Surgery and massive trauma are frequently associated with significant derangement of haemostatic capacity, caused by loss, consumption, endogenous inhibition (by the protein C pathway), dilution of coagulation factors, and increased clot breakdown (fibrinolysis). Haemostatic therapy in patients without pre-existing haemostatic disorders aims to substitute key components (clotting factors and other blood components) by transfusion of allogeneic blood products, including fresh frozen plasma (FFP), platelet concentrate (PC), red blood cells (RBC) and, in some countries, cryoprecipitate or coagulation factor concentrates. Most commonly, it is not a prophylactic administration of coagulation factors to supranormal levels but rather a correction of critically reduced coagulation factor activity or levels.1 Coagulopathy must be fixed fast in order to prevent an aggravating bloody vicious cycle leading to massive bleeding and massive transfusion.1,2 Of note, fibrinogen is the first coagulation factor to fall to a suboptimal level early during bleeding and dilutional coagulopathy; fibrinogen supplementation is therefore recommended early in patients with relevant bleeding to maintain plasma fibrinogen levels above 1.5–2.0g/L.1 Coagulation factors required for thrombin generation fall to suboptimal levels late; prothrombin complex supplementation is therefore not recommended as a first-line therapy in bleeding patients.
Alternatives for coagulation factor supplementationTypically, standard preparation FFP contains 2.0g/L of fibrinogen (equivalent to 0.6g in a 300mL unit), as well as other pro- and anti-coagulant factors found in plasma, acute phase proteins (cytokines), electrolytes, immunoglobulins and albumin. Concentrations are heterogeneous depending on the donor and mode of preparation.
Pasteurised and lyophilised human fibrinogen concentrate is typically reconstituted in 50mL of sterile water to a final concentration of 20g/L (10 times higher final concentration than FFP). Each vial of fibrinogen concentrate contains about 1.0g of fibrinogen, as well as albumin, l-arginine hydrochloride, sodium chloride and sodium citrate. The concentrated dose of fibrinogen provided by fibrinogen concentrate could be preferable to FFP for restoring plasma fibrinogen levels because of its rapid availability (no thawing), reduced volume (faster infusion times) and increased safety, and has recently been suggested to be more effective than administration of FFP.3
Prothrombin complex concentrates (PCC) contain coagulation factors II, VII, IX and X in concentrations depending on the manufacturing process but higher than in FFP. In perioperative bleeding PCC containing activated coagulation factors (factor eight bypassing activity) is not routinely used.
Clinical routine despite poor efficacyClinical use of FFP in component therapy has increased over the past four decades. Reports of the success of high FFP:RBC ratios may be responsible for some of this rise. The benefits of any intervention should outweigh the risks: FFP has been associated with increased risk of morbidity and mortality.4 The evidence to support the effectiveness of FFP in the perioperative setting or its appropriate dosing in the massive transfusion setting has been questioned3,5: Previous systematic reviews concluded that efficacy of FFP was inconsistent across all assessed outcomes. Overall, FFP showed a positive effect for 28% and a negative effect for 22% of outcomes. There was limited evidence that FFP reduced mortality: 50% of outcomes associated FFP with reduced mortality (typically trauma/massive bleeding), while 20% were associated with increased mortality (typically surgical/non-massive bleeding). The evidence for the efficacy of fibrinogen concentrate was consistently positive with no negative effects reported. When comparing FFP against fibrinogen concentrate the latter was superior for >50% of outcomes in terms of reducing blood loss, allogeneic transfusion requirements, length of intensive care unit and hospital stay, and increasing plasma fibrinogen levels. A Cochrane review assessed the use of fibrinogen concentrate in bleeding patients with the conclusion that it seems to reduce transfusion requirements but further studies are warranted to demonstrate its harms and benefits.6 However, it should be noted that there is currently no evidence that other therapies, such as therapeutic plasma or cryoprecipitate, are safer or more effective in the setting of acquired bleeding.
Clinical effectiveness of FFP is inferior to PCC, e.g. in the clinical scenario of warfarin reversal.1 Evidence is lacking comparing the efficacy of FFP versus PCC in restoring thrombin generation if reduced in massive bleeding. There are no factor concentrates currently available, e.g. for coagulation factor V, XI. After massive transfusion, replacement of these enzymes can only be achieved by FFP transfusion.7,8
From the perspective of the best available scientific evidence the conclusion could be as follows: the weight of evidence does not appear to support the clinical effectiveness of FFP for surgical/massive trauma patients, and suggests it can be detrimental. According to evidence-based medicine methodology it would be rational to recommending that FFP should not be used outside clinical trials for fibrinogen and prothombin complex substitution until further data demonstrate the applicability of plasma to treat coagulopathy in bleeding patients. Currently, 4 randomised controlled trials investigating the efficacy and safety of therapeutic plasma in comparison with other available choices of haemostatic therapy for fibrinogen supplementation are ongoing; no data have been released as yet.
Volume therapy in severe bleedingMassive transfusion ratio-driven protocols deliver per each combination of 1 unit of FFP, RBC and PC about 600mL. Reconstitution of these 3 allogeneic blood products results in dilution of erythrocytes, platelets and fibrinogen levels9; adequate and timely correction of coagulopathy is not feasible. Absolute hypovolaemia due to blood loss is not a recommended indication for FFP8; safer alternatives with colloidal/crystalloidal solutions exist.1 The European trauma guidelines recommend that plasma transfusion be avoided in patients without substantial bleeding (Grade 1B); irrespective of hypovolaemia.10 Recent data on beneficial effects of FFP on the glycocalyx lack the head-to-head comparison with albumin and synthetic colloidal solution which also have been shown to protect the endothelial lining.
Cost-effectivenessCost-effectiveness of a FFP ratio-driven transfusion protocol has not been investigated yet.1 Implementation of monitoring-guided goal-directed management algorithms primarily using clotting factor concentrates while avoiding allogeneic blood products including FFP can reduce costs in trauma, cardiac surgery and liver transplantation.1 Noteworthy, overall costs have been found to be reduced despite higher direct costs for clotting factor concentrates and viscoelastic tests.
Conclusion and outlookThere is a paucity of high-quality evidence reporting the clinical effectiveness of FFP in a perioperative or massive trauma setting, despite the long period of its usage. Controlled trials with robust blinding and randomisation procedures are warranted.
Currently, FFP is overused, FFP is abused for volume replacement, adequate laboratory parameters for assessing the indication for haemostatic therapy are underused (e.g. fibrinogen level or fibrinogen polymerisation; thrombin generation potential), adverse outcomes and harms related to plasma transfusion are not monitored and ignored.
An individualised, rational plasma transfusion behaviour with careful monitoring of indication, efficacy (e.g. laboratory parameters before and after transfusion), as well as side effects could help improving quality of care and patient safety.
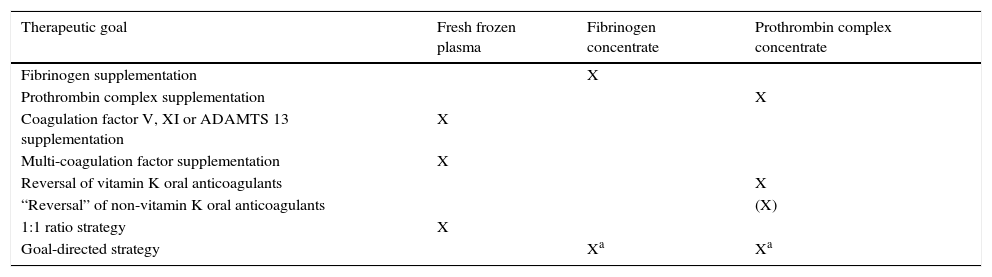
Final recommendations on when to use plasma and when coagulation factor concentrates are summarized in the table.
| Therapeutic goal | Fresh frozen plasma | Fibrinogen concentrate | Prothrombin complex concentrate |
|---|---|---|---|
| Fibrinogen supplementation | X | ||
| Prothrombin complex supplementation | X | ||
| Coagulation factor V, XI or ADAMTS 13 supplementation | X | ||
| Multi-coagulation factor supplementation | X | ||
| Reversal of vitamin K oral anticoagulants | X | ||
| “Reversal” of non-vitamin K oral anticoagulants | (X) | ||
| 1:1 ratio strategy | X | ||
| Goal-directed strategy | Xa | Xa |
SKL has received travel reimbursement and speakers fees for lecturing from Biotest, Octapharma, Baxter and CSL Behring; travel reimbursement and honoraria for consulting at a Biotest advisory board; and unrestricted educational grants for the e-learning platform, ‘perioperative bleeding’, from CSL Behring among others.