Right atrial thrombus is well-documented life-threatening complications associated with central venous catheters,1,2 yet unrecognized due to underreporting in asymptomatic patients and difficult diagnosis. The catheter induced right atrium thrombus has a reported risk of 40% for pulmonary thromboembolism and associated mortality rate as high as 28–31%.3 Although difficult to diagnose, right atrial thrombus is a complication with deadly consequences, like pulmonary embolus and right heart obstruction, that must be prevented by proper approach and management of the catheter, early clinical suspicion, diagnosis and appropriate intervention.1 Authors describe management of a 64-year-old man with hemodialysis catheter induced right atrial thrombus, conditioning cardiac arrest and a review of the existing literature.
Case reportA 64-year-old man was admitted to the Emergency Room (ER) in coma with acute respiratory failure. Family described cough and mucous expectoration from a week, without fever. The patient had past medical history of essential arterial hypertension, type 2 diabetes mellitus, chronic obstructive pulmonary disease (COPD), hypothyroidism, benign prostatic hyperplasia (BPH), lower limbs lymphedema and chronic constipation. Chronic medicated with Perindopril, Furosemide, Metformin, Silodosin, Levothyroxine and Alprazolam.
On hospital admission, patient had Glasgow Score of 8, was immediately intubated and mechanical ventilated. He was hemodynamically stable and pulmonary auscultation had diminished breath sounds on right hemithorax. Arterial blood gas analysis revealed respiratory acidosis (pH 7.28, pO2 112mmHg, pCO2 60mmHg, HCO3− 27.8, lactates 1.11). Laboratory evaluation showed hemoglobin 12.2g/dl, leukocytes 6.10×109/L, neutrophils 67.41%, C-reactive protein (CRP) 165.6mg/L, D-dimer 2511mcg/L, platelets 230×109/L, INR 1.22, urea 63mg/dL, creatinine 1.83mg/dL, potassium 6.6mEq/L, troponin 0.49ng/ml, myoglobin 673.8ng/mL, CK 403U/L, AST 111U/L, ALT 35U/L, LDH 483U/L. Normal thyroid function. Chest radiograph showed opacity of the right hemithorax. Blunt brain Computerized tomography (CT) – scan. A Chest Angio-CT was performed excluding pulmonary thromboembolism.
Patient was started on empirical antibiotherapy with Amoxicillin and Clavulanic acid for the hypothesis of aspiration pneumonia. He was also started on Acetylsalicylic acid 250mg 1id, Clopidogrel 75mg 1id and Enoxaparin 100mg 2id due to the diagnostic hypothesis of acute coronary syndrome. There was not indication for emergency coronariography. Condition progressed to refractory shock with development of acute renal failure with oliguria and maintenance of acute respiratory failure. Patient was admitted in an Intensive Care Unit. Resuscitation with aggressive fluid resuscitation and vasopressor support with norepinephrine were started with hemodynamic response. H2N3 virus was isolated and 10 days of treatment with Oseltamivir 150mg 2id were completed. There were not other isolated agents.
Progressive worsening of the renal function was observed and renal replacement therapy (hemodialysis – HD) started at 14th day. First HD catheter was placed in the right femoral vein and then changed to left femoral vein for infection suspicion. At 27th day of internment a deep venous thrombosis of the left femoral vein involving the HD catheter extremity and of the right femoro-popliteus system was documented and managed with continuous perfusion of unfractionated heparin (UFH), controlled by a PTT monitoring.
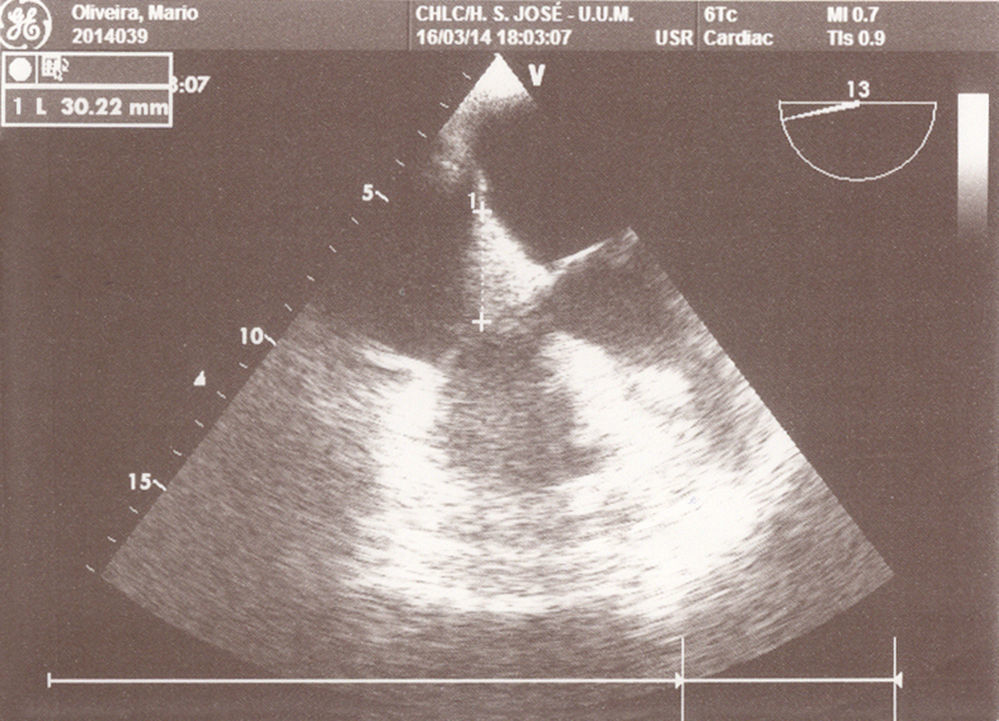
Other central venous HD catheter was placed in the right subclavian vein and catheterization progressed uneventfully. At 2nd day of this catheter, 15min after the initiation of renal replacement technique, patient developed desaturation, hypotension and bradycardia with evolution to cardiac arrest in asystolia, recovered after 2 cycles of advanced life support (ALS). Chest Angio-TC excluded pulmonary thromboembolism. At 3rd day patient was hemodynamically stable under continuous perfusion of norepinephrine but developed supraventricular tachycardia with hemodynamic instability after renal replacement technique was started, which converted to sinusal rhythm after synchronized cardioversion (100J). The transthoracic echocardiogram performed did not show any changes. At 4th day, 20min after the beginning of the renal replacement technique, new cardiac arrest in pulseless electrical activity. A transesophageal echocardiogram (TEE) was performed under ALS and clot in the right atrium (RA) with 30mm diameter adjacent to the hemodialysis catheter was documented (Fig. 1). It prolapsed into the tricuspid valve, causing obstruction of blood flow to the right ventricle and causing the cardiac arrest.
Fibrinolytic therapy was performed with Alteplase 100mg and continuous anticoagulation with UFH was maintained. Complete resolution of the clot was documented by TEE at 48h. After clot resolution, renal replacement therapy resumed on alternate days without complications.
DiscussionCentral venous catheters for renal replacement therapy are widely used in ICU. They have as known complication thrombus formation in right atrium.4 But it is a rare complication and the literature is scarce for this subject. The reported incidence for catheter-related right atrial thrombosis varies from 2% to 29%.5 Thrombus formation pathogenesis includes constant motion of the catheter tip, due to the movement of the heart, with friction of the distal catheter end to the endocardium and consequent irritation and damage of the atrial wall, resulting in mural thrombus formation at the contact point.2,6 In this particular patient with left femoral vein and right femoro-popliteus system thrombosis already documented, clot embolization with starting point in the lower limb venous system is other possible etiology.
Thrombus at the right atrium may cause obstruction to blood flow during renal replacement therapy that can complicate with cardiac arrest, as described in this case report. Immediate TEE performance allowed early diagnosis and timely treatment with resolution of the thrombus. TEE has better sensitivity and specificity when compared to TTE.7 Diagnosis was achieved only after performing the TEE, even after performing transthoracic echocardiography in the two previous days. Diagnosis confirmation could be made by Cardiac-MRI which allows tissue characterization. It wasn’t done in this case by the risk of nephrogenic systemic fibrosis.7
A review of literature revealed lack of uniformity in the treatments adopted. Removal of the catheter is the first recommendation but reduction of the risk of serious complications has not been proven.1 In this particular case, patient had no other available vascular access and the catheter removal could mean the loss of any venous access. Furthermore, Alteplase fibrinolytic therapy was effective in resolving the thrombus but prevented catheter removal, for the hemorrhagic risk. So immediate withdrawal of the catheter was not done. Patient started HD two days after fibrinolysis without complications.
Some authors suggest performing routine transthoracic echocardiography in case of having a hemodialysis catheter for long than 2 weeks, to avoid further lethal complications.6 However, as already mentioned, the imaging test with higher sensitivity and specificity is transesophageal echocardiography,7 with the possibility of false negatives in the implementation of transthoracic echocardiography.
Conflict of interestThe authors declare no conflict of interest.