The way to assess tissue perfusion during the resuscitation of patients with severe sepsis and septic shock is a current subject of research and debate. Venous oxygen saturation and lactate concentration have been the most frequently used criteria, though they involve known limitations. The venous-to-arterial difference of carbon dioxide (pCO2 delta) is a parameter than can be used to indicate tissue perfusion, and its determination therefore may be useful in these patients.
MethodsA qualitative systematic review of the literature was made, comprising studies that assessed pCO2 delta in adult patients with severe sepsis or septic shock, and published between January 1966 and November 2016 in the Medline-PubMed, Embase-Elsevier, Cochrane Library, and LILACS databases. There was no language restriction. The PRISMA statement was followed, and methodological quality was evaluated.
ResultsTwelve articles were included, all of an observational nature, and including 10 prospective studies (9 published since 2010). Five documented greater mortality among patients with high pCO2 delta values, in 3 cases even when achieving venous oxygen saturation targets. In 4 studies, a high pCO2 delta was related to lower venous oxygen saturation and higher lactate levels, and another 3 documented lesser percentage lactate reductions.
ConclusionThe parameter pCO2 delta has been more frequently assessed in the management of patients with severe sepsis during the last few years. The studies demonstrate its correlation to mortality and other clinical outcomes, defining pCO2 delta as a useful tool in the management of these patients.
La forma de evaluar la perfusión tisular durante la reanimación de pacientes con sepsis grave y shock séptico es tema de estudio y debate en la actualidad. La saturación venosa de oxígeno y el lactato han sido los criterios más utilizados; sin embargo, presentan limitaciones reconocidas. La diferencia venoarterial de dióxido de carbono (delta de pCO2) es una variable que puede indicar el estado de perfusión tisular, por lo que su evaluación puede ser útil en estos pacientes.
MétodosRevisión sistemática cualitativa de la literatura que incluyó estudios que evaluaron el delta de pCO2 en pacientes adultos con sepsis grave o shock séptico, publicados entre enero de 1966 y noviembre de 2016 en las bases de datos Medline-PubMed, Embase-Elsevier, Cochrane Library y LILACS. No tuvo restricción de idiomas. Se siguió la declaración PRISMA y se evaluó la calidad metodológica.
ResultadosDoce estudios fueron incluidos, todos observacionales, 10 prospectivos, 9 publicados a partir del 2010. Cinco documentaron una mayor mortalidad entre pacientes con delta de pCO2 alto, en 3 incluso cuando conseguían metas de saturación venosa de oxígeno. En 4 estudios, un delta de pCO2 alto se relacionó con una menor saturación venosa de oxígeno y niveles mayores de lactato, y otros 3 documentaron un menor porcentaje de disminución de lactato.
ConclusiónEl delta de pCO2 ha sido evaluado en el manejo de los pacientes con sepsis grave y shock séptico con mayor frecuencia en los últimos años. Los estudios demuestran su relación con la mortalidad y otros desenlaces clínicos, de tal forma que puede ser una herramienta útil en el manejo de estos pacientes.
Sepsis is one of the main causes of admission to Intensive Care Units (ICUs). This heterogeneous and complex syndrome can result in a 20–50% mortality rate, depending on the severity of the clinical condition,1,2 which in turn is conditioned to the presence of organ dysfunction mediated by different mechanisms of cell damage. The way in which the different individual mechanisms interact is not fully understood, though sepsis is known to involve microvascular anomalies, and a decrease in oxygen supply and/or deficient utilization of the available oxygen constitute a central element of such organ dysfunction.3 The early identification of tissue damage is therefore crucial in the management of these patients.
The measurement of certain physiological variables of use in assessing tissue perfusion status has been proposed in the initial care of such patients. In its early versions, the Surviving Sepsis Campaign recommended the measurement of venous oxygen saturation (SvO2), evaluated as mixed venous saturation or central venous oxygen saturation (SvcO2), and lactate concentration in this respect, with the definition of a series of target values intended to secure adequate patient resuscitation.4 This proposal was essentially based on the early intervention protocol published by Rivers et al., advocating the “normalization” of SvcO2, central venous pressure (CVP) and mean arterial pressure, with the purpose of improving tissue perfusion.5 Other investigators, fundamentally Jones et al., reinforced the idea that lactate can also be used in protocols of this kind.6,7
Although the usefulness of the protocol was evaluated in the context of randomized clinical trials, each of the mentioned variables has known limitations, and the use of a single variable does not seem to be the best way to assess tissue perfusion.8,9 More recently, multicenter clinical trials have been unable to confirm the usefulness of the protocol developed by Rivers et al., and the measurement of SvcO2 as a guide in patient resuscitation has been questioned.10–12 As a result of the above, the latest version of the Surviving Sepsis Campaign does not recommend the use of this variable as an initial resuscitation goal or target in the management of such patients.13
Other parameters for the assessment of tissue perfusion are therefore needed to guide therapy. One such parameter is the venous-to-arterial pressure difference of CO2 (pCO2 delta or ΔpCO2), which serves as a surrogate marker of the venous-to-arterial difference in CO2 content. Under physiological conditions, the venous CO2 concentration is higher than in arterial blood, due to CO2 production at peripheral level, coupled to oxygen consumption and metabolism in general. The measurement of these pressure values has been proposed since within normal ranges, the CO2 concentration is linearly correlated to pressure. In theory, low flow conditions and non-anaerobic sources of CO2 production can increase the venous concentration and thus increment the normal difference.14,15
The pCO2 delta value has been proposed as a parameter capable of indicating altered tissue perfusion in different clinical contexts,16,17 including sepsis.14,18 However, the evaluation of this parameter has not yet been recommended by the international Surviving Sepsis Campaign guide, and its usefulness during the initial resuscitation of these patients or as a resuscitation goal is not clear.4,14 The present study conducts a systematic literature review with the aim of identifying studies and outcomes referred to the use of pCO2 delta as a measure of prognostic or therapeutic value in patients with severe sepsis or septic shock.
MethodsA systematic search was made of the literature, including full-test original articles in which the primary objective was the evaluation of pCO2 delta during the initial management of patients specifically diagnosed with septic shock and/or severe sepsis. We excluded studies that evaluated patients under 18 years of age or pregnant women. There were no restrictions regarding the type of study or language of the publication.
The search covered the period between January 1966 and October 2015, with updating to November 2016, and was carried out in the Medline-PubMed, Embase-Elsevier, Cochrane Library and LILACS databases, using the terms of the strategy established in the research protocol (Annex A). Two authors reviewed the titles and abstracts, identifying those studies that met the screening criteria. Disagreements between the investigators were resolved by consensus with a third author. The references of the selected articles were in turn used to identify additional studies.
The information was entered in a datasheet including the study objective, sample size, characteristics of the study population, main outcomes and conclusions of each article. We specifically collected data referred to in-hospital mortality, mortality after 28 days, cardiac output (CO), cardiac index (CI) and other tissue perfusion variables such as lactate and SvO2, as well as therapeutic interventions defined in the methodology section of the studies. The PRISMA statement was followed in this systematic review.19 The risk of bias was assessed using the Altman proposal for the evaluation of prognostic variables, which uses a traffic light system with the application of 6 factors: patient sample, patient follow-up, evaluated outcome, prognostic factor, analysis of the data and treatment following inclusion in the cohort.20 The study was approved by the Institutional Review Boards of the Medical School of the Fundación Universitaria de Ciencias de la Salud and Hospital de San José de Bogotá (Colombia).
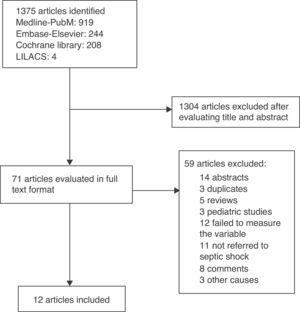
ResultsThe search yielded a total of 1375 articles, of which 1304 were discarded after evaluating the title and abstract. Of the 71 articles analyzed in full text format, 12 met the inclusion criteria.21–32 There were no disagreements between the reviewers. Fig. 1 shows the screening process of the included articles.
All the studies were of an observational nature, 10 were prospective, 10 were published in English, and two in Chinese. Convenience sampling was used in all the studies. The main potential sources of bias were a short duration of follow-up and the lack of statistical adjustment for other important prognostic factors (Annex A).
Table 1 shows the characteristics of the included studies. The main results are described below, according to specific aspects evaluated in relation to pCO2 delta.
Principal characteristics of the studies meeting the inclusion criteria.
| Author, year (ref.) | n | Population | Principal analytical characteristica |
|---|---|---|---|
| Mecher et al., 199021 | 37 | Severe sepsis and hypoperfusion | Comparison of 2 groups classified before fluid challenge ΔpCO2≤6 and ΔpCO2>6 |
| Bakker et al., 199222 | 64 | Septic shock | Comparison of 2 groups classified upon admission to ICU ΔpCO2≤6 and ΔpCO2>6 |
| Vallée et al., 200823 | 50 | Septic shock, MV, lactate>2mmol/L, SvcO2>70% | Comparison of 2 groups classified at T0 (start of monitoring) ΔpCO2≤6 and ΔpCO2>6 Evaluation at T0, T6 and T12 |
| Troskot et al., 201024 | 71 | Septic shock or severe sepsis | Comparison of 2 groups classified upon admission: with MV and without MV |
| Van Beest et al., 201325 | 53 | Septic shock or severe sepsis | Comparison of 2 groups classified upon admission ΔpCO2<6 and ΔpCO2>6 |
| Ospina-Tascon et al., 201326 | 85 | Septic shock | Classified according to change in ΔpCO2 between T0 and T6 (normal: ΔpCO2<6) Persistently normal=36 Decreasing (high-normal)=17 Persistently high=24 Increasing (normal-high)=8 Measurements at T0, T6, T12 and T24 |
| Du et al., 201327 | 172 | Septic shock | In T6 classified patients: Group 1: SvcO2<70% and ΔpCO2≥6 Group 2: SvcO2≥70% and ΔpCO2≥6 Group 3: SvcO2<70% and ΔpCO2<6 Group 4: SvcO2≥70% and ΔpCO2<6 |
| Zhao et al., 201228 | 45 | Septic shock | Comparison of 2 groups classified upon admission ΔpCO2<6 and ΔpCO2≥6 |
| Zhang et al., 201229 | 52 | Septic shock or severe sepsis and SvcO2>70% | Comparison of 2 groups classified upon admission ΔpCO2<6 and ΔpCO2>6 Measurements at T0, T6, T12 and T24 |
| Mallat et al., 201430 | 80 | Septic shock and MV | Comparison of 2 groups classified according to ΔpCO2≤6 and ΔpCO2>6 at T0 and T6 |
| Mallat et al., 201431 | 22 | Septic shock, MV, lactate<2mM, 24h evolution | Dobutamine infusion, initial dose: 5μg/kg/min Dose increments of 5μg/kg/min every 30min, to 15μg/kg/min Evaluation in the 3 dose ranges |
| Ospina-Tascón et al., 201632 | 75 | Septic shock | Comparison of 3 groups classified upon admission: ΔpCO2<6, ΔpCO2 6–9.9 and ΔpCO2≥10 |
SvcO2: central venous oxygen saturation; T0: time zero (time of patient entry to the study); T6: 6h after T0; T12: 12h after T0; T24: 24h after T0; ICU: Intensive Care Unit; MV: mechanical ventilation; ΔpCO2: venous-to-arterial difference of carbon dioxide.
Three articles evaluated in-hospital mortality in relation to pCO2 delta,22,24,25 while 5 assessed mortality after 28 days.23,26–28,30 Five studies compared mortality between groups of patients with high versus normal pCO2 delta values upon admission. These publications recorded greater percentage mortality among the patients with high pCO2 delta values, though the differences in the percentages were variable, and in two studies they failed to reach statistical significance (Table 2).
Percentage mortality according to pCO2 delta group.
| Study | Time of analysis | Percentage mortality | p-value | |
|---|---|---|---|---|
| High ΔpCO2 | Normal ΔpCO2 | |||
| Vallée et al.23 | T0 | 54 | 34 | 0.16 |
| Van Beest et al.25 | T0 | 29 | 21 | 0.53 |
| Du et al.27 | T0 | 53.6 | 23.3 | <0.001 |
| Zhao et al.28 | T0 | 60 | 63 | >0.05 |
| Mallat et al.30 | T0 | 59 | 50 | 0.42 |
| T6 | 75 | 42 | 0.003 | |
T0: time zero (time of patient entry to the study); T6: 6h after T0; ΔpCO2: venous-to-arterial difference of carbon dioxide.
Six studies conducted other analyses related to mortality. Troskot et al.24 showed pCO2 delta to be a mortality risk factor in non-ventilated patients, while Bakker et al.22 found pCO2 delta to be greater among non-survivors than among survivors (5.9±3.4 vs 4.4±2.3, respectively; p<0.05)—though the result was influenced by increased pulmonary impairment in the former group, and the authors concluded that the prognostic value of the parameter is modest.24 Van Beest et al.25 in turn showed that pCO2 delta >6mmHg 4h after admission exhibited an odds ratio (OR) of 5.3 (95% confidence interval [95%CI] 0.9–30.7; p=0.08) for in-hospital mortality, while Ospina-Tascon et al.26 found patients with persistently elevated pCO2 delta during the first 6h to have poorer survival after 28 days than those individuals that normalized this variable (log-rank, Mantel–Cox: 19.21; p<0.001). The study of Du et al.27 showed that among the patients that reached therapeutic targets referred to SvO2 during initial resuscitation, those presenting high pCO2 delta suffered greater mortality compared with those who normalized this variable. Similar results were reported by Ospina-Tascon et al.26 and Mallat et al.,30 though statistical significance was not reached in the latter case (Table 3).
Relationship between mortality and pCO2 delta.a
| Study | Time of analysis | Statistical analysis | p-value |
|---|---|---|---|
| Troskot et al.24 | T0 | HR 4.33 (CI 95% 1.33–14.11) (sin MV) | 0.015 |
| HR 1.25 (CI 95% 0.84–1.86) (con MV) | 0.27 | ||
| Van Beest et al.25 | T0 | OR 1.6 (0.5–5.5) | 0.53 |
| T4 | OR 5.3 (0.9–30.7) | 0.08b | |
| Ospina-Tascon et al.26c | T0 | RR 1.77 (0.97–3.22) | 0.06 |
| T6 | RR 2.23 (1.20–4.13) | 0.01 | |
| T12 | RR 2.41 (1.42–4.1) | 0.001 | |
| Du et al.27 | T6 | Percentage mortality according to groups: G1 SvcO2<70% and ΔpCO2≥6: 50% G2 SvcO2>70% and ΔpCO2≥6: 56.1% G3 SvcO2<70% and ΔpCO2<6: 50% G4 SvcO2>70% and ΔpCO2<6: 16.1% | <0.001 |
| Mallat et al.30 | T6 | Percentage mortality according to groups: SvcO2>70% and ΔpCO2>6: 57% SvcO2>70% and ΔpCO2 ≤ 6: 37% | 0.22 |
HR: hazard ratio; 95%CI: 95% confidence interval; OR: odds ratio; RR: relative risk; SvcO2: central venous oxygen saturation; T0: time zero (time of patient entry to the study); T4: 4h after T0; T6: 6h after T0; T12: 12h after T0; MV: mechanical ventilation; ΔpCO2: venous-to-arterial difference of carbon dioxide.
Nine studies evaluated pCO2 delta in relation to other tissue perfusion variables.22,23,25–30,32 Vallée et al.,23 Van Beest et al.,25 Mallat et al.30 and Zhao et al.28 recorded higher serum lactate levels and lower SvcO2 values when the patients presented pCO2 delta >6mmHg, compared with those showing pCO2 delta <6mmHg, while Bakker et al.22 reported no statistically significant differences for lactate – though mixed venous oxygen saturation was found to be lower in the high pCO2 delta group (Table 4). Ospina-Tascon et al. classified their patients according to the pCO2 delta value upon admission and after 6h. The group with persistently elevated pCO2 delta (high after 0 and 6h) showed greater lactate values compared with the patients that normalized their pCO2 delta value (high at 0h and normal after 6h) (Table 4).
Tissue perfusion variables and pCO2 delta.
| Study | Time of analysis | Variable | Normal ΔpCO2 | High ΔpCO2 | p-value | |
|---|---|---|---|---|---|---|
| Bakker et al.22 | T0 | Lactate | 5.6±3.9 | 6.2±5 | >0.05 | |
| SvmO2 | 66%±10 | 50%±14 | <0.01 | |||
| Vallée et al.23 | T0 | Lactate | 5.6±3.6 | 7.5±3.7 | 0.007 | |
| SvcO2 | 78%±5 | 75%±5 | 0.007 | |||
| Van Beest et al.25 | T0 | Lactate | 2.8±3.1 | 3.9±2.9 | <0.001 | |
| SvcO2 | 74.5%±9.3 | 71.1%±7.1 | <0.001 | |||
| Ospina-Tascon et al.26a | T6 | Lactate | G1: 1.3 (0.9–2.3) | G3: 3.3 (2.1–6.8) | <0.05 | |
| G2: 2 (1–3.5) | G4: 2.9 (1.1–7.1) | |||||
| Zhao et al.28 | T0 | Lactate | 3.4±2.1 | 5.7±4.5 | <0.05 | |
| SvcO2 | 74%±9 | 67%±8 | <0.05 | |||
| T24 | Lactate | 2.5±1.6 | 3.6±1.5 | |||
| SvcO2 | 77%±9 | 73%±6 | ||||
| Zhang et al.29 | T0 | Lactate | 3.12±0.88 | 4.57±1.61 | <0.01 | |
| T12 | Lactate | 2.66±0.78 | 4.31±1.43 | <0.01 | ||
| T24 | Lactate | 1.74±0.67 | 3.89±1.4 | <0.01 | ||
| Mallat et al.30 | T0 | Lactate | 3.2 (1.6–5.9) | 3.6 (2.2–8.5) | 0.26 | |
| SvcO2 | 73% (65–80) | 61% (53–63) | <0.0001 | |||
| T6 | Lactate | 2.0 (1.2–3.5) | 3.6 (2.1–8.4) | 0.002 | ||
| SvcO2 | 73% (70–76) | 63% (51–71) | <0.0001 | |||
| Ospina-Tascón et al.32 | ΔpCO2 (6–9.9) | ΔpCO2 (≥10) | ||||
| T0 | PPV | 83.9 | 56.8 | 40.1 | <0.05 | |
| FCD | 7.8 | 4.9 | 3.4 | <0.05 | ||
| HI | 0.24 | 0.46 | 0.57 | <0.05 | ||
| T6 | PPV | 85.5 | 61.7 | 43.2 | <0.05 | |
| FCD | 8.2 | 5.4 | 4.7 | <0.05 | ||
| HI | 0.15 | 0.52 | 0.54 | <0.05b |
FCD: functional capillary density; HI: heterogeneity index; PPV: percentage of perfused small vessels; SvcO2: central venous oxygen saturation; SvmO2: mixed venous oxygen saturation; T0: time zero (time of patient entry to the study); T6: 6h after T0; T12: 12h after T0; T24: 24h after T0; ΔpCO2: venous-to-arterial difference of carbon dioxide.
Three studies23,27,30 found the percentage decrease in lactate concentration to be greater in the presence of pCO2 delta <6mmHg. Vallée et al.23 recorded a decrease in lactate between 0 and 12h of −38±39 vs −17±33% (p=0.04), respectively, while Mallat et al.30 found the decrease in lactate between 0 and 6h to be 33.3±28.9 vs 7.8±41.2 (p=0.016). In turn, Du et al.27 classified their patients according to the SvcO2 target value and pCO2 delta after 6h. In the patients that reached the SvcO2 target, lactate clearance was greater in the subgroup with normal pCO2 delta versus the patients with high pCO2 delta (0.21±0.31 vs 0.01±0.61, respectively; p=0.023), while no such differences were noted in the group that failed to reach the SvcO2 targets (−0.04±0.43 vs −0.09±0.59, respectively).27
One study analyzed microvascular perfusion as assessed by videomicroscopy and the pCO2 delta value. The authors found increased pCO2 delta values to be correlated to a lesser proportion of perfused small vessels, a lower functional capillary density, and a greater heterogeneity index (Table 4).
pCO2 delta and cardiac output or cardiac indexNine articles evaluated the relationship between pCO2 delta and CO or CI.21,28–30 Five compared mean CO or CI in the groups of patients with high or low pCO2 delta values. All of them found pCO2 delta >6mmHg to be associated to lower CO or CI.22,23,28–30 In addition, the correlation between pCO2 delta and CO or CI was found to be discrete (Table 5). In no case did the correlation coefficient exceed 0.7.
Cardiac output or cardiac index and its correlation to pCO2 deltaa.
| Study | Time of analysis | Analytical group | Correlation statistic | |
|---|---|---|---|---|
| Normal ΔpCO2 | High ΔpCO2 | |||
| Mecher et al.21 | Basal | CI=3.0±0.2 | 2.3±0.2 | r=0.42 (p<0.01)b |
| Bakker et al.22 | T0 | CI=3.8±2.0 | 2.9±1.3 (p<0.01) | |
| Vallée et al.23 | T0 | CI=4.3±1.6 | 2.7±0.8 (p<0.0001) | r=0.57 (p<0.0001) |
| T6 | r=0.58 (p<0.0001) | |||
| T12 | r=0.58 (p<0.0001) | |||
| Troskot et al.24 | T0 | r=−0.21 (p=0.162) | ||
| Van Beest et al.25 | T0 | CI=4.1±1 | 3.3±1 (p=0.01) | R2=0.07 (p<0.001) |
| Ospina-Tascon et al.26 | T0 | r=0.16 (p<0.01) | ||
| Zhao et al.28 | T0 | CI=4.5±2.1 | 3.1±1.5 (p<0.05) | |
| T24 | CI=4.4±1.3 | 3.2±0.8 (p<0.05) | ||
| Zhang et al.29 | T0 | CO=7.19±1.34 | 5.23±0.84 (p<0.001) | r=−0.50 (p<0.0001) |
| T12 | CO=7.32±1 | 5.43±0.78 (p<0.001) | r=−0.62 (p<0.0001) | |
| T24 | CO=7.37±0.79 | 5.72±0.64 (p<0.001) | r=−0.46 (p<0.0001) | |
| Mallat et al.30 | T0 | CI=3.9 (3.3–4.7) | 2.9 (2.3–3.1). p=0.0001 | r=−0.69 (p<0.0001) |
| T6 | CI=4.2 (3.2–5.0) | 3.3 (2.5–4.2). p=0.004 | r=−0.54 (p<0.0001) | |
CO: cardiac output; CI: cardiac index; r: correlation coefficient; R2: coefficient of determination; T0: time zero (time of patient entry to the study); T6: 6h after T0; T12: 12h after T0; T24: 24h after T0; ΔpCO2: venous-to-arterial difference of carbon dioxide.
Mallat et al.31 recorded a statistically significant decrease in pCO2 delta on administering dobutamine at increasing doses between 5 and 15μg/kg/min, while Mecher et al.21 evaluated pCO2 delta response to fluid challenge – improvements in the variable being observed after the administration of colloids.
DiscussionThe present systematic review found most of the articles on pCO2 delta in patients with severe sepsis and septic shock to have been published in the last 6 years. Although evidence of its potential usefulness has been available since the late 1980s,16,17 and two of the identified articles date from the early 1990s,21,22 the more recent interest in the investigation of this parameter is possibly related to the limitations identified in the variables most commonly recommended in the management of these patients: lactate and SvO2.33,34 Evaluation of the microcirculation using more novel techniques, and the evidence of microcirculatory alterations despite apparent normality of the macrodynamic variables – including SvO2 – point to the need to explore other possible variables.8,9
The studies generally showed high pCO2 delta values to be associated to poorer clinical outcomes, including worsened hemodynamic parameters, poorer tissue perfusion, and greater in-hospital mortality and mortality after 28 days. With regard to mortality, the importance of the serial determination of pCO2 delta in terms of its prognostic usefulness must be underscored. The studies offering data referred to serial measurements found the second measurement of pCO2 delta to be more closely correlated to mortality than the first measurement.25,30 Although different studies have shown both SvO2 and lactate, considered individually, to be of prognostic value in terms of mortality,5,6,35 the recording of pCO2 delta appears to offer additional information. This was seen in three studies that analyzed patients that were able to reach adequate SvO2 goals after 6h of resuscitation, in which the presence of a normal pCO2 delta was seen to imply a better prognosis. This may indicate the usefulness of serial measurements during the initial resuscitation of septic shock patients, where first the SvO2 goal is reached, followed by the achievement an additional target based on pCO2 delta. The cutoff point of 6mmHg for stratifying the two groups (normal and high pCO2 delta) was quite consistent in all the studies – the publication of Bakker et al.22 generally being taken as the reference in recording this parameter.
However, it must be noted that the importance of serial measurements has been best established in the case of lactate concentration. In this regard, the importance of percentage lactate clearance, even as a resuscitation goal, has been documented both in sepsis and in the general critical patient population.6,36 In our investigation, the studies that analyzed pCO2 delta in relation to percentage lactose reduction23,27,30 found such reduction to be greater in patients with low pCO2 delta values, particularly when evaluated after 6h. This underscores the importance of the serial determination of pCO2 delta, and also evidences the potential usefulness of these measurements in combination with lactate concentration.
Taking into account that the increase in pCO2 delta is related to low flow states and the consequent accumulation of CO2, a series of studies evaluated the association of this parameter to CO or CI. These publications generally recorded an expected inverse relationship between the two variables, though some recorded low (but still statistically significant) correlation or determination coefficients between them,21,22,24 with additional important dispersion of the analyzed points. On the other hand, two studies reported no such relationship.24,26 The above may reflect the physiopathological complexity in interpreting pCO2 delta elevation in the context of these patients, as well as the evident individual variability found. As a result, the usefulness of pCO2 delta in indirectly assessing CO may prove inconsistent.
In addition to its prognostic implications, two studies evaluated the impact of therapeutic interventions upon pCO2 delta, showing fluid or inotropic drug administration to exert a positive effect upon the variable. This is important, since prior knowledge of how pCO2 delta can be modified is relevant when constructing a management algorithm for these patients in the context of clinical studies evaluating pCO2 delta as a resuscitation goal in septic shock patients.
Despite the evidence found, different authors have addressed the limitations of this variable in evaluation tissue hypoperfusion. In effect, pCO2 delta may be normal in cases of evident hypoperfusion and high CO, and may also be elevated in the absence of hypoperfusion, taking into account the Haldane effect.37 This is why the evaluation of CO2 content in relation to the oxygen levels has been proposed as another way to assess tissue perfusion status. The Cv-aCO2/Da-vO2 ratio is a variable that can identify patients with anaerobic metabolism under different critical conditions, including septic shock.38–40 This variable therefore might also be of clinical relevance in the management of patients with sepsis.
New methods for more directly evaluating tissue perfusion have been developed in recent years.41 One such method involves sidestream dark field sublingual videomicroscopy, which affords different parameters for assessing the microcirculation. This system has been used to document different microcirculatory alterations in patients with septic shock, and the improvements obtained as a result of certain interventions.42–44 However, the use of this technology at the patient bedside faces many challenges, and its relevance in the management of patients of this kind remains to be established. This explains why the easily measurable parameters commented above are still considered to be valid. In this regard, the study of Ospina-Tascon et al.32 offers important information, considering that pCO2 delta was the variable best correlated to microcirculatory alterations – though the clinical significance of this association has not been well defined.
Considering the above, should pCO2 delta be used as a resuscitation goal or target in patients with septic shock? The answer is still not clear, and further evaluation in the context of clinical trials is needed. The variables most widely evaluated in these patients are SvO2 and lactate, and the most recent Surviving Sepsis Campaign guide only recommends the measurement of lactate.4 Consequently, the way in which pCO2 delta should be implicated in the management of these patients and related to the abovementioned variables is not clear. What should be “standardized” first; how many resuscitation goals must be reached; or how they should be reached, are issues still waiting for an answer. However, this does not mean that pCO2 delta should not be taken into account in the management of patients with septic shock, in the context of a multimodal approach combined with other variables, involving serial measurements, on an individualized basis during initial resuscitation. In fact, the most recent circulatory shock and hemodynamic monitoring consensus document of the European Society of Intensive Care Medicine recommends the measurement of pCO2 delta as part of the evaluation and management of patients with septic shock in the presence of a central venous catheter.45
In the present study, we were unable to conduct a meta-analysis, due to the great heterogeneity of the study designs, the reporting of outcomes and the nature of the disease investigated. One of the limitations of our systematic review was that it included studies of pCO2 delta in patients exclusively presenting severe sepsis and septic shock—thereby precluding the possibility of extending the findings to a broader range of critically ill patients, and of expanding the analysis of its potential uses and limitations. However, this restriction was also an essential part of the purpose of the study. Some studies that analyzed pCO2 delta in critical care populations were not taken into account, despite the inclusion of cases of severe sepsis and septic shock, since the outcomes in these latter cases were not always described.38,46 Other publications involving septic shock patients analyzed pCO2 delta, though evaluation of the variable was limited, since it was not the primary objective of the study.39,47,48
It can be concluded that pCO2 delta applied to the management of patients with severe sepsis and septic shock has been evaluated more frequently in recent years, and that in most cases high pCO2 delta values have been correlated to poorer clinical outcomes, including lower CI, higher lactate levels, lower SvO2 values, lower lactate clearance rates and higher mortality – though the studies have been heterogeneous and involve some methodological limitations. Although the usefulness of pCO2 delta has not been assessed in the context of clinical research within an initial resuscitation protocol for patients with severe sepsis and septic shock, the overall results show that it may be a parameter to be considered in the management of these patients.
AuthorshipJuan José Diaztagle: research idea, protocol design, data search, analysis of results, discussion, drafting of the manuscript.
Jorge Camilo Rodríguez: protocol design, data search, analysis of results, discussion.
John Jaime Sprockel-Díaz: protocol design, analysis of results, discussion.
Conflicts of interestNone.
Thanks are due to Diana Buitrago, of the División de Investigaciones, Fundación Universitaria de Ciencias de la Salud, for her valuable contribution to the search for the study data.
Please cite this article as: Diaztagle Fernández JJ, Rodríguez Murcia JC, Sprockel Díaz JJ. La diferencia venoarterial de dióxido de carbono en la reanimación de pacientes con sepsis grave y shock séptico: una revisión sistemática. Med Intensiva. 2017;41:401–410.