To analyze characteristics, changes in oxygenation, and pulmonary mechanics, in mechanically ventilated patients with ARDS due to SARS-CoV-2 treated with prone position and evaluate the response to this maneuver.
DesignCohort study including patients with PaO2/FiO2 <150mmHg requiring prone position over 18 months. We classified patients according to PaO2/FiO2 changes from basal to 24h after the first prone cycle as: 1) no increase 2) increase <25%, 3) 25%–50% increase 4) increase >50%.
Setting33-bed medical-surgical Intensive Care Unit (ICU) in Argentina.
Patients273 patients.
InterventionsNone.
Main variables of interestEpidemiological characteristics, respiratory mechanics and oxygenation were compared between survivors and non-survivors. Independent factors associated with in-hospital mortality were identified.
ResultsBaseline PaO2/FiO2 was 116 [97–135]mmHg (115 [94–136] in survivors vs. 117 [98–134] in non-survivors; p=0.50). After prone positioning, 22 patients (8%) had similar PaO2/FiO2 values; 46(16%) increased PaO2/FiO2 ≤25%; 55 (21%) increased it 25%–50%; and 150 (55%), >50%. Mortality was 86%, 87%, 72% and 50% respectively (p<0.001). Baseline PaO2/FiO2, <100mmHg did not imply that patients were refractory to prone position. Factors independently associated with mortality were age, percentage increase in PaO2/FiO2 after 24h being in prone, and number of prone cycles.
ConclusionsOlder patients unable to improve PaO2/FiO2 after 24h in prone position and who require >1 cycle might early receive additional treatments for refractory hypoxemia. After the first 24h in the prone position, a low percentage of PaO2/FiO2 increase over baseline, beyond the initial value, was independently associated with higher mortality.
Analizar las características, cambios en la oxigenación y mecánica pulmonar, en pacientes ventilados mecánicamente con SDRA por SARS-CoV-2 tratados con posición prona, y evaluar la respuesta a esta maniobra.
DiseñoEstudio de cohorte que incluyó pacientes con PaO2/FiO2 <150mmHg que requirieron posición prona durante 18 meses. Se clasificaron los pacientes según los cambios de PaO2/FiO2 desde el basal y 24horas después del primer ciclo prono como: 1) Sin aumento 2) Aumento <25%, 3) 25–50% de aumento 4) Aumento >50%.
AmbitoUnidad de Cuidados Intensivos (UCI) médico-quirúrgica de 33 camas en Argentina.
Pacientes273 pacientes.
IntervencionesNinguna.
Principales variables de interésSe compararon características epidemiológicas, mecánica respiratoria y oxigenación entre sobrevivientes y no sobrevivientes. Se identificaron factores independientes asociados a la mortalidad hospitalaria.
ResultadosLa PaO2/FiO2 basal fue de 116 [97–135]mmHg (115 [94–136] en sobrevivientes vs. 117 [98–134] en no sobrevivientes; p=0,50). Después de la posición prona, 22 pacientes (8%) tenían valores similares de PaO2/FiO2; 46 (16%) aumentaron PaO2/FiO2 ≤25%; 55 (21%) lo aumentaron 25%–50%; y 150 (55%), >50%. La mortalidad fue de 86%, 87%, 72% y 50% respectivamente (p<0,001). La PaO2/FiO2 basal, <100mmHg no implicó que los pacientes fueran refractarios a la posición prona. Los factores asociados independientemente con la mortalidad fueron la edad, el aumento porcentual de PaO2/FiO2 después de 24horas en prona, y el número de ciclos prono.
ConclusionesLos pacientes mayores que no pueden mejorar PaO2/FiO2 después de 24 horas en posición prona y que requieren más de 1 ciclo podrían recibir tratamientos adicionales para la hipoxemia refractaria. Después de las primeras 24horas en decúbito prono, un bajo porcentaje de aumento de PaO2/FiO2 sobre el valor basal, más allá del valor inicial, se asoció de forma independiente con una mayor mortalidad.
The SARS-CoV-2 pandemic has increased the incidence of adult respiratory distress syndrome (ARDS) and has led to an increased need for rescue treatments for refractory hypoxemia, such as prone position. The physiological basis of the prone position lies in a more homogeneous distribution of the ventilation/lung tissue ratio, secondary to the more uniform opening of previously collapsed alveolar units. Prone position thus decreases overdistension and shear injury secondary to cyclic opening and closing in different alveolar populations and might be associated with lower mechanical ventilation induced injury.1–9
Prone position improves oxygenation and decreases mortality in patients with ARDS. However, there is no clear definition of failure to this treatment: the number of prone cycles that determine that the treatment is insufficient has not been determined. On the other hand, the early identification of patients who will exhibit a poor response to prone position allows the consideration of other therapeutic alternatives, which could potentially decrease mortality. The use of ECMO (extracorporeal oxygenation membrane) in this population with hypoxemic respiratory failure and severe ARDS could reduce mortality from 80% to 40% in selected candidates who present with severe, potentially reversible hypoxemia which is not associated with multi-organ failure.8–14
The need for a higher number of prone positioning cycles could be attributed to a more serious disease. However, it is unclear whether there are differences in mortality in patients requiring more cycles, or whether this is linked to treatment failure.15
The objective of our study was to analyze the epidemiological characteristics, oxygenation, and pulmonary mechanics, in patients with ARDS due to SARS-CoV-2 on mechanical ventilation who required prone position.
Secondary objectives were to identify independent determinants of mortality; and, particularly, whether the number of prone cycles was associated with a worse prognosis.
MethodsSettingsThis cohort study was conducted in a 33-bed medical-surgical Intensive Care Unit (ICU) in Argentina, which only admitted patients with SARS-CoV-2 pneumonia during the pandemic.
Inclusion and exclusion criteriaAll patients with ARDS on mechanical ventilation admitted between April 1 and September 1, 2021 in whom prone position was utilized due to severe hypoxemia were prospectively included.10
Additionally, patients with SARS-CoV-2 mechanically ventilated who had required prone position from March 1, 2020 to March 1, 2021 were retrospectively included.
The need for prone position was defined by attending physicians. Patients hospitalized outside the data collection period, those who did not have a record of respiratory mechanics values, and those who did not require prone position, were excluded.
VariablesWe recorded age, sex, comorbidities, APACHE II, and SOFA scores. Prior to turning patients into prone positioning, and 24h after the first session, the following variables were recorded: tidal volume (Vt, in mL/kg of predicted body weight [PBW]), respiratory rate (RR), blood gases, PaO2/FiO2, plateau pressure (cmH2O), PEEP (cmH2O), static compliance of the respiratory system (mL/cmH2O) and delta pressure in airway (ΔP) (Defined as End of Inspiration Pressure [Pinsp] – positive end-expiratory pressure [PEEP]) (cmH2O). A high ΔP was defined as >15cmH2O. Ventilatory ratio (VR), as a surrogate of deadspace, was estimated as Vt×RR×CO2/PBW (kg)×100×37.5; a value >1 correlate with increase deadspace.16
Respiratory variables were collected in a systematized paper instrument that is used for the daily monitoring and follow-up of patients on mechanical ventilation.
Each prone session lasted for 24h. Number of prone position cycles performed were registered.
After 24h in prone position, the percentage of increase in PaO2/FiO2 ratio related to the initial value was also recorded. PaO2/FiO2 ratio was obtained while the patient was in prone position. Hospital mortality, rate of tracheostomy, use of corticosteroids, use of neuromuscular blocking agents, length of hospitalization and UCI length of stay was also recorded.
Baseline characteristics, severity scores, respiratory variables before and after the first 24h in prone position, PaO2/FiO2 ratio changes and ΔP were compared between survivors and non-survivors. Because the subgroup of patients with PaO2/FiO2 <100mmHg (Severe ARDS) might be potential candidates for ECMO, they were also analyzed as a subgroup to discern if response to prone and outcomes were similar to those of the rest of the patients in the study.
Patients were classified according to the behavior of PaO2/FiO2 after the first session of prone position, according to the increase in PaO2/FiO2 in: 1) no increase, 2) increase <25%, 3) increase between 25 and 50%, 4) increase >50%.
Statistical analysisContinuous variables are expressed as mean±standard deviation or median and percentiles [0.25–0.75], according to the nature of the variables. Categorical variables are expressed as numbers and percentages [n (%)]. Statistical analysis was performed with t test for continuous variables of normal distribution, Mann–Whitney’s U test for continuous variables of non-normal distribution, Chi² for categorical variables and chi2 trend for evolutionary categorical variables. For the analysis of the effects of prone position on oxygenation and respiratory mechanics at baseline and at 24h, Wilcoxon signed-rank test was used.
A value of p<0.05 was defined as statistically significant.
Independent factors associated with in-hospital mortality were identified by means of Cox regression. Variables that presented a p<0.10 in univariate analysis or that were physiologically or clinically relevant were chosen for the regression model.
For the analysis of the data, the statistical software SPSS, version Statistics 25, was used.
Ethical issuesThis study was approved by the Institutional Review Board and informed consent was waived, since it was considered as posing minimal risk to the participants due to its retrospective study design.
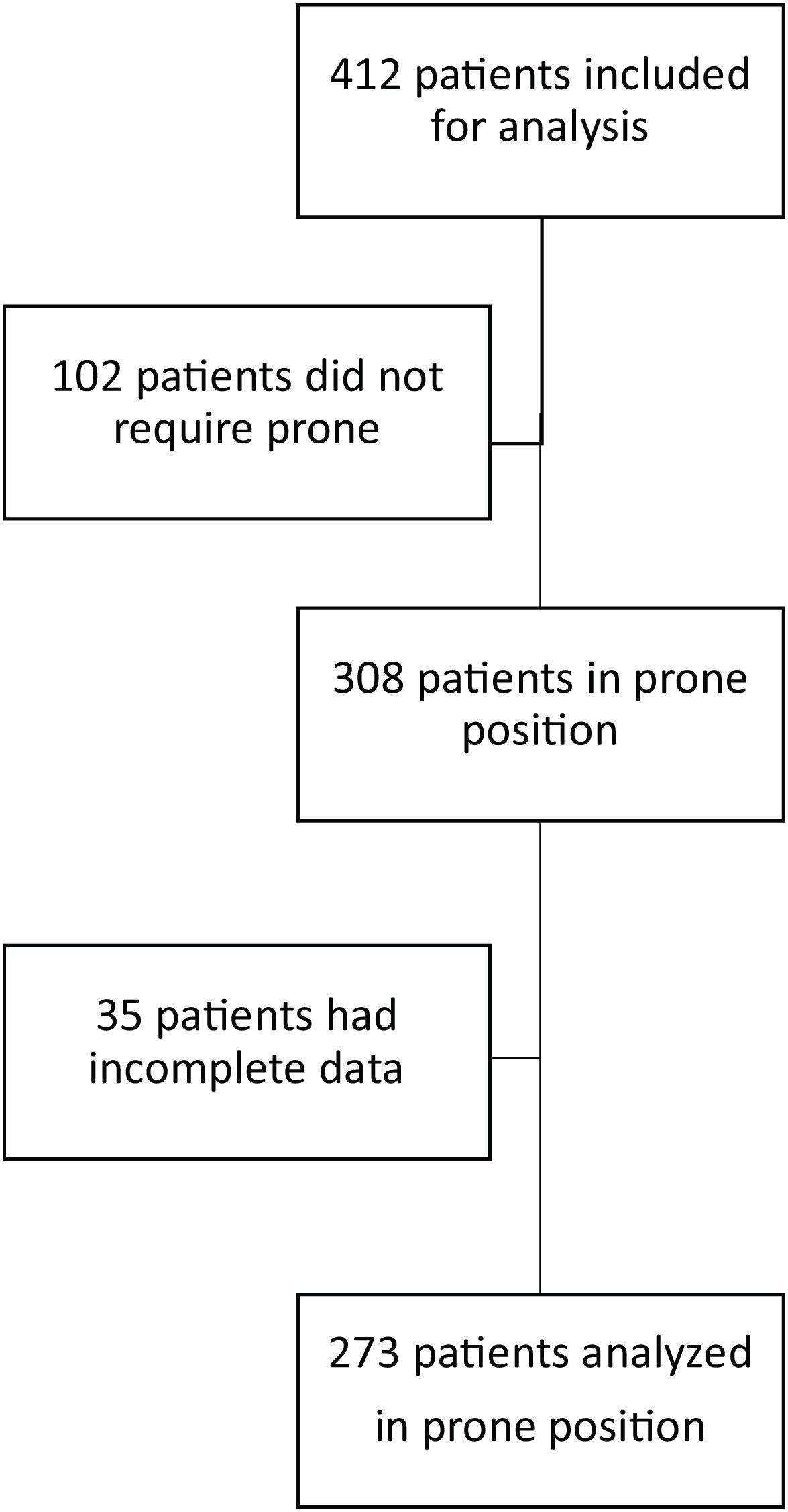
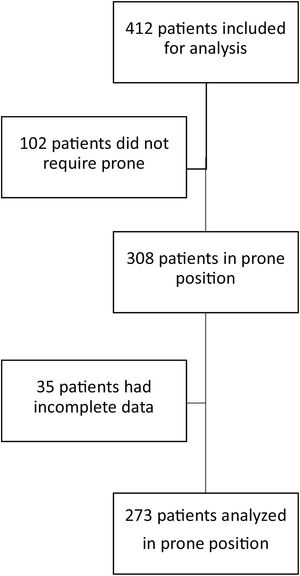
ResultsOver an 18-month period, 412 patients with SARS-CoV-2 requiring invasive mechanical ventilation were admitted to the ICU. In 308 of them prone position was used; however, in 35, clinical data were incomplete, so they were not included for analysis. We included 273 patients; of these, 126 were admitted between January 1, 2020, and March 1, 2021 and 147 between April 1, 2021, and September 1, 2021 (Fig. 1)
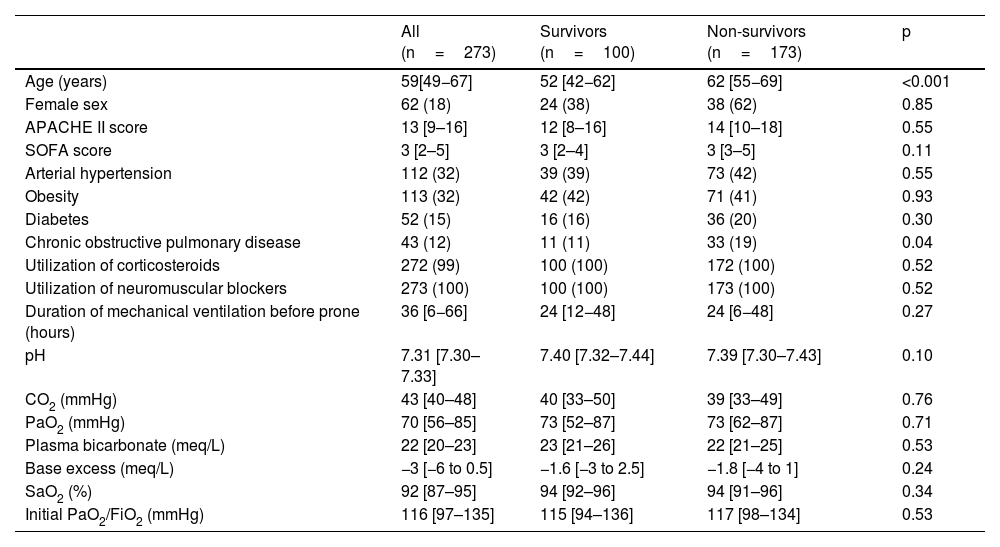
Of the 273 patients analyzed, 166 had moderate ARDS with PaO2/FiO2 ratio <150mmHg and 107 severe ARDS (PaO2/FiO2 <100mmHg). Age was 59 [49−67] years, with differences between survivors and non-survivors; 62 patients (18%) were female. Regarding comorbidities, 113 (32%) had obesity, 112 (32%) arterial hypertension, 52 (15%) diabetes type II, and 43 (12%) chronic obstructive pulmonary disease (COPD). This last comorbidity was the only one that differed between survivors and non-survivors. The scores APACHE II and SOFA were 13 [9–16] and 3 [2–5] respectively (Table 1).
Baseline characteristics of patients receiving prone position for ARDS due to SARS-CoV-2.
| All (n=273) | Survivors (n=100) | Non-survivors (n=173) | p | |
|---|---|---|---|---|
| Age (years) | 59[49−67] | 52 [42−62] | 62 [55−69] | <0.001 |
| Female sex | 62 (18) | 24 (38) | 38 (62) | 0.85 |
| APACHE II score | 13 [9–16] | 12 [8–16] | 14 [10–18] | 0.55 |
| SOFA score | 3 [2–5] | 3 [2–4] | 3 [3–5] | 0.11 |
| Arterial hypertension | 112 (32) | 39 (39) | 73 (42) | 0.55 |
| Obesity | 113 (32) | 42 (42) | 71 (41) | 0.93 |
| Diabetes | 52 (15) | 16 (16) | 36 (20) | 0.30 |
| Chronic obstructive pulmonary disease | 43 (12) | 11 (11) | 33 (19) | 0.04 |
| Utilization of corticosteroids | 272 (99) | 100 (100) | 172 (100) | 0.52 |
| Utilization of neuromuscular blockers | 273 (100) | 100 (100) | 173 (100) | 0.52 |
| Duration of mechanical ventilation before prone (hours) | 36 [6−66] | 24 [12−48] | 24 [6−48] | 0.27 |
| pH | 7.31 [7.30–7.33] | 7.40 [7.32–7.44] | 7.39 [7.30–7.43] | 0.10 |
| CO2 (mmHg) | 43 [40–48] | 40 [33–50] | 39 [33–49] | 0.76 |
| PaO2 (mmHg) | 70 [56–85] | 73 [52–87] | 73 [62–87] | 0.71 |
| Plasma bicarbonate (meq/L) | 22 [20–23] | 23 [21–26] | 22 [21–25] | 0.53 |
| Base excess (meq/L) | −3 [−6 to 0.5] | −1.6 [−3 to 2.5] | −1.8 [−4 to 1] | 0.24 |
| SaO2 (%) | 92 [87–95] | 94 [92–96] | 94 [91–96] | 0.34 |
| Initial PaO2/FiO2 (mmHg) | 116 [97–135] | 115 [94–136] | 117 [98–134] | 0.53 |
Data are expressed as mean (standard deviation) median [percentiles 0.25–0.75], or n (%).
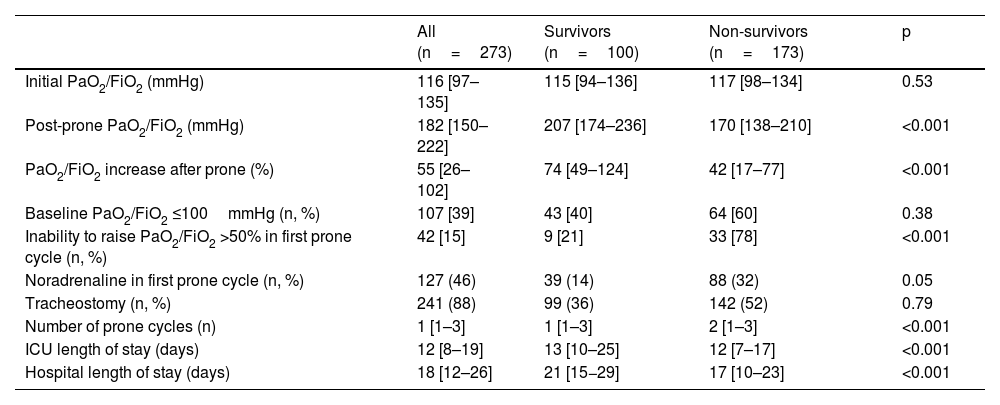
The PaO2/FiO2 immediately prior to the start of the first prone cycle was 116 [97–135]mmHg in the total group without differences between survivors and in non-survivors. After 24h in prone position, PaO2/FiO2 was 182 [150–222]mmHg in the total group; with significant differences between survivors vs. non-survivors (207mmHg [174−236] vs.170mmHg [138−210]; p<0.001) (Table 2). ICU and hospital length of stay for the entire population and for survivors and non-survivors is shown in Table 2.
Oxygenation before and after the first prone position cycle. Comparison between survivors and non-survivors.
| All (n=273) | Survivors (n=100) | Non-survivors (n=173) | p | |
|---|---|---|---|---|
| Initial PaO2/FiO2 (mmHg) | 116 [97–135] | 115 [94–136] | 117 [98–134] | 0.53 |
| Post-prone PaO2/FiO2 (mmHg) | 182 [150–222] | 207 [174–236] | 170 [138–210] | <0.001 |
| PaO2/FiO2 increase after prone (%) | 55 [26–102] | 74 [49–124] | 42 [17–77] | <0.001 |
| Baseline PaO2/FiO2 ≤100mmHg (n, %) | 107 [39] | 43 [40] | 64 [60] | 0.38 |
| Inability to raise PaO2/FiO2 >50% in first prone cycle (n, %) | 42 [15] | 9 [21] | 33 [78] | <0.001 |
| Noradrenaline in first prone cycle (n, %) | 127 (46) | 39 (14) | 88 (32) | 0.05 |
| Tracheostomy (n, %) | 241 (88) | 99 (36) | 142 (52) | 0.79 |
| Number of prone cycles (n) | 1 [1–3] | 1 [1–3] | 2 [1–3] | <0.001 |
| ICU length of stay (days) | 12 [8–19] | 13 [10–25] | 12 [7–17] | <0.001 |
| Hospital length of stay (days) | 18 [12–26] | 21 [15−29] | 17 [10–23] | <0.001 |
Data are expressed as median [percentiles 0.25–0.75], or n (%).
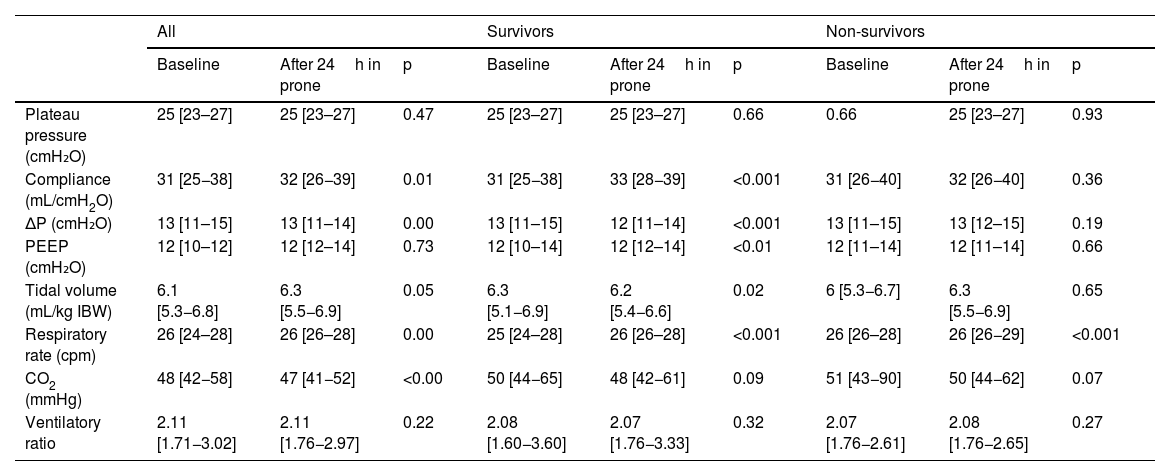
There were no differences in baseline ΔP between survivors and non-survivors (13 [11–15] vs. 13 [11–15], respectively, but the response of both subgroups to prone position was different (12 [11–14] vs. 13 [12–15]; p<0.00. Fifty-nine (21%) patients presented with values of ΔP >15cmH2O and in 37 (13%) patients ΔP was elevated after proning. In the entire group, static compliance of the respiratory system in supine position was 30 [25−38]mL/cmH2O, and 32 [26−39]mL/cmH2O after prone positioning (p<0.01); displaying significant differences between survivors and nonsurvivors. Survivors had 31 [25−38] in supine and 33[28−39]mL/cmH2O after prone (p<0.001). Non survivors had 29 [24–35] in supine and 31[23–35]mL/cmH2O after prone position (p=0.36) (Table 3).
Respiratory mechanics based on mortality and mechanical ventilation variables. Comparison between survivors and non-survivors.
| All | Survivors | Non-survivors | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 24h in prone | p | Baseline | After 24h in prone | p | Baseline | After 24h in prone | p | |
| Plateau pressure (cmH₂O) | 25 [23–27] | 25 [23–27] | 0.47 | 25 [23–27] | 25 [23–27] | 0.66 | 0.66 | 25 [23–27] | 0.93 |
| Compliance (mL/cmH2O) | 31 [25−38] | 32 [26−39] | 0.01 | 31 [25−38] | 33 [28−39] | <0.001 | 31 [26−40] | 32 [26−40] | 0.36 |
| ΔP (cmH₂O) | 13 [11–15] | 13 [11–14] | 0.00 | 13 [11–15] | 12 [11–14] | <0.001 | 13 [11–15] | 13 [12–15] | 0.19 |
| PEEP (cmH₂O) | 12 [10–12] | 12 [12–14] | 0.73 | 12 [10–14] | 12 [12–14] | <0.01 | 12 [11–14] | 12 [11–14] | 0.66 |
| Tidal volume (mL/kg IBW) | 6.1 [5.3−6.8] | 6.3 [5.5−6.9] | 0.05 | 6.3 [5.1−6.9] | 6.2 [5.4−6.6] | 0.02 | 6 [5.3−6.7] | 6.3 [5.5−6.9] | 0.65 |
| Respiratory rate (cpm) | 26 [24–28] | 26 [26–28] | 0.00 | 25 [24–28] | 26 [26–28] | <0.001 | 26 [26–28] | 26 [26−29] | <0.001 |
| CO2 (mmHg) | 48 [42−58] | 47 [41−52] | <0.00 | 50 [44−65] | 48 [42−61] | 0.09 | 51 [43−90] | 50 [44−62] | 0.07 |
| Ventilatory ratio | 2.11 [1.71−3.02] | 2.11 [1.76−2.97] | 0.22 | 2.08 [1.60−3.60] | 2.07 [1.76−3.33] | 0.32 | 2.07 [1.76−2.61] | 2.08 [1.76−2.65] | 0.27 |
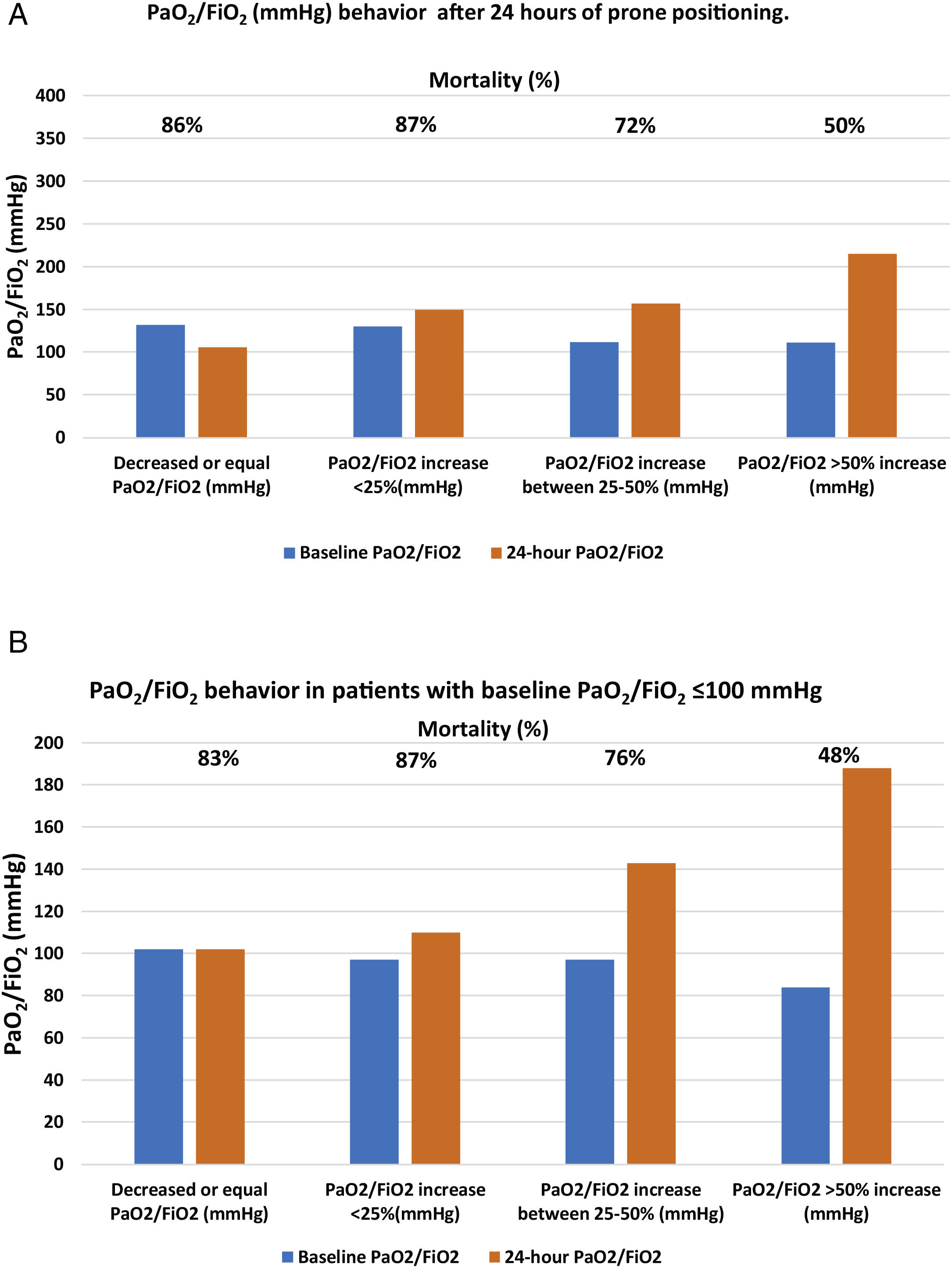
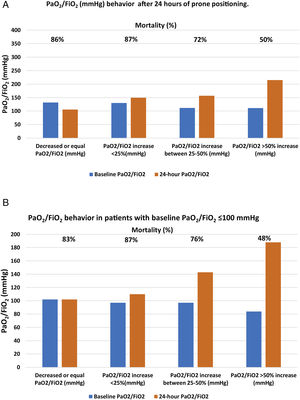
| Panel A. Patient mortality according to the different PaO2/FiO2 (mmHg) behavior after 24h of prone positioning. | |||||
|---|---|---|---|---|---|
| N (%) | Baseline PaO2/FiO2 | 24-h PaO2/FiO2 | Mortality (%) | p | |
| Decreased or equal PaO2/FiO2 (mmHg) | 22 (8) | 132 [112–145] | 106 [95–139] | 19 (86) | <0.001 |
| PaO2/FiO2 increase <25% (mmHg) | 46 (16) | 130 [118–143] | 150 [132–166] | 40 (87) | |
| PaO2/FiO2 increase between 25%–50% (mmHg) | 55 (21) | 112 [101–130] | 157 [144–185] | 40 (72) | |
| PaO2/FiO2 >50% increase (mmHg) | 150 (55) | 111 [86–128] | 215 [185−228] | 75 (50) | |
| Panel B. Mortality based on PaO2/FiO2 behavior in patients with baseline PaO2/FiO2 <100mmHg | |||||
|---|---|---|---|---|---|
| N (%) 107(39) | Baseline PaO2/FiO2 (mmHg) | PaO2/FiO2 24h of prone (mmHg) | Mortality (%) | p | |
| PaO2/FiO2 decreased or equal | 6 (5) | 100 [100–105] | 102 [100–104] | 5 (83) | <0.001 |
| PaO2/FiO2 increase <25% | 8 (7) | 97 [82–98] | 110 [117–132] | 7 (87) | |
| PaO2/FiO2 increase between 25%−50% | 25 (23) | 97 [84−105] | 143 [117−153] | 19 (76) | |
| PaO2/FiO2 increase >50%g | 67 (55) | 84 [72−97] | 188 [140−221] | 32 (48) | |
Data are expressed as median [percentiles 0.25−0.75], or n (%).
Regarding the surrogates of dead space, baseline CO2 was 48 [42−58] and after the first prone cycle it was 47 [41–52] (p<0.01). There were differences between survivors and non-survivors but they did not reach statistical significance. We did not find differences in the respiratory ratio before and after prone treatment (Table 3).
After 24h in prone position, and compared to baseline values, 22 patients (8%) had the same value or experienced a decrease in PaO2/FiO2; 46 patients (16%) showed an increase in PaO2/FiO2 ≤25%; 55 patients (21%) had an increase of 25%–50%; and 150 patients (55%) had an increase >50%. Mortality in each group was, respectively, 86%, 87%, 72%, 50% (p<0.001) (Table 3 panel A and Fig. 2, panel A).
Of the 107 patients who presented with severe ARDS with baseline PaO2/FiO2 values, <100mmHg, 64 (60%) died; however, that initial PaO2/FiO2 value was not associated with higher mortality. Patients who managed to raise their oxygenation by more than 50% presented a mortality rate comparable to those with initial PaO2/FiO2 >100mmHg and a similar increase in oxygenation. Having debuted with PaO2/FiO2, ratio <100mmHg did not imply that the patient was refractory to prone position. The baseline absolute value of PaO2/FiO2 <100mmHg showed no association with higher mortality in this cohort of patients but the inability to raise PaO2/FiO2 more than 50% from baseline after the first prone cycle did (Table 2, Table 3 panel B and Fig. 2, panel B).
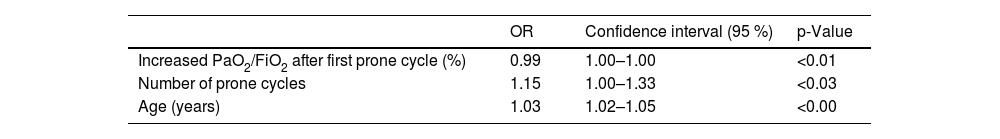
Variables that were independently associated with mortality according to Cox regression were age, percentage of increase in PaO2/FiO2 after 24h in prone position, and number of prone cycles (Table 4).
DiscussionOur most important finding was that after the first 24h in the prone position, the percentage of PaO2/FiO2 increase over baseline was independently associated with higher mortality, beyond the absolute value. That might suggest that patients who raise PaO2/FiO2 <50% above baseline could be refractory to, or failed to, prone treatment. In addition, with each consecutive, additional cycle of prone the chances of survival decreased significantly. Older age was also a factor that was associated with lower survival.
A higher mortality in severe ARDS with PaO2/FiO2 values <100mmHg has been described in the literature.17–19 In our cohort, the initial value of PaO2/FiO2 was not related to prognosis; instead, the determinant was the change in the PaO2/FiO2 after having been in prone position for 24h. In addition, this was an early determinant, since it evident at 24h of treatment―which could help identifying patients who would most benefit in terms of mortality.
Our study includes a large population of patients undergoing prone position comprehensively analyzed from the physiological point of view; and it presents an exhaustive analysis of oxygenation, number of prone cycles and respiratory mechanics changes. Other authors have described a similar change in mortality associated with the percentage increase in PaO2/FiO2 ratio in patients with ARDS and prone.20,21
Whereas Langer et al. studied 648 patients in prone position, only 78 were extensively analyzed. Additionally, these authors included a small number of patients and a mix of mild, moderate and severe ARDS, therefore the final effect of prone position on oxygenation might have been attenuated.22 Camporota et al. described a mixed population of patients, with COVID-19 and non-COVID-19 ARDS.24 Cunha et al. included 412 patients in prone position with COVID-19 ARDS; while the authors describe a positive response on oxygenation, they attribute their high mortality rates to pre-existing health status and comorbidities.23
Langer et al., in a subgroup of COVID-19 patients who received prone position, considered those who presented a 20mmHg increase in PaO2/FiO2 as responders to the treatment. However, this improvement can be considered as having little significance in clinical practice.22 In our cohort of patients, the group that increased PaO2/FiO2 by 20mmHg (which corresponded to a 25% increase in PaO2/FiO2 from baseline approximately) had 87% mortality, and a 30% higher risk of dying than those who increased PaO2/FiO2 by 50%. Patients who were unable to raise PaO2/FiO2 values to more than 50% exhibited a very high mortality rate, between 72 and 87%. That behavior was similar to that described by Park et al. and Lee et al.20,21
In the Brazilian multicenter observational study of 574 patients with COVID-19 who received prone positioning, the authors classified the response the same way as Langer et al. While they had a larger number of patients who responded to treatment (72%) mortality was similar in both responders and non-responders.23
We believe that using an absolute value of increase in PaO2/FiO2 to define responders to treatment does not adequately reflect the response of patients with varying degrees of ARDS severity. For example, in a patient starting prone treatment with PaO2/FiO2 of 50mmHg, a 20mmHg-increase represents a response of almost 50%, while in a patient starting with PaO2/FiO2 of 140mmHg an increase in PaO2/FiO2 of 20mmHg is barely an increase of 15%. In our cohort of patients, the evolution of these cases was very different.
We had classified patients according to the behavior of PaO2/FiO2 after the first session of prone position, the subgroup of patients that increased PaO2/FiO2 by 20mmHg (corresponding to a 25% increase in PaO2/FiO2 from baseline had approximately 87% mortality, and a 30% higher risk of death than those that increased PaO2/FiO2 by 50%. In our population, patients who were unable to raise the values of PaO2/FiO2 by more than 50%, had a remarkably high mortality, between 72 and 87%.
Our findings regarding the change of oxygenation in response to prone position are consistent with the results of other researchers, both in COVID-19 and non-COVID-19 populations.20,21 A recently published observational study comparing both diseases demonstrate, after a sensitivity analysis, that the percentage of change in PaO2/FiO2 after the first prone cycle predicted weaning of mechanical ventilation at 90 days, with an AUROC of 0.87.21 Similarly, a retrospective study in moderate and severe COVID-19 and non-COVID-19 ARDS patients suggests a similar response to oxygenation after administering the first prone position cycle.20
The duration of prone position required to improve oxygenation in a sustained manner is unknown. Short cycles have been described as ineffective25; but it has not been established how many cycles, and how prolonged they should be, to consistently reverse hypoxemia. For example, Guerin et al. utilized 4.4 cycles per patient of 17±3h each in the PROSEVA study.9
It has been suggested that SARS-CoV-2 as a cause of ARDS behaves more aggressively and might require a higher number of cycles. Langer et al., in the abovementioned study, reported an utilization of 3 [1–4] prone position cycles, although the percentage of patients who received this therapeutic maneuver was strikingly high, and this may have affected the results.22 Camporota et al. reported a higher number of prone cycles in the COVID-19 population compared to non-COVID-19 ARDS (4 [2–6] vs. 2 [1–3], respectively).24 Cunha et al. reported 2 [1–3] sessions in COVID-19 patients, and found, as we did, that the number of prone sessions needed was independently associated with higher mortality.23
On the other hand, the need to extend prone treatment identifies patients with poorer response in terms of oxygenation, or with an initial adequate response that cannot be sustained over time. Patients with a higher need for prone cycles or with a requirement for longer cycles are likely to have more severe lung disease. In addition, the chance of surviving with each subsequent cycle of prone treatment decreases. The delay in early identification of these patients might exclude them from other rescue therapies for severe hypoxemia which might have been applied earlier, such as ECMO.13,14 Therefore, patients who fail to increase PaO2/FiO2 by more than 50%, or who increase it but subsequently deteriorate oxygenation and require additional prone cycles, should be readily evaluated for other treatments for refractory hypoxemia, since the mortality rate in these group might exceed 80%.17
Patient age might be another factor contributing to decision-making. In our study, older patients had higher mortality, in agreement with what other researchers found: from the beginning of the pandemic, increasing age was a risk factor for severe illness due to COVID-19.4,25–27 Also, in the subgroup of patients requiring critical care, older age was associated with higher mortality. Recently, a study showed that elderly patients with COVID-19 who required life-sustaining treatments in the ICU had higher mortality compared to other elderly patients with similar treatments who were admitted for other diseases than COVID-19.27,28
Our study has strengths and weaknesses. Since it is an observational study carried out in a single centre, it is subjected to biases and possible residual confounders. As we did not make a sample size calculation, lack of power for some comparisons might have occurred. A subgroup of data, corresponding to the first wave of COVID-19, was collected retrospectively. Most of the data, however, were recorded prospectively, using the same collection instrument used for routine daily monitoring in the ICU. Finally, our conclusions should only apply to patients with ARDS secondary to COVID-19 since there is no control group composed of patients with ARDS from other causes. Nevertheless, this study has several strengths: we include a large number of patients, and our findings are in line with the results of other researchers’ results. We provide novel information regarding prone treatment in patients with ARDS, which might be useful for early decision-making in severely compromised patients, underscoring the need of escalating therapies in this group.
ConclusionsThe response in prone after the first 24h defined ARDS trajectory, rather than the baseline PaO2/FiO2: an increase of PaO2/FiO2 >50% after the first cycle of prone position is associated with lower mortality, even if the baseline PaO2/FiO2 is low.
Authors’ contributionsCL is the study lead and guarantor for this paper. CG, GM, JMD, MM, contributed to conception and design of the study. JRL, SC, AL, JR, GS, LF, AR, EB, AA, LC. CG, JMD and EE performed the main statistical analysis, and EE has critically evaluated those results. CG, GM mainly wrote the first draft of this paper, and MM, GM, CL, EE revised it critically for important intellectual content. All authors read and approved the final manuscript.
FundingThere was no funding for this study.
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participateThis study was approved by the institutional review board and has informed consent. This study was considered as posing minimal risk to the study participants because of its retrospective study design.
All authors consent publication of this paper.
Conflict of interestThe authors have no conflicts of interest to declare.