To compare intensive care unit (ICU) mortality in patients with severe community-acquired pneumonia (SCAP) caused by Legionella pneumophila receiving combined therapy or monotherapy.
MethodsA prospective multicenter study was made, including all patients with sporadic, community-acquired Legionnaires’ disease (LD) admitted to the ICU. Admission data and information on the course of the disease were recorded. Antibiotic prescriptions were left to the discretion of the attending physician and were not standardized.
ResultsTwenty-five cases of SCAP due to L. pneumophila were included, and 7 patients (28%) out of 25 died after a median of 7 days of mechanical ventilation. Fifteen patients (60%) presented shock. Levofloxacin and clarithromycin were the antibiotics most commonly used in monotherapy, while the most frequent combination was rifampicin plus clarithromycin. Patients subjected to combination therapy presented a lower mortality rate versus patients subjected to monotherapy (odds ratio for death [OR] 0.15; 95%CI 0.02–1.04; p=0.08). In patients with shock, this association was stronger and proved statistically significant (OR for death 0.06; 95%CI 0.004–0.86; p=0.04).
ConclusionsCombined antibiotic therapy decreases mortality in patients with SCAP and shock caused by L. pneumophila.
comparar la mortalidad de los pacientes ingresados en unidad de cuidados intensivos (UCI) por neumonía comunitaria severa (NCS) causada por Legionella pneumophila que recibieron tratamiento combinado o monoterapia.
Metodosestudio prospectivo multicentrico que incluye los pacientes con Enfermedad del Legionario comunitaria, esporádica, que requiere ingreso en UCI. Se recogieron datos en el momento del ingreso y durante la evolución en la UCI. El tipo y el numero de antibióticos a administrar no fue estandardizado y fue decidido por el medico responsable del paciente.
Resultadosse incluyeron veinticinco casos de NCS causada por Legionella pneumophila, 7 pacientes (28%) de los 25 falleció tras una mediana de 7 días de ventilación mecánica. Quince pacientes (60%) presentaron shock. Los antibióticos mas prescritos en monoterapia fueron levofloxacino y claritromicina, mientras que la asociación mas frecuente fue rifampicina mas claritromicina. Los pacientes que recibieron tratamiento combinado presentaron una mortalidad inferior con respecto a los tratados con monoterapia (odds ratio para fallecimiento [OR] 0.15; IC95% 0.02 hasta 1.04; p=0.08). En el subgrupo de pacientes con shock la asociación fue mas fuerte y estadísticamente significativa (OR para fallecimiento 0.06; IC95% 0.004 hasta 0.86; p=0.04).
Conclusionesel tratamiento antibiótico combinado disminuye la mortalidad de los pacientes con NCS y shock causados por Legionella pneumophila.
In patients with SCAP admitted to the ICU, mortality ranges from 25% to 40%.1–4 In many series, Legionella spp. ranks second after Streptococcus pneumoniae in the list of causative agents of SCAP.5–9 The incidence of legionellosis has increased in the United States for the last decades.10 Although it has been suggested that a presumptive diagnosis of LD may be done even in cases of SCAP, most authors believe that clinical and laboratory features of LD are not distinctive5–9,11–13; that is why empiric coverage of Legionella spp. is strongly recommended in most international guidelines for management of SCAP.1–4,13 Once Legionella pneumophila has been confirmed as the etiologic agent of severe pneumonia some experts suggest that combined therapy would be preferable to monotherapy, although there is no solid evidence to confirm it.1–9,11–15 In case of combined therapy, macrolide or fluoroquinolone in addition with rifampicin is the approach that is usually suggested; again, scientific evidence supporting this assertion is scarce.14,16 To the best of our knowledge, only a few monocentric and retrospective studies have focused on ICU patients with SCAP due to L. pneumophila.17–21 Our hypothesis was that combination antibiotic therapy improves outcome in critically ill patients with SCAP caused by L. pneumophila; the primary outcome of the present study was to compare ICU mortality; the analysis was done in all patients admitted to the ICU and subsequently only in patients with shock. Secondary objectives were to document the epidemiology and therapeutic options.
Patients and methodsCAPUCI study collected all patients admitted for SCAP to thirty-three hospitals in Spain, from December 1st 2000 to February 28th 2002.22 In CAPUCI2 study, an ECCRN endorsed project, data were recorded from patients admitted to ICU for SCAP, from 2008 to 2011. We analyzed patients enrolled in these large series to gain insight into the current therapy and outcomes for severe CAP admitted in the ICU caused by L. pneumophila. Informed consent was waived by the ethics committee due to the observational nature of the study. Patients were admitted to the ICU either to undergo mechanical ventilation or because they were in an unstable condition requiring intensive medical care.23 Patients with severe chronic illness in whom pneumonia was an expected terminal event were not included. At least one of the following tests was required to establish a diagnosis of LD: isolation of L. pneumophila from any respiratory sample culture on buffered charcoal yeast extract selective medium; a positive detection of urinary antigen test by enzyme immunoassay; an indirect immunofluorescent antibody test showing a fourfold increase in IgG antibodies, using a commercial ELISA kit against L. pneumophila. The antibiotic prescriptions and the decision to initiate monotherapy or combination therapy were left to the discretion of the attending physician and were not protocolized. Patients were observed until death or ICU discharge. Data of antibiotic doses were not registered.
DefinitionsCAP was defined as an acute lower respiratory tract infection characterized by: (1) an acute pulmonary infiltrate on chest X-ray, (2) confirmatory findings of a clinical examination, and (3) acquisition of the infection outside of a hospital, long-term care facility, or nursing home. Diagnosis of active smoker, alcoholism and chronic obstructive pulmonary disease (COPD) was done with criteria reported elsewhere.24,25 Immunocompromise was defined as primary immunodeficiency or immunodeficiency secondary to radiation treatment, use of cytotoxic drugs or steroids (daily doses of >20mg of prednisolone or equivalent for >2 weeks),26 or AIDS. Shock was defined as the need for vasopressor during >4h after fluid replacement; rapid radiographic spread was defined as an increase in the size of opacities on chest radiograph by >50% at 48h. Monotherapy was defined as administration of the same single antibiotic during the first 2 days of ICU admission. Combination therapy was defined as administration of the same two antibiotics within the first 2 days of ICU admission.
Statistical analysisICU mortality was chosen as primary endpoint. All data spreadsheets, analysis codes and outputs, were electronically stored and archived. Data validation consisted of searching out-of-range and missing values, and lack of consistency between related variables detection. General characteristics obtained at baseline, risk factors and other variables were compared and summarized. Qualitative variables were summarized using absolute and relative frequencies for each group. Differences between groups were tested using Fisher's exact test. Quantitative variables were summarized using mean, standard deviation and valid cases for each group. Differences between groups were tested using Mann–Whitney–Wilcoxon test. The Kaplan–Meier product limit method was used to construct survival curves for patients receiving combination and monotherapy regimens. All statistical decisions were based on a significance level of 5%. All data management and statistical analysis were performed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA).
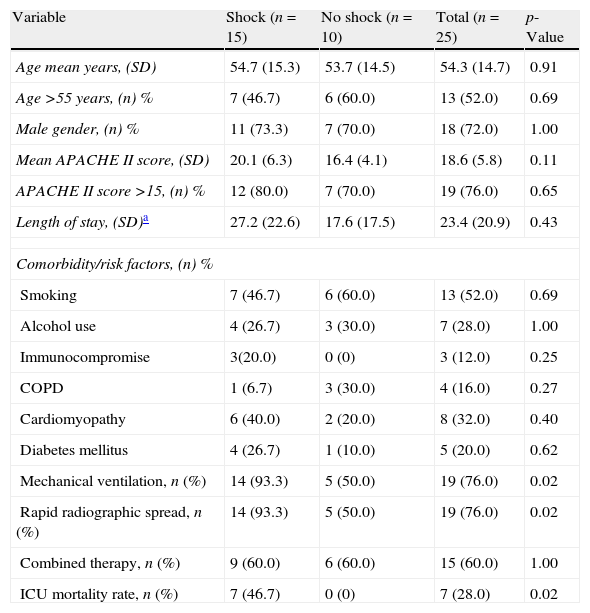
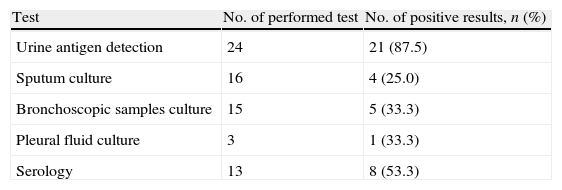
ResultsData from 779 ICU patients with SCAP were extracted. A total of 25 patients with diagnosis of L. pneumophila pneumonia were recruited, of whom 15 (60%) presented with shock on admission. All patients presented sporadic forms of Legionnaires’ disease. Seven patients (28%) out of 25 died after a median of 7 days of mechanical ventilation; all deceases were secondary to multi-organ failure and were pneumonia-related. Median age of the patients was 55 years and median APACHE II score was 19. Thirteen patients (52%) were smokers. The commonest comorbitiy conditions were: cardiomyopathy (32%) and diabetes mellitus (20%). Nineteen patients (76%) required MV. Age was significantly similar in patients with shock and without shock. Mean APACHE II score and length of stay had a trend to be higher in group of patients with shock, but without achieving significant statistic differences. Need for mechanical ventilation, rapid radiographic spread and ICU mortality was significantly higher in the shock subset. Acute kidney injury was documented in 2 patients (8%). Other baseline characteristics of the study population are described in Table 1. Table 2 shows the diagnostic methods used for detecting infection by Legionella spp.; 87.5% of patients were positive for antigen urinary detection.
Demographic characteristics of the study population.
| Variable | Shock (n=15) | No shock (n=10) | Total (n=25) | p-Value |
| Age mean years, (SD) | 54.7 (15.3) | 53.7 (14.5) | 54.3 (14.7) | 0.91 |
| Age >55 years, (n) % | 7 (46.7) | 6 (60.0) | 13 (52.0) | 0.69 |
| Male gender, (n) % | 11 (73.3) | 7 (70.0) | 18 (72.0) | 1.00 |
| Mean APACHE II score, (SD) | 20.1 (6.3) | 16.4 (4.1) | 18.6 (5.8) | 0.11 |
| APACHE II score >15, (n) % | 12 (80.0) | 7 (70.0) | 19 (76.0) | 0.65 |
| Length of stay, (SD)a | 27.2 (22.6) | 17.6 (17.5) | 23.4 (20.9) | 0.43 |
| Comorbidity/risk factors, (n) % | ||||
| Smoking | 7 (46.7) | 6 (60.0) | 13 (52.0) | 0.69 |
| Alcohol use | 4 (26.7) | 3 (30.0) | 7 (28.0) | 1.00 |
| Immunocompromise | 3(20.0) | 0 (0) | 3 (12.0) | 0.25 |
| COPD | 1 (6.7) | 3 (30.0) | 4 (16.0) | 0.27 |
| Cardiomyopathy | 6 (40.0) | 2 (20.0) | 8 (32.0) | 0.40 |
| Diabetes mellitus | 4 (26.7) | 1 (10.0) | 5 (20.0) | 0.62 |
| Mechanical ventilation, n (%) | 14 (93.3) | 5 (50.0) | 19 (76.0) | 0.02 |
| Rapid radiographic spread, n (%) | 14 (93.3) | 5 (50.0) | 19 (76.0) | 0.02 |
| Combined therapy, n (%) | 9 (60.0) | 6 (60.0) | 15 (60.0) | 1.00 |
| ICU mortality rate, n (%) | 7 (46.7) | 0 (0) | 7 (28.0) | 0.02 |
APACHE, acute physiology and chronic health evaluation; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease.
Yield of diagnostic tests for 25 patients with severe Legionnaires’ disease admitted to the ICU.
| Test | No. of performed test | No. of positive results, n (%) |
| Urine antigen detection | 24 | 21 (87.5) |
| Sputum culture | 16 | 4 (25.0) |
| Bronchoscopic samples culture | 15 | 5 (33.3) |
| Pleural fluid culture | 3 | 1 (33.3) |
| Serology | 13 | 8 (53.3) |
ICU, intensive care unit.
Ten patients were treated with monotherapy and 50% of this group died: levofloxacin and clarithromycin were the most administered antibiotics; each medication was prescribed in 4 patients. All patients treated with levofloxacin died while only 1 patient of 4 who received clarithromycin did not survive. Fifteen patients received combined therapy; 2 patients out of 15 expired. The most used combination therapy was rifampicin and clarithromycin, this combination was given in 3 patients: 1 person out of 3 who received this combination died. The second most used combined therapy were: clarithromycin, ciprofloxacin plus rifampicin, and clarithromycin, levofloxacin plus rifampicin. Each one the previous combinations was administered in 2 patients. One patient expired after being treated with clarithromycin, ciprofloxacin and rifampicin.
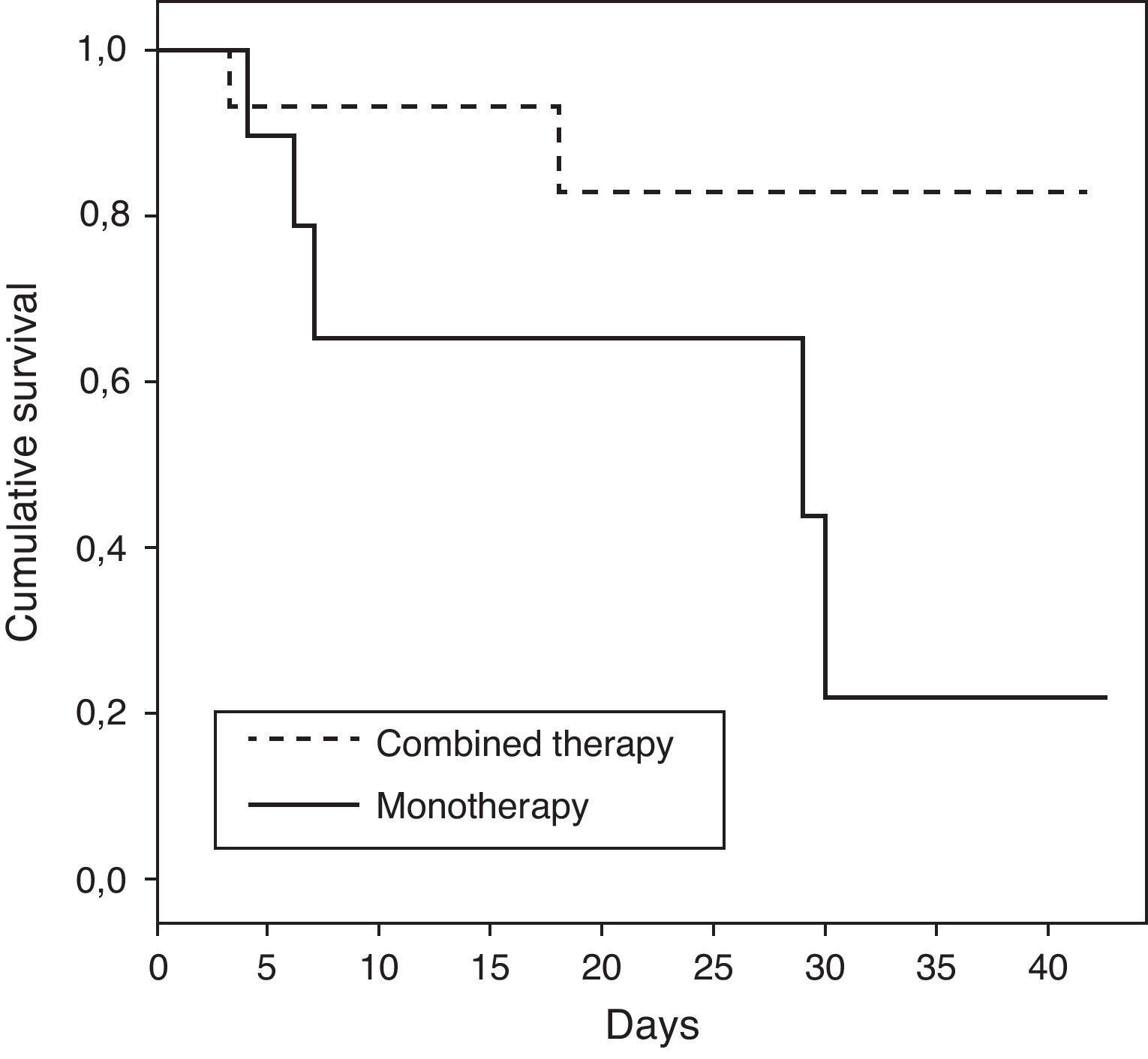
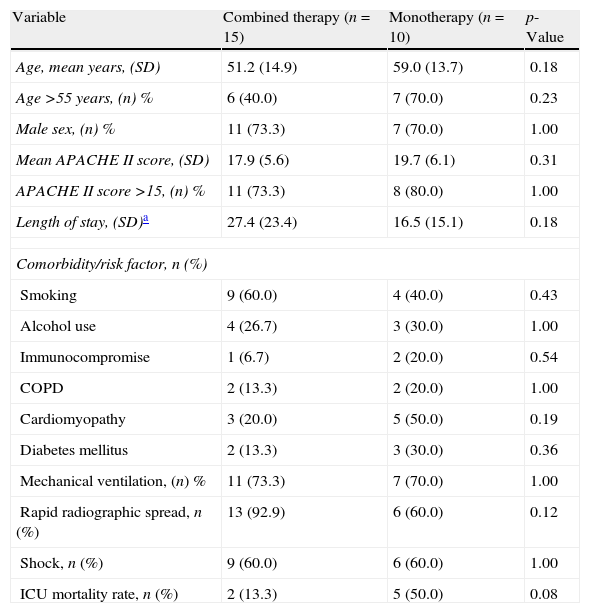
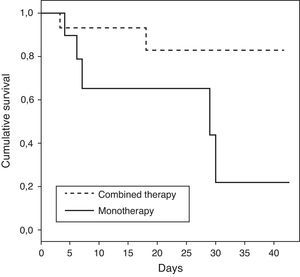
The characteristics of patients who received monotherapy or combination therapy are shown in Table 3. Patients who received combination therapy presented a lower ICU mortality rate versus patients treated with monotherapy (OR of death 0.15; 95%CI 0.02–1.04; p=0.08). When the analysis was done in patients with shock, the association between combination therapy and decrease of mortality was stronger with statistical significance (OR of death 0.06; 95% CI 0.004–0.86; p=0.04). The demographics for the patients with shock who received combination therapy versus monotherapy were comparable, as shown in Table 4. Survival time for patients receiving combination therapy versus monotherapy is represented (p=0.04) using a Kaplan–Meier survival curve (Fig. 1).
Characteristics of 25 patients with SCAP caused by Legionella pneumophila receiving combination therapy or monotherapy.
| Variable | Combined therapy (n=15) | Monotherapy (n=10) | p-Value |
| Age, mean years, (SD) | 51.2 (14.9) | 59.0 (13.7) | 0.18 |
| Age >55 years, (n) % | 6 (40.0) | 7 (70.0) | 0.23 |
| Male sex, (n) % | 11 (73.3) | 7 (70.0) | 1.00 |
| Mean APACHE II score, (SD) | 17.9 (5.6) | 19.7 (6.1) | 0.31 |
| APACHE II score >15, (n) % | 11 (73.3) | 8 (80.0) | 1.00 |
| Length of stay, (SD)a | 27.4 (23.4) | 16.5 (15.1) | 0.18 |
| Comorbidity/risk factor, n (%) | |||
| Smoking | 9 (60.0) | 4 (40.0) | 0.43 |
| Alcohol use | 4 (26.7) | 3 (30.0) | 1.00 |
| Immunocompromise | 1 (6.7) | 2 (20.0) | 0.54 |
| COPD | 2 (13.3) | 2 (20.0) | 1.00 |
| Cardiomyopathy | 3 (20.0) | 5 (50.0) | 0.19 |
| Diabetes mellitus | 2 (13.3) | 3 (30.0) | 0.36 |
| Mechanical ventilation, (n) % | 11 (73.3) | 7 (70.0) | 1.00 |
| Rapid radiographic spread, n (%) | 13 (92.9) | 6 (60.0) | 0.12 |
| Shock, n (%) | 9 (60.0) | 6 (60.0) | 1.00 |
| ICU mortality rate, n (%) | 2 (13.3) | 5 (50.0) | 0.08 |
SCAP, severe community-acquired pneumonia; APACHE, acute physiology and chronic health evaluation; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease.
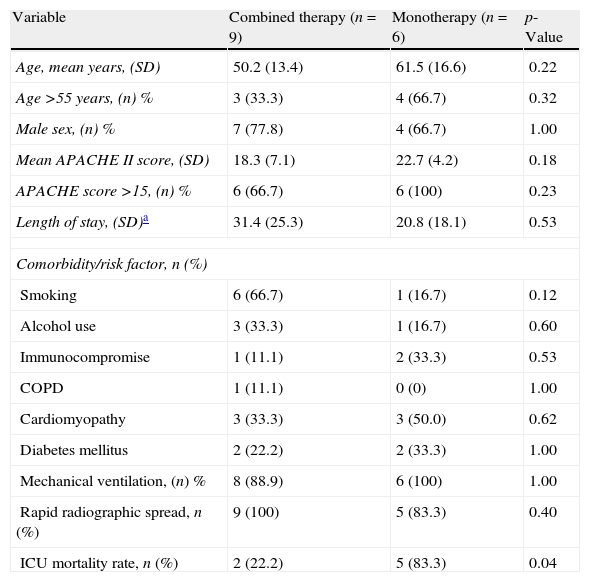
Characteristics of 15 patients with SCAP caused by Legionella pneumophila and shock receiving combination therapy or monotherapy.
| Variable | Combined therapy (n=9) | Monotherapy (n=6) | p-Value |
| Age, mean years, (SD) | 50.2 (13.4) | 61.5 (16.6) | 0.22 |
| Age >55 years, (n) % | 3 (33.3) | 4 (66.7) | 0.32 |
| Male sex, (n) % | 7 (77.8) | 4 (66.7) | 1.00 |
| Mean APACHE II score, (SD) | 18.3 (7.1) | 22.7 (4.2) | 0.18 |
| APACHE score >15, (n) % | 6 (66.7) | 6 (100) | 0.23 |
| Length of stay, (SD)a | 31.4 (25.3) | 20.8 (18.1) | 0.53 |
| Comorbidity/risk factor, n (%) | |||
| Smoking | 6 (66.7) | 1 (16.7) | 0.12 |
| Alcohol use | 3 (33.3) | 1 (16.7) | 0.60 |
| Immunocompromise | 1 (11.1) | 2 (33.3) | 0.53 |
| COPD | 1 (11.1) | 0 (0) | 1.00 |
| Cardiomyopathy | 3 (33.3) | 3 (50.0) | 0.62 |
| Diabetes mellitus | 2 (22.2) | 2 (33.3) | 1.00 |
| Mechanical ventilation, (n) % | 8 (88.9) | 6 (100) | 1.00 |
| Rapid radiographic spread, n (%) | 9 (100) | 5 (83.3) | 0.40 |
| ICU mortality rate, n (%) | 2 (22.2) | 5 (83.3) | 0.04 |
SCAP, severe community-acquired pneumonia; APACHE, acute physiology and chronic health evaluation; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease.
This is the first study reporting that patients with legionellosis and shock can benefit from two agents instead of one. Moreover, to our knowledge, this is the largest series of SCAP by L. pneumophila in ICU patients that was analyzed in a prospective, multicentric study: previous studies were usually monocentric and retrospective.14,17–21 Furthermore, our population was homogeneous since all patients fulfilled inclusion criteria of sporadic, community-acquired LD.27 A generalized pitfall of previous series on severe LD is that Legionella antigen detection was not used as part of diagnostic methods. This drawback should be taken into account since it has been suggested that positive urine antigen detection could be associated with a more severe form of the disease.28
Results from at least two studies demonstrated that combined antibiotic therapy improve survival in critically ill patients with severe infection and shock.29,30 A recent study including mild-to-moderate LD in 49 cancer patients confirms that combination therapy is correlated with better outcome, especially in patients with severe pneumonia.31 Gacouin et al. reported in 2002 a retrospective series of 43 cases of severe Legionella spp. pneumonia admitted to the ICU: authors concluded that combined treatment with quinolones was the best therapeutic option for severe LD.21 Data from our study suggest that combination therapy is better than monotherapy in the setting of SCAP by L. pneumophila in patients with shock.
Laboratory and experimental data support the concept that both fluoroquinolones and newer macrolide/azalides agents against L. pneumophila are superior to erythromycin.5–7,14,32 Azithromycin, an excellent therapeutic option in severe LD,5–7,14,32–34 was administered in only one patient. The reason is because azithromycin was not available in Spain when the CAPUCI study was realized. The patient who received azithromycin was enrolled in CAPUCI II study.
In our series, differently to prior similar studies, new fluoroquinolones or macrolides were administered in all cases. Clarithromycin has been found to be an effective anti-Legionella agent in non-severe LD, but clinical efficacy in the setting of the ICU has not been established until now.14,35,36 Three patients out of four who received clarithromycin survived. Although the small number of patients in our series is clearly a major drawback, our results suggest that clarithromycin could be a good therapeutic agent in severe LD.
Recent studies suggest that levofloxacin should be the drug of choice for the treatment of mild-to-moderate LD35,37,38; on the other hand, there is certain lack of consistency about its use in monotherapy in severe cases of LD, especially when mechanical ventilation is needed.14,35–37 The finding of death in our four patients exclusively treated with levofloxacin raises some concerns on its use in monotherapy in this subset of severely ill patients, although the small number of patients does not allow definitive conclusions. Controversy between new macrolides versus fluoroquinolones as treatment of choice of severe LD is beyond the scope of this discussion since this study was not designed to focus on this issue.
Rifampicin is very active against L. pneumophila both in vitro and in animal model5,14; however, its clinical use in monotherapy has been precluded because of concerns of increased resistance to the drug. Nevertheless, at least one study in an animal model denied an increase in antibiotic resistance.39 Experimental data in animal models show that the bacterial killing rate is dramatically higher when rifampicin is added to other antibiotic.40 The association of macrolides and rifampicin has been the recommended combined therapy for treating severe LD.5,14 On the contrary, in cases of non-severe LD, this association was not superior versus monotherapy.36
There are some limitations to our study. First, as with other publications exploring this issue, results are not from a randomized controlled study. Second is the small sample size; however, it is unlikely that an adequate comparative clinical trial could be completed. Data for our study were collected from two different chronological periods so there may be confounding factors that cannot be corrected nor adjusted. Another relevant limitation is that azithromycin, an excellent therapeutic option for treating severe LD, was only administered in one patient because it was not available in Spain in intravenous formulation until recently. Finally, delay of antibiotic therapy from pneumonia onset was not recorded. Newer studies should be performed comparing recent antibiotics, but this is not feasible due to the small number of patients currently complicated with MV.
ConclusionsIn summary, our findings show a reduction of ICU mortality in patients with SCAP caused by L. pneumophila and shock, when combined therapy is administered instead of monotherapy. Our results are consistent with other observational studies suggesting that combination therapy improves survival in the subset of the most severe critically ill patients with SCAP.
Financial support2001/SGR414, RED RESPIRA ISCIII (RTIC 03/11), and FISS (PI 04/1500).
Conflicts of interestDr Rello serves in the speaker's bureau and advisory boards for Pfizer. The remaining authors declared no conflicts of interest.
CAPUCI 2 was an ECCRN (European Critical Care Research Network) endorsed project by the ESICM (European Society of Intensive Care Medicine).
Investigators of Community-Acquired Pneumonia in the Intensive Care Unit 2 (CAPUCI 2) study:
J. Almirall, MATARÓ HOSPITAL, MATARÓ (BARCELONA); R. Alonso, GENERAL DE ASTURIAS HOSPITAL, OVIEDO; B. Alvarez, GENERAL HOSPITAL, ALICANTE; F. Alvarez Lerma, DEL MAR HOSPITAL, BARCELONA; J.R. Badia, CLINIC HOSPITAL, BARCELONA; F. Barcenilla, ARNAU DE VILANOVA HOSPITAL, LLEIDA; M. Bassetti, SAN MARTINO HOSPITAL, GENOA; J. Blanquer, CLINIC HOSPITAL, VALENCIA; M.A. Blasco, PESET ALEIXANDRE HOSPITAL, VALENCIA; F. Bobillo, CLINIC HOSPITAL, VALLADOLID; M. Bodi, JOAN XXIII HOSPITAL, TARRAGONA; M. Borges, SON LLATZER HOSPITAL, MALLORCA; E. Bouza, GREGORIO MARANON HOSPITAL, MADRID; M.J. Broch, SAGUNTO HOSPITAL, VALENCIA; N. Carrasco, PRINCESA HOSPITAL, MADRID; M. Catalan, 12 DE OCTUBRE HOSPITAL, MADRID; V de la Torre, VIRGEN DE LA VICTORIA HOSPITAL, MALAGA; E. Diaz, JOAN XXIII HOSPITAL, TARRAGONA; A. Doblas, JUAN RAMON JIMENEZ HOSPITAL, HUELVA; J. Fierro, PONIENTE HOSPITAL, ALMERIA; F. Garcia, GENERAL HOSPITAL, ALBACETE; J. Garnacho, VIRGEN DEL ROCIO HOSPITAL, SEVILLA; M.A. Herranz, RIO HORTEGA HOSPITAL, VALLADOLID; M.J. Huertos, PUERTO REAL HOSPITAL, CADIZ; J. Jimenez, VIRGEN DEL ROCIO HOSPITAL, SEVILLA; R. Jorda, SON DURETA HOSPITAL, PALMA DE MALLORCA; D. Koulentis, KAT HOSPITAL, ATHENS; D. Lopez, FUNDACION GIMENEZ DIAZ, MADRID; M.J. Lopez Cambra, GENERAL HOSPITAL, SEGOVIA; M.J. Lopez Pueyo, GENERAL DE YAGÜE HOSPITAL, BURGOS; A. Lores, BELLVITGE HOSPITAL, BARCELONA; F. Lucena, VALME HOSPITAL, SEVILLA; P. Luque, LOZANO BLESA CLINIC HOSPITAL, ZARAGOZA; R. Maiez, BELLVITGE HOSPITAL, BARCELONA; E. Maravi, VIRGEN DEL CAMINO HOSPITAL, PAMPLONA; A. Margarit, VIRGEN MERITXELL HOSPITAL, ANDORRA; A. Martin, SANTIAGO APOSTOL HOSPITAL, VITORIA; G. Masdeu, VERGE DE LA CINTA HOSPITAL, TORTOSA (TARRAGONA); A. Mendia, NUESTRA SENORA DE ARANZAZU HOSPITAL, SAN SEBASTIAN; E. Mesalles, TRIAS I PUJOL HOSPITAL, BADALONA (BARCELONA); J. Pereira SAN JOAO HOSPITAL, OPORTO; G. Poulakou, ATTIKON UNIVERSITY GENERAL HOSPITAL, ATHENS; J. Rello, VALL D’HEBRON UNIVERSITY HOSPITAL, BARCELONA; F. Renedo, LEON HOSPITAL, LEON; J.M. Ricard, ROTGER CLINIC, PALMA DE MALLORCA; J.C. Robles, REINA SOFIA HOSPITAL, CORDOBA; L. Rocha, JUAN CANALEJO HOSPITAL, LA CORUNA; R. Sierra, PUERTA DEL MAR HOSPITAL, CADIZ; J.M. Sirvent, JOSEP TRUETA HOSPITAL, GIRONA; J. Sole, DR. NEGRIN HOSPITAL, GRAN CANARIA; B. Suberviola, MARQUES DE VALDECILLA HOSPITAL, SANTANDER; A. Torres, CLINIC HOSPITAL, BARCELONA; J. Valles, HOSPITAL PARC TAULI, SABADELL (BARCELONA); L. Vidaur, DONOSTIA HOSPITAL, DONOSTIA; R. Zaragoza, PESET ALEIXANDRE HOSPITAL, VALENCIA.