinical improvement than the landmark technique.71 Other joint effusions can also be best delineated and aspirated with ultrasound guidance when compared with landmarks such as the hip, elbow, wrist, or ankles.72–74
PreprocedureWith a linear probe (or a convex probe in obese or edematous patients), ultrasound aids in differentiating subcutaneous tissue edema, soft-tissue fluid collections (e.g., abscesses), periarticular disease (e.g., bursitis), or an intra-articular effusion.
In transverse and longitudinal views, soft-tissue fluid collections are measured, as well as the distance to the skin, to estimate the needle depth of insertion and to choose an adequate needle length (Fig. 5A,B). Color Doppler aids in defining the presence of vascular structures near the lesion, to avoid puncturing when inserting the needle.
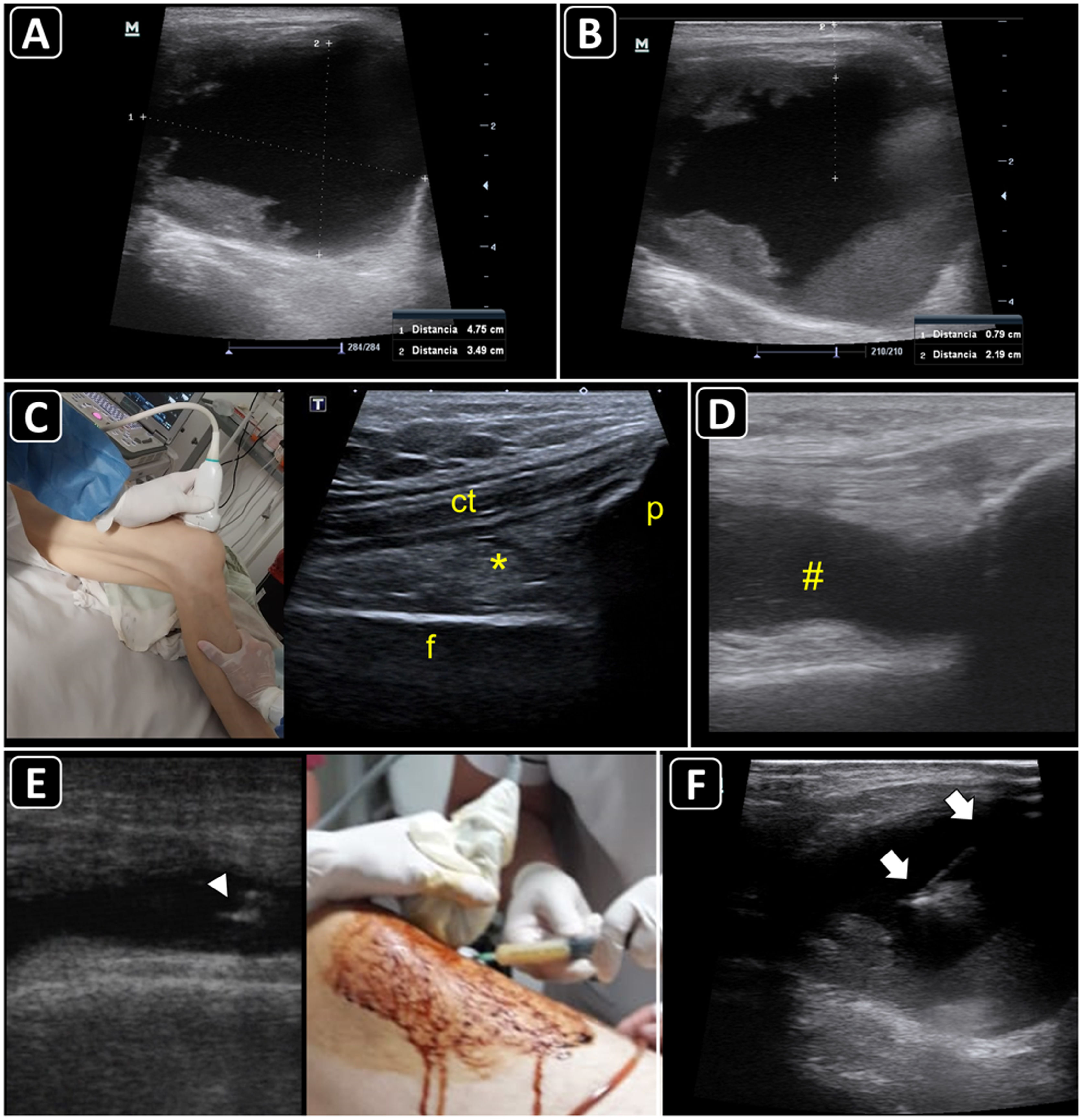
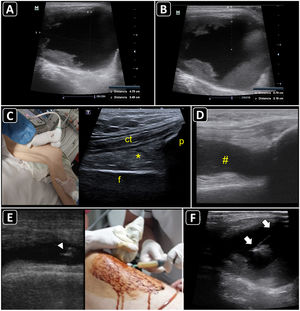
Ultrasound-guided aspiration of soft tissue collections and arthrocentesis. A) Soft tissue fluid collection is identified and measured. B) Distances are measured to estimate the needle depth of insertion and length to be used. C) Technique for finding the suprapatellar articular recess of the knee and its corresponding ultrasound images; p, patella; t, cuadriceps tendon; f, femur; asterisk, fat pad (articular recess). D) Knee effusion is indicated by the symbol #. E) Real-time ultrasound-guided arthrocentesis of the knee, obtaining a purulent fluid. The arrowhead indicates the bevel of the needle that reached the articular effusion. F) Real-time ultrasound-guided aspiration of soft tissue collection. The needle is indicated by arrows.
Ultrasound anatomy of each joint should be well known for assessing effusion. Given its frequency and ease of access, knee ultrasound is reviewed herein. With the linear probe placed longitudinally above the patella and the probe marker pointing cephalad, the superior portion of the patella, quadriceps femoris tendon (long axis), fat pad, and femur should be observed (Fig. 5C). The effusion will be located below the quadriceps tendon and above the femur (Fig. 5D). Once fluid effusion is recognized, the probe is rotated 90 degrees into a transverse view so that the probe marker faces to the patient's right side. The distance from the skin to the effusion is then measured to estimate the needle depth of insertion and length to be used.
ProcedureReal-time technique is used to aspirate soft-tissue collections and perform arthrocentesis. The out-of-plane and in-plane approaches are selected on a case-by-case basis. For example, aspiration of abscess and knee arthrocentesis are best performed using the in-plane approach (Fig. 5E and 5F), whereas the out-of-plane approach is preferred for aspiration of effusion from the wrist joint. A fully sterile barrier technique is required to do so.
PosprocedureInvestigation of post-procedural soft-tissue hematomas should be ruled in or out, as well as reassessing the joint effusion size and the presence of hemarthrosis, comparing with pre-procedural evaluation.
The technique for ultrasound-guided arthrocentesis is provided in the Supplementary Material, Appendix D.
Lumbar punctureLumbar puncture (LP) is often performed in critical care patients to rule in or out meningitis, Guillain-Barre syndrome, and in selected cases for providing spinal or epidural anesthesia. Although many practitioners successfully perform the procedure using anatomical landmarks, the failure rate reaches up to 19%.75 Ultrasound guidance has demonstrated to significantly reduce the risk of traumatic taps, number of insertion attempts, and number of needle redirections,76 with the greatest benefits demonstrated in patients with poorly palpable landmarks, as in obesity, and in patients with scoliosis, previous spinal surgery, or a history of difficult LP.77
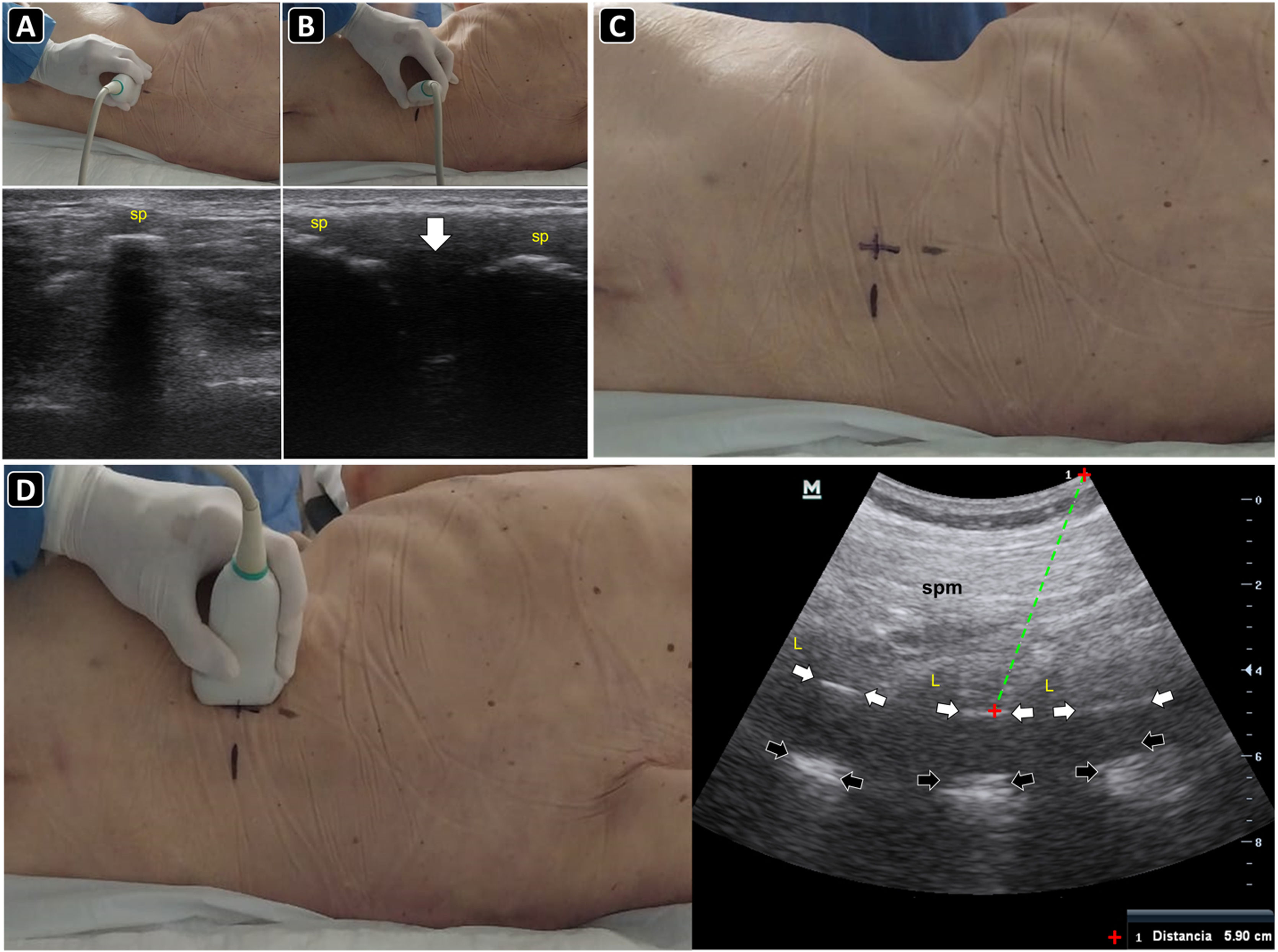
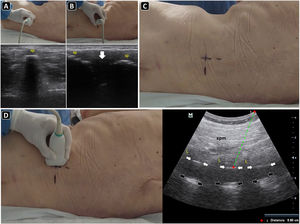
PreprocedureWith the sitting patient or in a lateral decubitus (the latter often in critical care patients), a convex probe is placed in the transverse plane (perpendicular to the long axis of the spine) in the midline of the lower back. Here, the spinal processes (SPs) are recognized as superficial hyperechoic peaked structures with posterior acoustic shadowing (Fig. 6A), while the laminae or articular processes (“bat sign”) are seen as hyperechoic structures deep and lateral to the SPs. The hypoechoic interspinal spaces can be delineated by moving the transducer cephalad or caudally to the SPs. With the SPs centered on the ultrasound screen, the skin is marked with a pen at the transducer midpoint over several vertebrae (Fig. 6A).
Ultrasound-guided lumbar puncture. A) With the linear transducer placed transversally over the midline of the lower back, the spinal processes (sp) are recognized and the skin marked with a pen. B) After rotating the transducer 90 degrees clockwise and placing it longitudinally over the midline of the lower back, the spinal processes (sp) are seen, and the interspinal space (arrow) is delineated, and the skin is marked with a pen. C) Intersecting the skin markings obtained in A) and B), the needle insertion site is marked with a pen. D) Parasagittal approach and the corresponding ultrasound image. To obtain this view, the transducer is slightly basculated to one side from the longitudinal midline view. In the ultrasound image, the spinal muscles (spm), laminae (L), posterior complex (white arrows), and posterior longitudinal ligament (black arrows) are well recognized. The distance to the posterior complex is measured (dotted green line), to approximate the needle depth of insertion during the lumbar puncture.
The transducer is then placed longitudinally (parallel to the long axis of the spine). Again, the SPs are observed as bright peaked structures with posterior acoustic shadowing; between them, there are the hypoechoic interspinal spaces (Fig. 6B). To recognize the sacrum, the transducer is moved caudally until a roughly hyperechoic line is observed. With the interspinal space centered on the ultrasound screen, the skin is marked with a pen at the transducer midpoint over several vertebrae.
After marking the skin in the transverse and longitudinal planes, both lines are continued until they intersect the midline, where the puncture will be performed over the broad spinal space (Fig. 6C).
Slightly angling the transducer to the midline, the paramedian longitudinal approach is obtained (Fig. 6D). Here, the pedicles and posterior complex (including the ligamentum flavum, epidural space, and posterior dura) are easily recognized (Fig. 6D) Measuring the distance from the skin to the posterior complex aids in estimating the needle depth of insertion and length to be used.(Fig. 6D)
ProcedureThe procedure can be performed with the patient sitting (preferably) or lying in a lateral decubitus position.
The use of the static technique is largely supported in the literature; however, real-time technique have shown comparable or even better outcomes.78
The static procedure technique is performed following the mark on the skin, advancing the needle until a loss of resistance is felt and the cerebrospinal fluid (CSF) starts coming out.
For the real-time technique, the LP is performed using the paramedian approach. The needle is inserted in-plane, until it reaches and passes through the posterior complex when a loss of resistance is felt, and CSF fluid is obtained.
PosprocedurePost-procedural evaluation is mainly clinical, such as the presence of a visible hematoma on the skin, postdural puncture headache, back pain, and eventually spinal hematoma, which is rare but devastating complication of LP. While there are publications in pediatrics for recognizing epidural hematomas using ultrasound,79 there are no data on the adult population in whom magnetic resonance imaging is typically indicated.
Table 6 summarizes the steps involved in ultrasound-guided lumbar puncture.
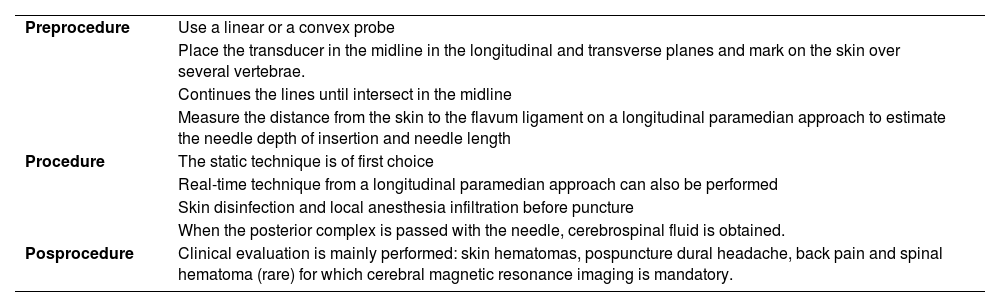
Steps for performing ultrasound-guided lumbar puncture.75,76
| Preprocedure | Use a linear or a convex probe |
| Place the transducer in the midline in the longitudinal and transverse planes and mark on the skin over several vertebrae. | |
| Continues the lines until intersect in the midline | |
| Measure the distance from the skin to the flavum ligament on a longitudinal paramedian approach to estimate the needle depth of insertion and needle length | |
| Procedure | The static technique is of first choice |
| Real-time technique from a longitudinal paramedian approach can also be performed | |
| Skin disinfection and local anesthesia infiltration before puncture | |
| When the posterior complex is passed with the needle, cerebrospinal fluid is obtained. | |
| Posprocedure | Clinical evaluation is mainly performed: skin hematomas, pospuncture dural headache, back pain and spinal hematoma (rare) for which cerebral magnetic resonance imaging is mandatory. |
Newer ultrasound technologies may be applied for procedural guidance, such as biplane imaging (also known as Xplane®), which simultaneously shows two-orthogonal planes. Matrix array transducers are required for this purpose, although they are typically not available in the ultrasound machines of the ICU. Anecdotic evidence using a miniaturized device with biplane imaging has shown its usefulness for vascular cannulation.80
ConclusionsUltrasound guidance is key to guaranteeing the success and safety of several procedures performed in the ICU. With time, the procedures performed by intensivists may extend beyond the core competencies depicted in this review.
FundingThis work has not been supported by any grants.
Conflicts of interestAuthors have no conflicts of interest to disclose.
This work has not been presented at any conferences.
Authors’ contributionsAnselmo Abdo-Cuza (AAC) revised the manuscript for important intellectual content and provided raw material for creating the figures and editing the videos. AAC read and approved the final manuscript.
Cristina Martínez Díaz (CMD) revised the manuscript for important intellectual content and provided raw material for creating the figures and editing the videos. CMD read and approved the final manuscript.
Elena Abril Palomares (EAP) revised the manuscript for important intellectual content and provided raw material for creating the figures and editing the videos. EAP read and approved the final manuscript.
Pablo Blanco (PB) performed the literature review, wrote the drafts, coordinated the input of all authors, created figures/edited the videos, and revised the manuscript and appendices for important intellectual content. PB read and approved the final manuscript.
Virginia Fraile Gutiérrez (VFG) revised the manuscript for important intellectual content and provided raw material for creating the figures and editing the videos. VFG read and approved the final manuscript.
The following are Supplementary data to this article:
The flush test, confirming the intravascular position of a peripheral venous catheter. Reproduced from Blanco P. Ultrasound-guided peripheral venous cannulation in critically ill patients: a practical guideline. Ultrasound J. 2019 Oct 17;11(1):27. https://theultrasoundjournal.springeropen.com/articles/10.1186/s13089-019-0144-5 (CC-BY-4.0).