To evaluate variability in the detection and prevention of acute kidney injury (AKI) in the intensive care unit (ICU), and application of the international recommendations in this field (Acute Dialysis Quality Initiative [ADQI] and Acute Kidney Injury Network [AKIN]).
DesignA prospective, observational, multicenter study.
SettingA total of 42 ICUs in 32 hospitals (78% in third level hospitals and 70.7% general units) recruited for a study on the prevalence of AKI (COFRADE).
InterventionsSurvey.
VariablesAspects related to AKI detection and prevention and renal replacement therapy (RRT) protocols.
ResultsThe method used for estimating glomerular filtration rate was serum creatinine (Crs) in 36.6%, creatinine clearance in 41.5% and equations in 22%; none reported using cystatin-C. Only 39.1% ICUs acknowledged the use of stratification systems (13 RIFLE and 3 AKIN).
A total of 48.8% ICUs had no written protocols for AKI prevention, 31.7% reported using them only for contrast nephropathy, 7.3% for nephrotoxic drugs and 12.2% for both.
In contrast, 63.4% participants had written protocols for RRT, 70.7% had implemented a training program, and 53.7% had some method for adjusting doses of drugs when on RRT.
ConclusionsWe observed an important variability regarding diagnostic criteria and prevention of AKI in Spanish ICUs, the application of ADQI or AKIN recommendations still being low in our units. RRT seems to generate more concern among our intensivists than AKI management.
Evaluar la variabilidad en la detección y prevención de la disfunción renal aguda (DRA) en las unidades de cuidados intensivos (UCI), así como la aplicación de recomendaciones internacionales en este campo (Acute Dialysis Quality Initiative [ADQI] y Acute Kidney Injury Network [AKIN]).
DiseñoEstudio multicéntrico prospectivo descriptivo.
ÁmbitoUn total de 42 UCI (70,7% generales) en 32 hospitales (78% de tercer nivel), participantes en un estudio de prevalencia de DRA (COFRADE).
IntervencionesEncuesta.
Variables de interésMétodos de detección y prevención de DRA y existencia de protocolos para depuración extrarrenal (TDE).
ResultadosPara la estimación del filtrado glomerular se usa la creatinina sérica en el 36,6%, el aclaramiento de creatinina en el 41,5%, ecuaciones basadas en una muestra de creatinina sérica en 22% y creatinina sérica aislada en el resto; en ningún caso se utiliza la cistatina-C.
Solo el 39,1% de las UCI aplica sistemas de estratificación (13 RIFLE y 3 AKI). El 48,8% no cuenta con protocolos de prevención de DRA, el 31,7% los tiene para nefropatía por contraste, el 7,3% para manejo de nefrotóxicos y el 12,2% para ambos.
Por contra, el 63,4% de las unidades cuenta con protocolos de manejo de TDE, el 70,7% aplica programas de formación continuada para estas y el 53,7% cuenta con protocolos de ajuste de dosis de fármacos en estos casos.
ConclusionesExiste amplia variabilidad en cuanto al método de detección de la DRA en nuestras unidades, con escasa aplicación de sistemas de estratificación de daño. El manejo de las TDE despierta más inquietud que la prevención o el diagnóstico de la DRA.
The development of AKI in critical patients produces an increase in morbidity–mortality and in the costs of associated treatment.1–4 According to the FRAMI study,4 the incidence in our setting was 5.7% ten years ago—this figure being very similar to the incidence reported by the BEST survey,1 conducted at the time of the FRAMI study4 and likewise involving the use of a Crs cutoff value of 2mg/dl for defining AKI.
At that time the differences in diagnostic thresholds were considerable, and this lack of consensus explains why the incidences reported in different studies varied greatly from 5 to 25%, approximately.1,4,5
At present, and thanks to the recommendations of the ADQI6 and posteriorly the AKIN,7 the panorama has changed, and the detection of AKI is now based on standardized criteria—with the adoption of a common language that is facilitating access to uniform and easily reproducible information, but which 10 years later obliges us to reconsider the true impact of AKI in our setting and to reassess the results obtained from the FRAMI survey.4 To this effect, we have started a study (COFRADE) designed to determine the prevalence of AKI in our ICUs, based on current diagnostic criteria. In this context, and in a first phase, we have wished to define the current situation regarding AKI diagnostic and preventive methods, as well as the standardization of RRT in the different Units participating in the study.
Our aim is to present the results of this initial survey and thus offer a perspective of the current situation found in our ICUs regarding the diagnosis and management of AKI.
Materials and methodsThe present work represents the initial phase of a prospective observational study on the prevalence of renal dysfunction in the ICU (the COFRADE study), designed to analyze the prevalence of AKI and the application of RRT. This study comprises two phases: (a) a first phase in which the participating Units were asked to complete a questionnaire designed to define the diagnostic habits and preventive measures applied in each Unit; and (b) a second phase in which an AKI prevalence study is planned.
The study was made to evaluate application in our Units of the current criteria for the detection of renal dysfunction (stratification using the RIFLE6 and AKIN7 systems) and the use of protocols designed to detect or prevent AKI. The investigator in charge in each of the participating Units was requested to supply information on the existence of defined AKI prevention protocols (referred to toxic agents or contrast media), the method used to estimate renal function, the application of AKI stratification systems, and the existence of protocols referred to RRT management, drug adjustment, or continued personnel training in the use of the techniques.
The study was approved by the Clinical Research Ethics Committees of each of the participating centers.
The data are presented as values (percentages), and comparisons were made using the chi-squared test, with a significance level of 95% (p<0.05).
ResultsA total of 32 hospitals participated in the COFRADE study: 10 in the Community of Madrid, 9 in Andalusia, three in Catalonia and the Valencian Community, and one in the Canary Islands, Castilla La Mancha, Castilla-León, the Community of Murcia, Extremadura, Galicia and the Basque Country. Twenty-three of these hospitals were third-level centers.
A total of 42 ICUs were involved, with a total of 826 beds. Of these ICUs, 30 were general Units, three medical Units, three surgical Units, and 6 specialized Units.
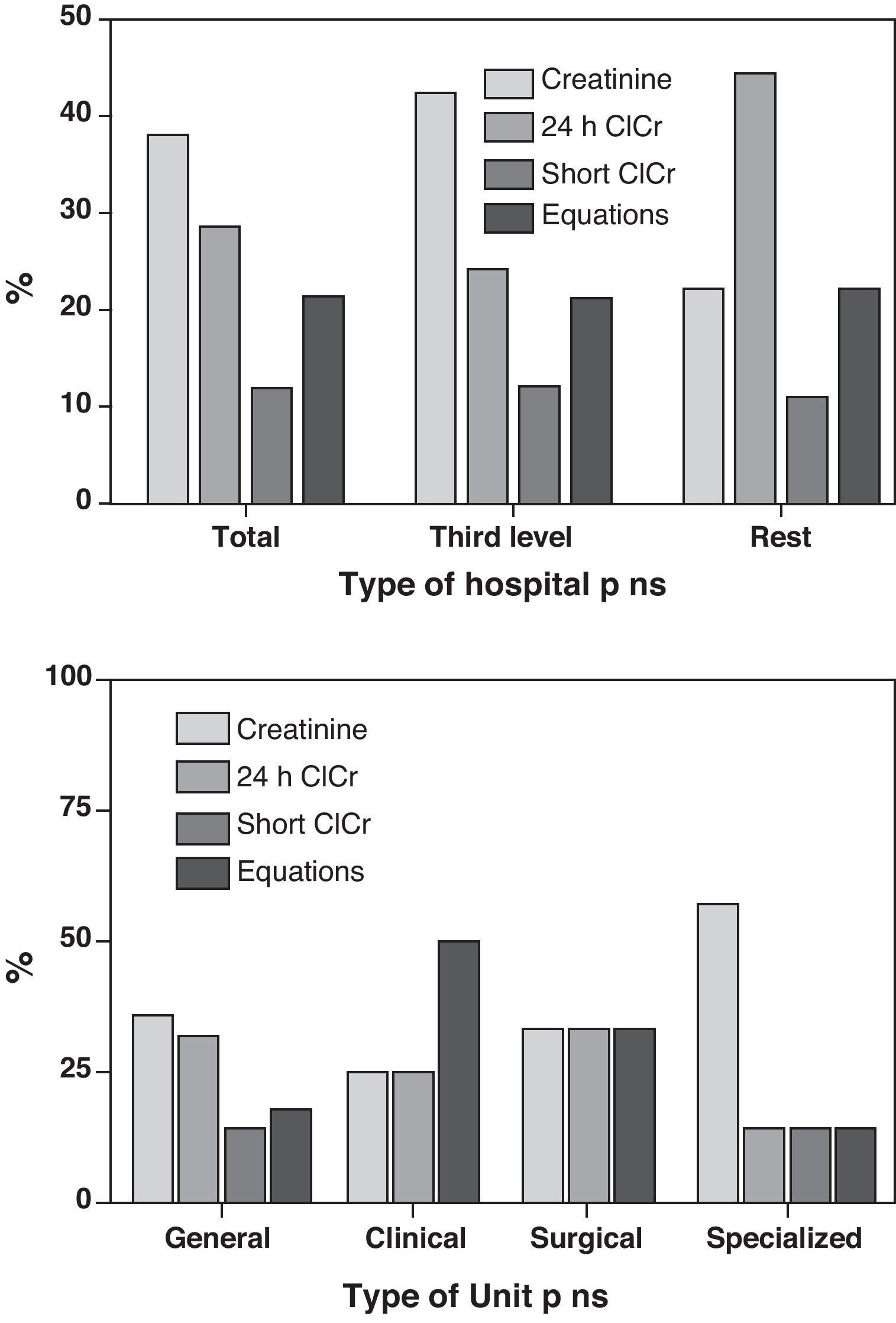
In relation to the estimation of glomerular filtration, 24-h urine creatinine clearance was applied in 12 Units (28.6%), and short duration urine creatinine clearance in 5 Units (11.9%). In turn, 9 Units (21.4%) made use of equations involving the determination of plasma creatinine (the Cockroft–Gault8 formula in 7 Units and the MDRD9 in two Units), while the remaining 16 Units (38.1%) only used Crs for this purpose. In no case was cystatin-C used as estimation method. There were no differences dependent upon the type of hospital or Unit surveyed (Fig. 1).
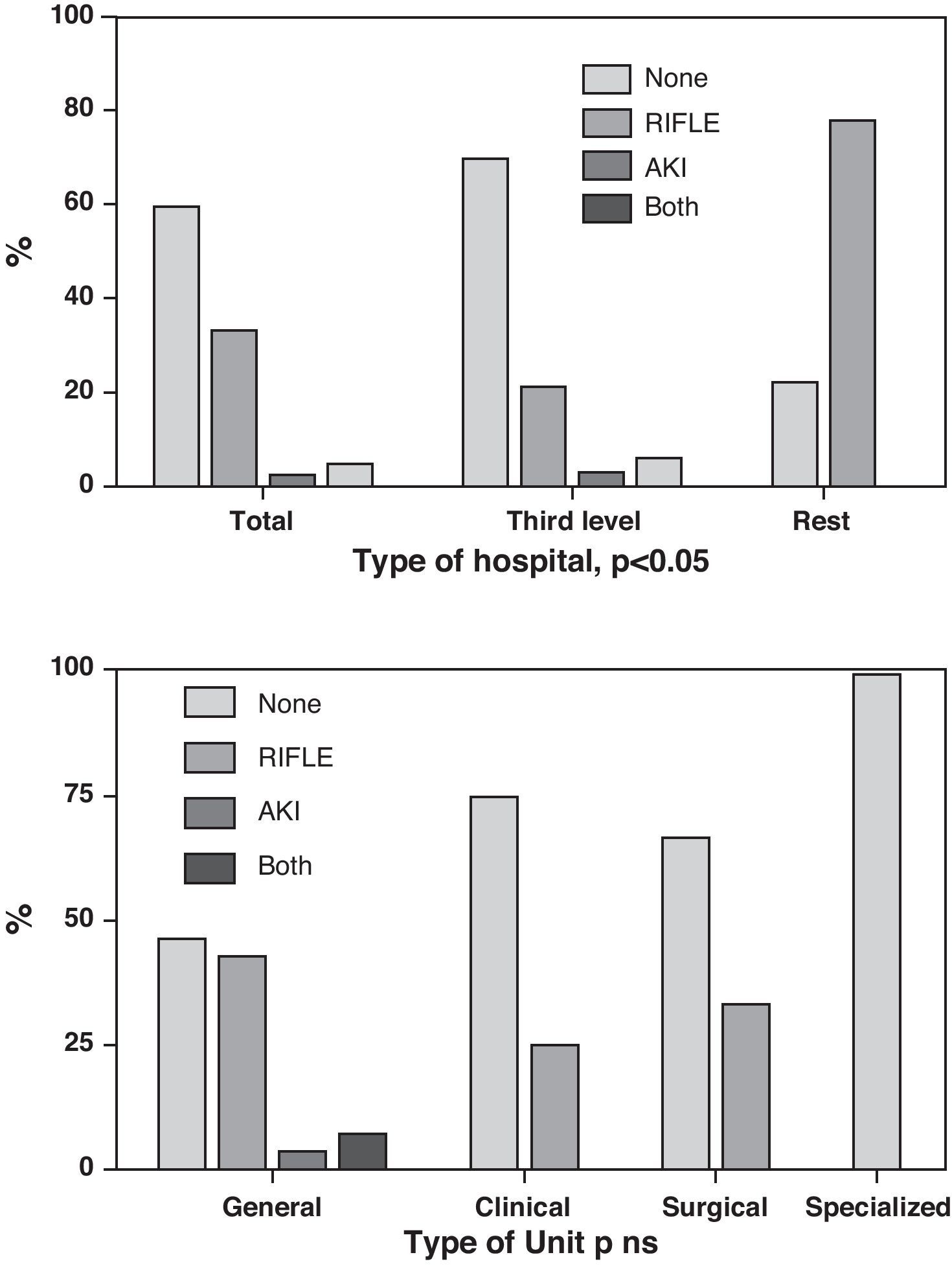
Fourteen ICUs (33.3%) adopted the RIFLE scale, while one Unit (2.4%) incorporated the AKIN system, and two Units (4.8%) adopted both scales. In contrast, 25 Units (59.5%) admitted that they applied neither system. Again in this case there was no correlation between the type of Unit and the use of the different scales, though there were differences referred to the type of hospital (p<0.05) (Fig. 2).
Twenty Units (47.6%) had no defined protocols for the prevention of AKI, while 14 Units (33.3%) presented contrast utilization protocols, three Units (7.1%) had nephrotoxic drug use protocols, and 5 Units (11.9%) had both contrast and drug use protocols. The hospital or Unit type was not related to this variable.
Thirty Units (71.4%) claimed to have continued training programs for the management of RRT. In 26 Units (61.9%) there were specific protocols for application of the technique (without differences dependent upon the type of hospital or ICU), and of these Units, 24 had a drug adjustment protocol for patients subjected to RRT.
DiscussionFollowing the publication of the recommendations of the ADQI6 and posteriorly the AKIN7 referred to the need to stratify AKI, different studies have described a significant change in the way to approach renal dysfunction, and in fact the incidence figures10 are now considered to be far higher than the previous reference values1,4 for general critical care patients. According to our results, and despite the existence of these recommendations, there is still great variability regarding the AKI detection methods used, with little application of the injury stratification systems. In this context, it is clear that the management of RRT (although only a part of the problem in patients with AKI) generates more concern than the prevention or diagnosis of AKI among intensivists in this country.
In recent years there have been changes in the physiopathological concepts of AKI, particularly referred to dysfunction of ischemic origin. This is important, considering that acute tubular necrosis is the main cause of AKI in critical patients, especially in relation to severe sepsis.11,12 This increased knowledge of the physiopathology of renal dysfunction from the moment of primary injury has led to an interest in improving the diagnosis and developing new and better methods to facilitate early diagnosis and thus minimize secondary kidney damage. In this context, the study of new kidney injury biomarkers is one of the fields of interest in AKI.13,14
The recommendations of the ADQI6 referred to the diagnosis and risk stratification of AKI, as reflected in the RIFLE system, were published in 2004. This system is based on the detection of evolutive changes referred to patient basal glomerular filtration (GF) through changes in urine volume or based on the estimation of GF from Crs or creatinine clearance (ClCr). One year later, in 2007, the AKIN7 group redefined this system once it became clear (initially in studies in heart surgery and posteriorly in other population groups)15,16 that also small Crs increments imply a poorer prognosis, independently of the basal situation. The AKIN system stratifies patients into three levels according to changes in diuresis or Crs increments, without considering other GF estimators, and incorporating absolute Crs elevations (an increase of 0.3mg/dl would classify a patient as AKIN 1, independently of the previous basal level).
On furthermore considering that when Crs rises to above 2mg/dl, the functional kidney mass (i.e., the “kidney reserve”) has dropped to 50%,17 it is not surprising that the RIFLE and AKIN systems, by detecting small Crs increments, are of great interest, and that the previous incidence data—mostly based on this 2mg/dl cutoff value—possibly greatly underestimated the true incidence of AKI. It also must be considered that both systems (RIFLE and AKIN) have already been widely validated and show good correlation to mortality.18–22 However, despite the interest in these new defining systems, to date they have shown scant penetration in Spanish ICUs, since less than 40% of our Units use such instruments.
Another aspect to be taken into account in assessing the detection of AKI is the GF estimator used. In clinical practice, the measurement of urine output is a simple method and in most cases anticipates Crs elevation; as a result, it is widely used for the initial detection of renal dysfunction,23 although as a parameter it offers very poor specificity. Both systems define Crs as an alternative estimator, since it is easy to determine and is universally available. However, Crs elevation shows a delay or lag after the lowering of GF, and does not exhibit a linear relationship with decreasing GF. These aspects may pose an important problem for a system precisely aiming to secure the early detection of reductions in GF.
Taking into account that kidney function in critical patients is not stable, it seems reasonable that serial measurements of ClCr (which can be made with short duration diuresis, thereby allowing for easy reproducibility)21 are able to detect such changes earlier than Crs – though the use of this estimator is only contemplated in the RIFLE (which in our opinion represents an advantage over AKIN). In our experience there are discrepancies in stratifying patients with the RIFLE system according to whether it is based on Crs or ClCr, in the sense that utilization of the latter parameter is more closely related to the prognosis.22 It is notorious that in the surveyed ICUs, ClCr was the parameter most commonly used to estimate GF, and it is even more surprising that 70% of the Units used determinations based on 24-h urine—these data being discordant with those obtained 10 years ago in the FRAMI study,4 where few Units made use of ClCr.
Crs is the next most common estimator used in our ICUs, though in fact it constitutes the most common estimator (59%) on considering those Units that use it incorporated to equations. This is not surprising, since the method is simple and reproducible, and very familiar in the clinical setting. What is surprising is that Crs is not incorporated in either of the available and validated systems (RIFLE or AKIN) with the purpose of stratifying patients and of offering a tool for follow-up capable of contributing relevant clinical information (this in our view being the most attractive aspect of these classification systems).
On the other hand, we see that the use of formulas remains a generalized practice despite the fact that they were specifically developed for application in chronic patients. However, a significant percentage of centers (21%) apply them to estimate GF in patients with AKI, even though none of them are useful in critically ill patients.24,25
None of the Units, regardless of their size, have incorporated biomarkers or cystatin-C in routine use, though we have evidenced that their application is being studied in some Units (including our own), in concrete patient groups. The incorporation of these new biomarkers may give added impulse to the early detection of AKI, though considering the current situation found in Spanish ICUs (as reflected by our data), their incorporation to the clinical setting does not appear due anytime soon.
Of note is the observation that no intervention for the diagnosis, stratification or prevention of AKI has been protocolized in the majority of our Units, since less than 50% of them admit the use of protocols in this sense. Furthermore, those protocols that do exist are particularly centered on the prevention of nephropathy referred to contrast injection, and in only very sporadic cases to drug adjustment. Specifically, only 12% of the Units have protocols which include these two very important aspects. This is in stark contrast to the use of RRT, since most of the Units have written protocols for applying such techniques. This may be attributed to the fact that the diagnosis of AKI is well assimilated in our usual clinical practice, while RRT is a relatively recent practice with which we have had to become quickly familiarized in view of the advances made in this field. This has created the need to learn and reflect the practice in written protocols; indeed, almost 60% of the Spanish Units have protocols for drug adjustment associated to the use of RRT, while only 7% have protocols for drug adjustment in relation to AKI. In fact, in relation to training, over 70% of the Units reported having active training programs in RRT.
The described results of course are based on the analysis of a survey, and so must be viewed with caution. On the other hand, and as the main weakness of this study, the sample comprised a highly selected group of Units—which questions the external validity of the findings. Nevertheless, this bias further emphasizes the problem, since the fact that the Units in the study largely participated because of their involvement and interest in AKI leads us to suspect that the results obtained in a larger study would have proven even less adequate.
We consider it necessary to change the strategy of our approach to AKI, with incorporation of the recommendations of the ADQI and AKIN conferences to our daily clinical practice, since this would directly result in early detection. Simply acquiring the habit of detecting patients at risk and of reflecting the fact in their case history can help improve management and reduce secondary renal damage, independently of the notion that different software applications (where available) can draw our attention through specific alerts when kidney function is altered—as is the case in our center. Undoubtedly, the incorporation of written protocols for the prevention and detection of AKI, and for RRT, would have a positive effect upon patient safety—facilitating secondary prevention (limitation of damage) and avoiding classification error and iatrogenic problems (“dialytrauma”)26 in RRT.
Conflicts of interestThe authors declare no conflicts of interest.
Investigators of the COFRADE study working group: Coordination: Herrera Gutiérrez ME, Seller Pérez G, Sánchez-Izquierdo Riera JA, Maynar Moliner J. Investigators: Hospital Clínic Barcelona: Nephrology: Pérez N; Hepatology: Acevedo J, Mas A, Castro M; General ICU: Nicolas JM; Surgical ICU: Zavala E, Adalia R, Tercero FJ; Nephrological ICU: Poch E, Serra N; Cardiological ICU: Bosch Genover X; Respiratory ICU: Badia JR; Cardiac surgery ICU: Cartana R; Anesthesia and resuscitation: Fontanals J. Hospital Clínico San Carlos Madrid: ICU: Ortuño Andériz F. Hospital Costa del Sol Málaga: ICU: Fernández García MI. Hospital de Alcorcón Madrid: ICU: Núñez Reiz D. Hospital de Cruces Bilbao: Anesthesia and resuscitation: Lekerika N; ICU: Sánchez A. Hospital de Guadalajara Guadalajara: ICU: Benito Puncel C, Borrallo JM. Hospital de la Santa Creu i San Pau Barcelona: ICU: Roglan Piqueras A, Rodríguez López M. Hospital del Norte Madríd: ICU: González Arenas MP. Hospital del Sureste Madrid: ICU: Ochoa Calero M, Albert de la Cruz P, Cruz Tejedor M. Hospital del Tajo Madrid: ICU: Ballesteros Ortega D. Hospital Fundación Jiménez Díaz Madrid: ICU: Alcalá Llorente MA, Pérez Calvo C, Oeding Angulo G. Hospital General Universitario Asociado Castellón: ICU: Mas Font S, González Luis R, Ferrandiz Sellés A. Hospital General Yagüe Burgos: ICU: López Pueyo MJ, Llata Rodríguez L, Perea Rodríguez ME. Hospital Germans Trias i Pujol Badalona: ICU: Tomasa Irriguible TM. Hospital Ramón y Cajal Madrid: Anesthesia and resuscitation: Candela-Toha A; ICU: Liétor Villajos JA. Hospital Severo Ochoa Madríd: ICU: López Martínez J, Chamorro Borraz N, Suárez Saiz J. Hospital Torrecárdenas Almería: ICU: Ramos Cuadra JA, Calderón Rodríguez A, Rodríguez Castaño R. Hospital Universitario 12 de Octubre Madrid: ICU: Flordelis Lasierra JL, Mohedano Gómez A, Pérez Vela JL, Terceros L. Hospital Universitario Carlos Haya Málaga: ICU: Lozano Sáez R, Olalla García R. Hospital Universitario de Canarias Tenerife: ICU: Lorenzo de la Peña L, Pérez Martínez N. Hospital Universitario Doctor Peset Valencia: ICU: Zaragoza R., Casanoves Laparra E, Montoro Lozano Y. Hospital Universitario Infanta Cristina Badajoz: ICU: Robles Marcos M, Almaraz Velarde R, Trasmonte Martínez MV. Hospital Universitario La Paz Madrid: ICU: García-de-Lorenzo A, Sánchez M, Perales E. Hospital Universitario Puerta del Mar Cádiz: ICU: Sierra Camerino R, Sánchez Rodríguez AC. Hospital Vega Baja Orihuela: ICU: Giménez-Esparza Vich C, Pérez Martínez D, Portillo Requena C. Hospital Universitario Virgen de la Arrixaca Murcia: ICU: Ros Martínez J, Llamas Lázaro C. Hospital Virgen de la Victoria Málaga: ICU: Daga Ruiz D, Vidal Hernández Rodríguez J. Hospital Virgen de las Nieves Granada: ICU: Guerrero López F. Hospital Universitario Virgen de Valme Sevilla: ICU: Herrera Rojas D, Úbeda Iglesias A, Contreras del Pino T. Hospital Virgen del Rocío Sevilla: ICU: Hinojosa Pérez R. Hospital Universitario Virgen Macarena Sevilla: ICU: Arenzana Seisdedos A, Ibáñez Cuadros S. Hospital Xeral Lugo: ICU: Álvarez Montero L, Nespereira Jato V, Saornil Agote O.
Please cite this article as: Herrera-Gutiérrez ME, et al. Variabilidad en los criterios de definición y métodos de detección de la disfunción renal en las unidades de cuidados intensivos ¿se aplican los consensos internacionales para el diagnóstico de la disfunción renal? Med Intensiva. 2012;36:264–9.
This study has the scientific support of the SEMICYUC.