Central vascular cannulation is not a risk-free procedure, especially in pediatric patients. Newborn and infants are small and low-weighted, their vascular structures have high mobility because of tissue laxity and their vessels are superficial and with small diameter. These characteristics, together with the natural anatomical variability and poor collaboration of small children, make this technique more difficult to apply. Therefore, ultrasound imaging is increasingly being used to locate vessels and guide vascular access in this population.
Objective(a) To present a model that simulates the vascular system for training ultrasound-guided vascular access in pediatrics patients; (b) to ultrasound-guided vascular cannulation in the model.
ResultsThe model consisted of two components: (a) muscular component: avian muscle, (b) vascular component: elastic tube-like structure filled with fluid. 864 ecoguided punctures was realized in the model at different vessel depth and gauge measures were simulated, for two medical operators with different degree of experience. The average depth and diameter of vessel cannulated were 1.16 (0.42)cm and 0.43 (0.1)cm, respectively. The average number of attempts was of 1.22 (0.62). The percentage of visualization of the needle was 74%. The most frequent maneuver used for the correct location, was the modification of the angle of the needle and the relocation of the guidewire in 24% of the cases. The average time for the correct cannulations was 41 (35.8)s. The more frequent complications were the vascular perforation (11.9%) and the correct vascular puncture without possibility of introducing the guidewire (1.2%). The rate of success was 96%.
ConclusionsThe model simulates the anatomy (vascular and muscular structures) of a pediatric patient. It is cheap models, easily reproducible and a useful tool for training in ultrasound-guided puncture and cannulation.
La canalización vascular central es una técnica no exenta de riesgos sobretodo en la población de los pacientes pediátricos. El tamaño y peso de los pacientes más pequeños (recién nacidos y lactantes), la mayor movilidad de algunas de sus estructuras vasculares, la posición más superficial y diámetros más pequeños de sus vasos, la variabilidad anatómica que pueden presentar, asociado a la poca colaboración que presentan estos pacientes, hace más difícil la realización de esta técnica. A pesar de ello la ecografía está instaurándose para asistir a la realización de la punción vascular en dichos pacientes.
Objetivoa) Diseñar un modelo experimental que permita la simulación vascular, la punción vascular ecodirigida y que sirva como método de aprendizaje y entrenamiento para la canalización ecoguiada de los vasos sanguíneos de los pacientes pediátricos. b) Realizar la punción ecoguiada en el modelo.
ResultadosSe presenta un modelo compuesto por una porción muscular aviar a la que se introduce un sistema tubular elástico. Se simulan distintas profundidades del vaso así como diferentes diámetros de los mismos. Se realizaron 864 punciones ecoguiadas en el modelo con diferentes niveles de profundidad y de diámetro vascular por dos operadores con distinto grado de experiencia. La media de profundidad y diámetro de los vasos canalizados fue de 1,6 (0,42) cm y de 0,43 (0,1) cm respectivamente. El número medio de intentos fue de 1,22 (0,62). El porcentaje de visualización de la aguja fue del 74%. La maniobra más frecuentemente utilizada para la correcta canalización fue la recolocación de la aguja y la guía en el 24% de los casos. El tiempo medio hasta la correcta canalización fue de 41 (35,8) segundos. La complicación más frecuente fue la perforación vascular (11,9%) y la adecuada punción sin conseguir la introducción de la guía. La tasa de éxito fue del 96%.
ConclusionesEl modelo presentado simula la anatomía (estructuras vasculares y musculares) del paciente pediátrico; es barato, fácilmente reproducible; permite la canalización y el aprendizaje de la técnica de la punción ecoguiada.
The use of ultrasound guidance reduces the number of punctures required for vascular access, as well as the associated failure and complication rates,1 although requires considerable training.2 However, the associated learning curve and technical preparation required for this procedure (ultrasound equipment, probe sterilization, selection of suitable preset, puncture plane, previsualize, etc.) often lead healthcare professionals to choose traditional “blind” cannulation despite its associated complications, which frequently emerge in critical situations (coagulopathy, thrombocytopenia, obesity), especially in pediatric patients whose vessels are smaller and more superficial than those of adults.
Surprisingly, non-human simulated models are scarcely used for training in invasive techniques such as ultrasound-guided vascular access. Some training models may be expensive or scarcely available or transmit ultrasound poorly.3–5 Much like other recently adopted techniques, few experimental models are available to simulate actual procedures. Most of biologic models consist of chicken or turkey thighs,3 and others (sintetic models) are performed by latex, gelatin or silicone rubber models,6 where plastic structures simulating central vessels are inserted.
In this article, we present: (a) a low-cost reproducible training model that reliably simulates the central vascular structures of pediatric patients, where different depth and diameter measures (corresponding to children's vessels) can be represented; (b) the outcome of ecoguided puncture in the models and the useful tool for training in ultrasound-guided vascular cannulation.
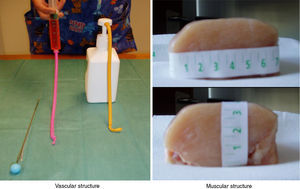
Patients and methodThe training model consisted of: (a) Muscular component: a portion of avian chest muscle of approximately 7cm×7cm×3cm (long, wide, high), which can be purchased in any food store. (b) Vascular component: a tube-like structure made of an elastic material (latex free ballon), filled-up with 10ml of colored water using a syringe or manual pumping dispenser and sealed at both ends with knots. Both components simulated the muscular and vascular structures of pediatric patients (Fig. 1).
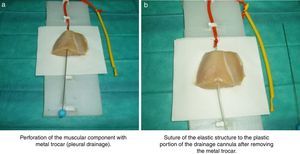
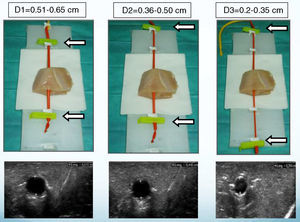
A thoracic drainage cannula (Argyle® 8 French – 2.7mm×9inch – 23cm) was inserted with a puncture trocar (to different depths) by longitudinally piercing the muscular component. Then the trocar was removed while the plastic portion of the drainage cannula remained in the muscle. The distal end of the drainage cannula was then sutured to the distal end of the elastic tube-like structure at the knot zone. As the drainage cannula was pulled toward the opposite side, the whole device was inserted into the muscular structure and could be used as a training model for training in ultrasound-guided vascular access (Fig. 2). By fixing a clamp system at different lengths, three different diameter ranges could be obtained, similar to the blood vessel diameters of pediatric patients (Fig. 3). Puncture and cannulation were performed with a catheter Vigon® 3 French (gauge)×11cm (length), a 30cm radio-opaque guidewire and a 5.5mm needle. The total cost of the model system was 3 Euros (avian muscle 1€, 15 elastic tube-like structures 1€, fixing support and clamp 1€). Every system could be used for more than 100 punctures.
Three different depth and diameter values were established on the basis of 300 central vessels measurements, previously taken in 70 pediatrics patients of different weight and sizes after requesting the informed consent garents by the parents of the infants.
24 punctures weekly were realized in the model during 18 weeks for each of operators in the three ultrasound scan axis more frequent.
The time required for successful wire insertion was calculated from the time that the skin was penetrated, until the guidewire was successfully inserted. The number of needle passes, the success rate, the number of cases with success achieved, the time, the visualization of the needle and the incidence of complications were noted.
The quantitative variables were expressed in averages and standard deviation and the qualitative variables in percentages.
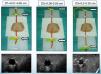
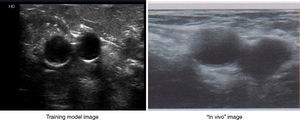
ResultsThe structure (vascular and muscular) in the training model was visualized with a Philips HD 7® ultrasound equipment with a linear multi-frequency probe L12-5, selecting “preset vascular” and 2cm depth (Fig. 4).
By ultrasound visualization, the depth and diameter of the elastic tubular vessel-like structure could be measured. Three different depth and diameter values were established on the basis of 300 central vessels depth and diameter measurements previously taken in pediatric patients of different weights and sizes. The averaged measurements (Table 1) determined 9 depth and diameter ranges for the vessel in the training model (Table 2).
Mean depth and diameter of major central vessels in the pediatric population (300 measures in 70 pediatric patients).
| Weight range (kg) | Depth m (SD) | Diameter m (SD) |
|---|---|---|
| <10 | 0.56cm (0.14) | 0.30cm (0.03) |
| 10–30 | 0.65cm (0.17) | 0.50cm (0.18) |
| 30–50 | 0.90cm (0.24) | 0.69cm (0.08) |
| >50 | 1.65cm (0.14) | 0.70cm (0.03) |
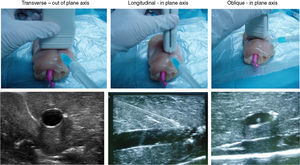
Ultrasound-guided vessel puncture and cannulation in the model could be conducted in the three more frequently used axes in vascular access: transverse out-of-plane axis, oblique in-plane axis and longitudinal axis (Fig. 5).
The training model also allowed visualizing the needle in the selected ultrasound axes as well as the probe inserted for vessel cannulation, which made it possible to maneuver the probe or the needle to avoid difficulties that could possibly be encountered during the cannulation procedure, thus facilitating vessel catheterization (Fig. 5).
864 ecoguided punctures was realized in the model at different vessel depth and gauge measures were simulated, for two medical operators with different degree of experience.
The average depth and diameter of vessel cannulated were 1.16 (0.42)cm and 0.43 (0.1)cm, respectively. The average number of attempts was of 1.22 (0.62). The percentage of visualization of the needle was 74%. The most frequent maneuver used for the correct location, was the modification of the angle of the needle and the relocation of the guidewire in 24% of the cases. The average time for the correct cannulations was 41 (35.8)s. The more frequent complications were the vascular perforation (11.9%) and the correct vascular puncture without possibility of introducing the guidewire (1.2%). The rate of success was 96%.
DiscussionAlthough whole-body ultrasound examination was mainly developed for adults, in pediatric patients it may help evaluating cardiac function and volemia, performing cardiopulmonary resuscitation (protocol FEER),7 monitoring and guiding endotracheal intubation (protocol TRUE),8 assessing pleural and pulmonary involvement, detecting free fluid in the peritoneum (protocol FAST)9 and guiding invasive procedures such as vascular access, thoracocentesis, pericardiocentesis, endotracheal intubation or lumbar puncture, in a safer and easier way at the bedside. Advances in vascular visualization lead to a more widespread use of vascular ultrasound as a helpful tool for central vascular access in critical pediatric patients.
Cannulation of central vessels in critical pediatric patients is a usual technique in Pediatric Intensive Care, Anestesiology and Emergency Services. In general, “blind” vascular access is performed, which is not free of complications, even for most experienced profesionals.10 It is widely accepted that ultrasound-guided central vascular access is associated with higher success and lower complication rates, as well as higher quality and safety for pediatric patients in the Intensive Care Unit.11–13 Actually, the “Guidelines on the use of ultrasound guidance for vascular access” recommended that cannulation of the internal jugular vein and the femoral vein in pediatric patients should be performed under ultrasound guidance,10 and other consensus document for training in ultrasound-guided vascular access have recently been published.14–18 The Agency for Healthcare Research and Quality confirmed that training in central vessel cannulation, both “blind” or ultrasound-guided, reduces the required number of vessel punctures and the associated complications.1,15 Compared with performing ultrasound-guided puncture directly to the patient at the bedside, training with an experimental model is less stressful and risky and offers an opportunity to improve ultrasound use.3 All of this contributes to the safety of invasive procedures in critical pediatric patients.
Our model obtain similar outcomes than others publications in pediatrics patients or in training models of ecoguided vascular access at the time of correct location, though his rate of success is 100% as in another model of training, but in this one the mean diameter of the vessels was 8mm, bigger than that of our model. The number of attempts ranges between 1 and 3 similar to our study and the maneuver to facilitate the location of the catheter is the replacement of the needle and the guide.19–20
The here-presented training model is low cost and readily available to any service of critical patient transfer, anesthesiology, intensive care or emergency care with an ultrasound equipment and suitable probe for vascular puncture, where vascular access is regularly performed and whose personnel need training. Additionally, this model is helpful to improve ultrasound use and probe sterilization and protection skills, as well as “eye-hand” coordination.16 Furthermore, the puncture and cannulation procedures can be repeated many times and several operators can work on a model system, since several tube-like structures can be placed, as necessary. Though the model this one designed for pediatric patients, can apply without problems in adult patients fitting the depths (1.50–1.75cm) and vascular diameters (0.8–1cm).
Several synthetic (silicone, gelatin, latex) and tissue models (chicken, turkey or pork thigh – “pork belly”)3,6,16 are commercially available for training in ultrasound-guided vascular access. However, unlike other models where semi-rigid plastic structures (cannulae or probes) are used to simulate the vessels, in our model the feeling is closely similar to that of puncturing pediatric patients on the usual zones (cervical, infraclavicular, brachial or inguinal), both in the muscular and vascular structures. Furthermore, the path of the needle is not marked and vascular fluid extravasation does not affect visualization, as it happens in synthetic models.
One of the limitations of the model – just like other mentioned models – is the fact that the anatomical structure (avian chest muscle) does not vary, whereas the structures of real pediatric patients may vary in size, location (cervical, inguinal, brachial), mobility or restlessness (except in sedated patients). Moreover, unlike real pediatric patients, the model remains motionless, which facilitates ultrasound visualization and guidance of vascular cannulation. However, in our opinion the model is a helpful training tool, both for physicians experienced in the traditional “blind” technique and residents who are starting with vascular access.
ConclusionsThe here-presented experimental model for ultrasound-guided vascular access is rather similar to the vascular structures of pediatric patients in terms of vessel anatomy, depth and diameter in relation to body weight. It also facilitates ultrasound-guided vascular cannulation and supports the learning curve to acquire the required skills. Furthermore, the model allows for needle path visualization and correction when necessary. On the basis of these conclusions, we postulate that our experimental model may be considered and used as reliably as other available models, such as the “pork belly”,16 for training in ultrasound-guided vascular access. In future studies, we expect to collect data to confirm whether training with this experimental model reduces the complication rates associated with vascular access in critical pediatric patients, thus enhancing children safety.
Conflicts of interestThe authors report no conflicts of interest.