The current pandemic of coronavirus disease 2019 (Covid-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is characterised by a spectrum of clinical presentations which differ greatly in severity.1 These range from an asymptomatic or mild upper respiratory tract infection to progressive inflammatory pulmonary cellular infiltration, severe hypoxaemia and the development of an acute respiratory distress syndrome (ARDS) or ARDS-like phenomenon.2 A subset of these patients will progress to multi-organ failure and death.
Whilst there is on-going and emerging observational data from China, Europe and the UK on the characteristics of patients who are admitted to ICU, it is not yet clear and remains a question of some urgency how to identify those patients who are likely to require high levels of critical care supports.
This was a cross-sectional study, conducted during the period from 23 March to 26 April 2020. We have seen a high level of admissions of critically ill patients with Covid-19 pneumonia to our intensive care unit (ICU) over the past 30 days. Herewith, we present the clinical and biochemical characteristics of a consecutive cohort of patients (n=39) with polymerase chain reaction (PCR) proven symptomatic Covid-19 infection admitted to our ICU.
Most patients were generally considered young, with a median of 60 years of age (IQR 53–66) and a predominance of male gender (74%). Patients with a BMI greater than 30 represented 42% of all admissions. The most common comorbid factor was hypertension with a prevalence of 36%, followed by type 2 diabetes mellitus with a prevalence of 23%. However in 26% of cases there was an absence of any known pre-existing medical conditions. A total of 15% of patients were healthcare workers.
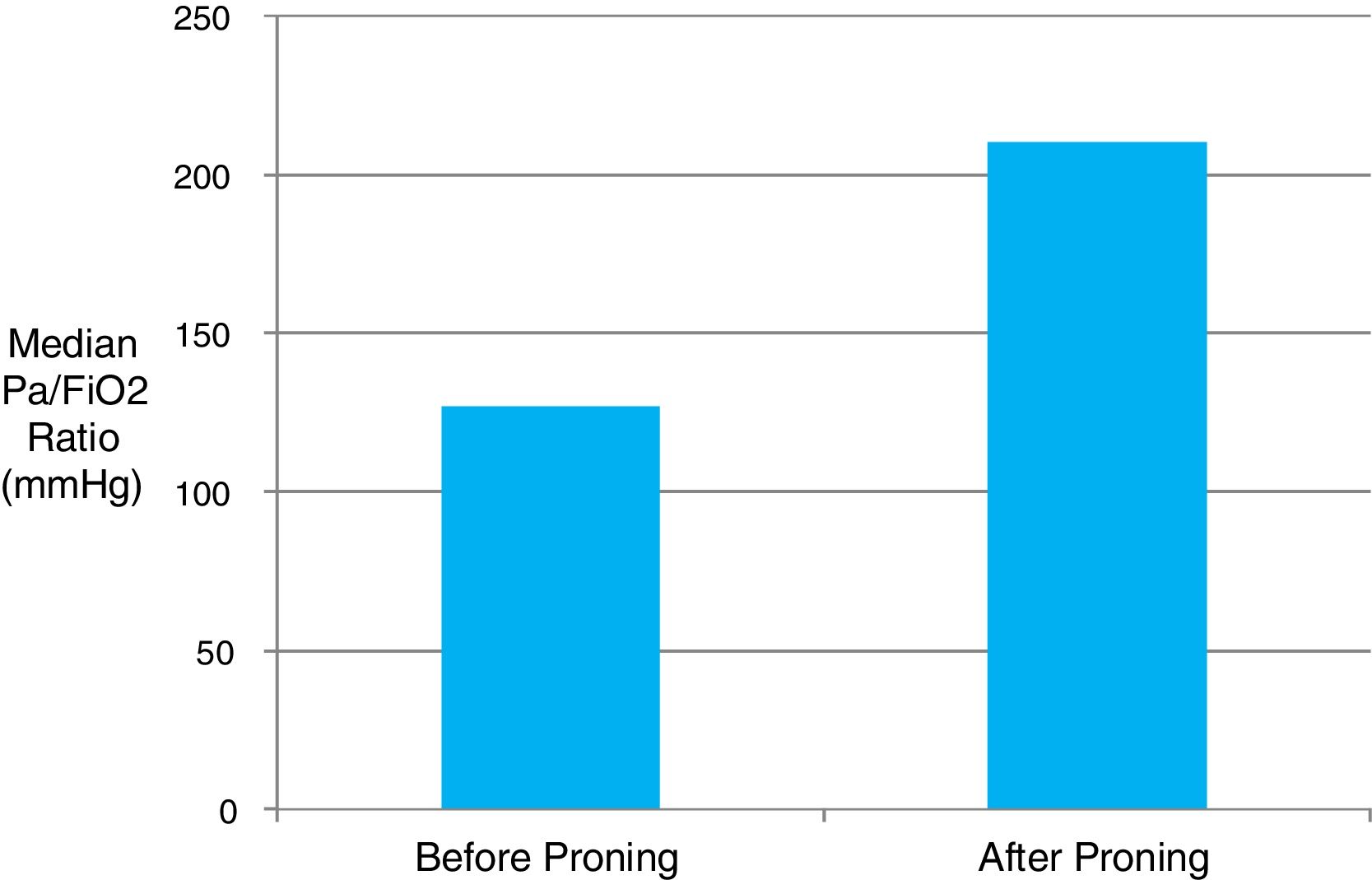
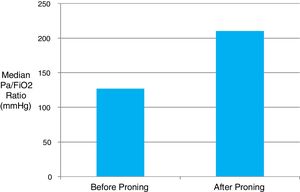
Patients admitted from the ward spent a median of 2 days (IQR 1–3.75) there prior to ICU admission. The median time interval from the first reported onset of symptoms to ICU admission was fairly consistent at 10 days (IQR 7–11). Patients presented with progressive respiratory failure, hypoxaemia and bilateral pulmonary infiltrates on plain radiograph. Invasive mechanical ventilation was provided in 74% of cases, and 76% of these required intubation within the first 24h of ICU admission. A rapid deterioration in respiratory function over the several hours preceding ICU admission was common. Proning position was utilised in 79% of patients with a median of 6×16-hour proning sessions provided per patient. Continuous neuromuscular blockade was provided in 86% of mechanically ventilated patients. Inhaled nitric oxide was delivered to 17% of patients, and one patient was referred for extracorporeal membrane oxygenation (ECMO). Median PaO2/FiO2 ratio on admission was 130mmHg (IQR 97.5–180) reflecting a high proportion of moderate–severe ARDS. The majority of patients were prone responsive with a median rise in the PaO2/FiO2 ratio of 83mmHg (11kPa) observable immediately following the first proning (Fig. 1). The information regarding the response similar in the first proning than in the rests was not obtained.
In terms of other organ supports, vasopressors were required in 54% of cases, 59% developed an acute kidney injury (AKI) using KDIGO definitions, and renal replacement therapy was required in 38% of cases. The ICU mortality in our series is 21% out of the 24 patients who have either died or been discharged from critical care to date.
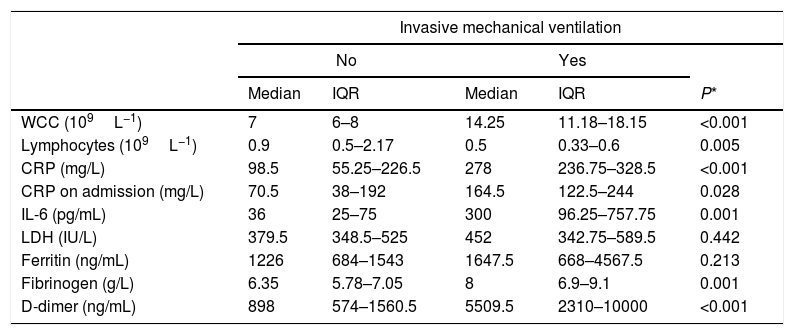
Those patients who required mechanical ventilation (representing the sickest patients) had significantly higher levels throughout ICU admission in CRP, interleukin 6 (IL-6), D-dimer, white cell count (WCC) and fibrinogen, and significantly lower lymphocyte counts than non-ventilated patients (Table 1).
Biochemical markers throughout ICU admission of ventilated and non-ventilated patients.
| Invasive mechanical ventilation | |||||
|---|---|---|---|---|---|
| No | Yes | ||||
| Median | IQR | Median | IQR | P* | |
| WCC (109L−1) | 7 | 6–8 | 14.25 | 11.18–18.15 | <0.001 |
| Lymphocytes (109L−1) | 0.9 | 0.5–2.17 | 0.5 | 0.33–0.6 | 0.005 |
| CRP (mg/L) | 98.5 | 55.25–226.5 | 278 | 236.75–328.5 | <0.001 |
| CRP on admission (mg/L) | 70.5 | 38–192 | 164.5 | 122.5–244 | 0.028 |
| IL-6 (pg/mL) | 36 | 25–75 | 300 | 96.25–757.75 | 0.001 |
| LDH (IU/L) | 379.5 | 348.5–525 | 452 | 342.75–589.5 | 0.442 |
| Ferritin (ng/mL) | 1226 | 684–1543 | 1647.5 | 668–4567.5 | 0.213 |
| Fibrinogen (g/L) | 6.35 | 5.78–7.05 | 8 | 6.9–9.1 | 0.001 |
| D-dimer (ng/mL) | 898 | 574–1560.5 | 5509.5 | 2310–10000 | <0.001 |
Initial data from the early stages of this pandemic had suggested that unlike SARS-CoV infection, a severe clinical phenotype was manifested only in the elderly and in those with underlying comorbidities. This has not been borne out by our experience, with a significant minority of our ICU patients relatively young and without any pre-existing risk factors. The complex host immunological response to SARS-CoV-2 is not yet fully understood, but studies of SARS-CoV have demonstrated a dysregulated inflammatory response with elevated levels of pro-inflammatory cytokines, along with increased monocyte–macrophage and neutrophil accumulation in the lungs as being important factors leading to acute lung injury.3,4 Inflammatory markers such as IL-6, D-dimers, CRP, WCC, lymphocyte count and fibrinogen levels were strong discriminators for a requirement for ventilation in our patients with Covid-19 disease whilst Ferritin and LDH levels were not statistically different in patients with and without mechanical ventilation. This finding is not being found in other case series however probably this is related to a limited sample size. D-dimer, CRP and WCC levels showed the greatest statistical significance, indicating that they may be useful surrogate markers for risk stratification for healthcare systems and ICUs looking after Covid-19 patients, where routine IL-6 assays are not available.
Conflicts of interestNone declared.