To establish the correlation and validity between PaO2/FiO2 obtained on arterial gases versus noninvasive methods (linear, nonlinear, logarithmic imputation of PaO2/FiO2 and SpO2/FiO2) in patients under mechanical ventilation living at high altitude.
DesignAmbispective descriptive multicenter cohort study.
SettingTwo intensive care units (ICU) from Colombia at 2600m a.s.l.
Patients or participantsConsecutive critically ill patients older than 18 years with at least 24h of mechanical ventilation were included from June 2016 to June 2019.
InterventionsNone.
VariablesVariables analyzed were demographic, physiological messures, laboratory findings, oxygenation index and clinical condition. Nonlinear, linear and logarithmic imputation formulas were used to calculate PaO2 from SpO2, and at the same time the SpO2/FiO2 by severe hypoxemia diagnosis. The intraclass correlation coefficient, area under the ROC curve, sensitivity, specificity, positive predictive value, negative predictive value, positive and negative likelihood ratio were calculated.
ResultsThe correlation between PaO2/FiO2 obtained from arterial gases, PaO2/FiO2 derived from one of the proposed methods (linear, non-linear, and logarithmic formula), and SpO2/FiO2 measured by the intraclass correlation coefficient was high (greater than 0.77, p<0.001). The different imputation methods and SpO2/FiO2 have a similar diagnostic performance in patients with severe hypoxemia (PaO2/FiO2 <150). PaO2/FiO2 linear imputation AUC ROC 0,84 (IC 0.81–0.87, p<0.001), PaO2/FiO2 logarithmic imputation AUC ROC 0.84 (IC 0.80–0.87, p<0.001), PaO2/FiO2 non-linear imputation AUC ROC 0.82 (IC 0.79–0.85, p<0.001), SpO2/FiO2 oximetry AUC ROC 0.84 (IC 0.81–0.87, p<0.001).
ConclusionsAt high altitude, the SaO2/FiO2 ratio and the imputed PaO2/FiO2 ratio have similar diagnostic performance in patients with severe hypoxemia ventilated by various pathological conditions.
Establecer la correlación y validez entre PaO2/FiO2 obtenida en gases arteriales versus métodos no invasivos (imputación lineal, no lineal, logarítmica de PaO2/FiO2 y SpO2/FiO2) en pacientes bajo ventilación mecánica que viven en altitudes elevadas.
DiseñoEstudio de cohorte multicéntrico descriptivo ambispectivo
ÁmbitoDos unidades de cuidados intensivos de Colombia a 2.600 m s.n.m.
Pacientes o participantesSe incluyeron pacientes consecutivos en estado crítico mayores de 18 años con al menos 24h de ventilación mecánica desde junio de 2016 a junio de 2019.
IntervencionesNinguna.
VariablesLas variables analizadas fueron demográficas, fisiológicas, hallazgos de laboratorio, índice de oxigenación y estado clínico. Se utilizaron fórmulas de imputación no lineales, lineales y logarítmicas para calcular la PaO2 a partir de la SpO2, y al mismo tiempo la SpO2/FiO2 mediante el diagnóstico de hipoxemia severa. Se calculó el coeficiente de correlación intraclase, el área bajo la curva ROC, la sensibilidad, la especificidad, el valor predictivo positivo, el valor predictivo negativo, la razón de verosimilitud positiva y negativa.
ResultadosLa correlación entre PaO2/FiO2 obtenida a partir de gases arteriales, PaO2/FiO2 derivada de uno de los métodos propuestos (fórmula lineal, no lineal y logarítmica) y SpO2/FiO2 medida por el coeficiente de correlación intraclase fue alta (mayor a 0,77, p<0,001). Los diferentes métodos de imputación y SpO2/FiO2 tienen un rendimiento diagnóstico similar en pacientes con hipoxemia severa (PaO2/FiO2<150). PaO2/FiO2 imputación lineal AUC ROC 0,84 (IC 0,81-0,87; p<0,001), PaO2/FiO2 imputación logarítmica AUC ROC 0,84 (IC 0,80-0,87; p<0,001), PaO2/Imputación no lineal de FiO2 AUC ROC 0,82 (IC 0,79-0,85; p<0,001), oximetría de SpO2/FiO2 AUC ROC 0,84 (IC 0,81-0,87; p<0,001).
ConclusionesA gran altitud, el cociente SaO2/FiO2 y el cociente PaO2/FiO2 imputado tienen un rendimiento diagnóstico similar en pacientes con hipoxemia severa bajo ventilación mecánica invasiva por diversas patologías.
Acute respiratory insufficiency (ARI) is diagnosed in 11.3% of hospitalizations in the United States. Its incidence calculated for 2001 was 502 cases per 100,000 inhabitants, increasing to 704 cases per 100,000 inhabitants in 2009.1 The relationship between the pressure of oxygen dissolved in the blood (PaO2) and the fraction of inspired oxygen (FiO2) (PaO2/FiO2), an index that classifies the severity of the disease in patients with acute respiratory failure, was incorporated in 2011 in the Berlin Consensus definition of adult acute respiratory distress syndrome (ARDS)2 and has been included in severity scores, such as in the sequential multiple organ damage assessment (SOFA) scale.3,4
The measurement of arterial blood gases is required to determine PaO2 and calculate the PaO2/FiO2 ratio.5,6 However, this technique can present limitations in its use, either because of difficulty in taking the sample (either because it requires trained personnel, complicated arterial accesses, constant puncture requirements, pain at the time of sample collection and skin laceration) or because it represents an increase in hospital costs.7,8
A non-invasive substitute for the PaO2/FiO2 ratio is the ratio of the percentage of hemoglobin saturation measured by pulse oximetry (SpO2) and the inspired fraction of oxygen (FIO2) (SpO2/FiO2).9 That allows the assessment and classification of the severity of respiratory failure10 without the need for arterial blood sampling.11 Other methods have been proposed to estimate PaO2 without direct measurement of oxygen in arterial blood. These models ensure their equivalence by imputing PaO2 from SpO2. These imputation techniques can be classified into those that simulate the hemoglobin dissociation curve (non-linear)12,13 and those that take into account other types of relationships (linear and logarithmic).14,15 The studies that have compared these formulas show an adequate relationship between measured and imputed PaO2 values, with high correlation coefficients ranging from 0.75 to 0.9.12,13,15
It must be taken into account that about 140 million people live at high altitudes (over 2500m above sea level), which is why it is essential to understand how the oxygenation indices (PaO2/FiO2 and SpO2/FiO2) behave in these regions.16 However, we do not know of previous studies conducted in populations living at altitudes above 1500m a.s.l., which evaluate the performance of different imputation methods and SpO2/FiO2 for the diagnosis of severe hypoxemia. Therefore, we aimed to establish the correlation and validity between PaO2/FiO2 obtained in arterial gases vs. non-invasive methods (linear, non-linear, logarithmic imputation of PaO2/FiO2 and SpO2/FiO2) in patients living at 2600 a.s.l. and under invasive mechanical ventilation.
MethodologyAmbispective cohort study was conducted in patients hospitalized in intensive care units with invasive ventilatory support in two third-level care hospitals in Colombia, the Santa Clara Hospital in the city of Bogota (Altitude: 2640m above sea level) and the Clínica Universidad de La Sabana in the city of Chia (Altitude: 2562m above sea level). The data was initially collected retrospectively, from June 2016 to April 2019, and prospectively from April to June 2019; information was obtained from the electronic medical records from the period of hospitalization.
PatientsSubjects over 18 years old, with at least 24h of invasive mechanical ventilation from any cause, with simultaneous arterial gases and SpO2 measurements, were included, regardless of the saturation value, severity of the disease, type of ventilatory mode, vasopressor support, or radiological involvement. Patients without concomitant measurements of arterial gases, SpO2 and ventilatory parameters were excluded. Patients with terminal disease, extracorporeal membrane oxygenation and those in whom FiO2, PaO2, and SpO2 data had not been reported in the clinical records or had bad quality in the pulse wave were also excluded.
VariablesInformation was collected on age, sex, skin pigmentation, type of pathology of entry, weight, height, vital signs, values of hemoglobin, hematocrit, bilirubin, and creatinine, findings on chest radiography, with verification of the presence of infiltrates and the number of quadrants involved, SOFA severity score, vasopressor support, ventilatory support parameters (PEEP level >10 and <10cmH20,17,18 peak pressure, plateau pressure, tidal volume), total arterial gas values and oxygenation rates, days of mechanical ventilation, intensive care unit (ICU) and hospital stays. Severe hypoxemia was the value of the PaO2/FiO2 ratio obtained from arterial gases less than 150.19,20
Severingaus-Ellis(no linear),21,22 Rice (linear)14 and Pandharipande (logarithmic linear)15 formulas were used to calculate imputed PaO2 from SpO2. The values of imputed PaO2 were used for the calculation of imputed PaO2/FiO2; besides, SpO2/FiO2 was calculated simultaneously. The blood sample for arterial gases analysis was obtained through an arterial line; the oximeters used measure the saturation through an infrared sensor with a wavelength of 905nm 2.0mW with an error range of 1%; verification and calibration were performed daily during the study.
All the subjects admitted to the ICU of both institutions during the study period were included. In order to reduce errors in data collection and transcription, the data were recorded by double typing and reviewed by two members of the research team. This protocol was approved by the ethics committee of the Clínica Universidad de La Sabana.
Statistical analysisAn initial descriptive analysis was carried out, summarizing the qualitative variables in frequencies and percentages, the quantitative variables with normal distribution in means and standard deviation and if they did not meet the criteria of normality in medians and interquartile ranges. The qualitative variables were compared through the chi-square test and the quantitative ones with Student's t-test or Mann–Whitney U test according to their distribution.
We applied the intraclass correlation coefficient between the values obtained from imputed PaO2, PaO2/FiO2 derived from the imputation methods, and the SpO2/FiO2 with the PaO2/FiO2 of the arterial gases. We considered the correlations thus: 0–0.3 null; 0.31–0.50 low; 0.51–0.70 moderate; 0.76–1.00 strong. The Bland–Altman method was used to evaluate the concordance. For the validity, the area under the ROC curve of the quantitative values obtained through the three imputation methods and the SpO2/FiO2 value was calculated, comparing it with the value of the arterial gases of severe hypoxemia (PaO2/FiO2<=150), to obtain the best cut-off point using the Youden index and calculating the values of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive and negative likelihood ratio. The ROC area was also used to establish the equivalence ranges between the different SpO2/FiO2 measurements. A p-value of less than 0.05 was considered significant. The statistical analysis was performed with the SPSS 22.0 licensed program (Statistical Package for the Social Sciences, Chicago, IL).
ResultsWe included a total of 664 patients during the study period, the general characteristics of the patients were described according to positive end-expiratory pressures (PEEP) greater or less than 10cm H2O and are showed in Table 1. Among the general characteristics, it was found that the patients had an mean age of 56±20 years, with a male predominance 444/664 (67%), most of the pathological conditions on admission were non-surgical (81%), the mean SOFA was 15±2 points, and 56% of the patients required vasoactive support.
General characteristics of the cohort.
| Characteristics | General population n=664 | PEEP<10 cmH2O n=438 | PEEP>10 cmH2O n=226 | p-Value | |||
|---|---|---|---|---|---|---|---|
| Age (years) (SD) | 56.0 | (19.9) | 55.8 | (20.1) | 56.6 | (19.5) | 0.603 |
| Sex (male), n (%) | 444 | (0.6) | 291 | (0.6) | 153 | (0.6) | 0.744 |
| Condition on admission n (%) | |||||||
| Elective surgery | 54 | (0.0) | 41 | (0.1) | 13 | (0.0) | 0.272 |
| Emergency surgery | 75 | (0.1) | 49 | (0.1) | 26 | (0.1) | |
| Non-surgical | 535 | (0.8) | 348 | (0.7) | 187 | (0.8) | |
| Height cm (SD) | 163.0 | (9.0) | 162.9 | (9.1) | 163.0 | (8.8) | 0.876 |
| Weight kg (SD) | 66.8 | (157.2) | 66.6 | (12.4) | 67.3 | (12.8) | 0.493 |
| Vital signs (DE) | 87.4 | (17.7) | 87.3 | (18.0) | 87.5 | (17.0) | 0.862 |
| SBP mmHg | 119.0 | (24.0) | 119.1 | (24.4) | 119.1 | (23.2) | 0.856 |
| DBP mmHg | 71.6 | (17.0) | 71.4 | (17.4) | 71.9 | (16.3) | 0.493 |
| MAP mmHg | 87.4 | (17.7) | 87.3 | (18.0) | 87.5 | (17.0) | 0.862 |
| HR beats×min | 86.8 | (21.3) | 84.9 | (20.6) | 90.6 | (22.2) | 0.001 |
| RR resp×min | 18.8 | (4.7) | 18.6 | (4.7) | 19.2 | (4.7) | 0.175 |
| Temperature | 36.9 | (0.7) | 36.8 | (0.6) | 36.9 | (0.8) | 0.037 |
| Laboratory workup (SD) | |||||||
| Leukocytes cell/ml | 11,979.7 | (7224.7) | 12,145.1 | (6975.9) | 11,659.0 | (7689.8) | 0.426 |
| Hb (g/dL) | 11.90 | (3.0) | 11.79 | (3.0) | 12.11 | (3.0) | 0.200 |
| Hematocrit (%) | 36.4 | (9.0) | 36.2 | (9.0) | 37.0 | (9.0) | 0.280 |
| Platelet count cell/ml | 231,737.6 | (102,667.8) | 233,050.3 | (98,410.4) | 229,199.9 | (110,618.9) | 0.659 |
| Total bilirubin (mg/dL) | 1.27 | (1.9) | 1.22 | (1.7) | 1.34 | (2.2) | 0.483 |
| Creatinine (mg/dL) | 1.40 | (1.4) | 1.40 | (1.5) | 1.39 | (1.3) | 0.920 |
| Sodium (mEq) | 143.5 | (8.5) | 143.3 | (9.1) | 143.9 | (7.1) | 0.327 |
| Potassium (mEq) | 3.97 | (0.8) | 3.97 | (0.7) | 3.97 | (0.8) | 0.917 |
| Infiltrates on chest X-ray Quadrants involved n(%) | |||||||
| 1 | 111 | (0.3) | 41 | (0.3) | 70 | (0.4) | |
| 2 | 127 | (0.4) | 43 | (0.3) | 84 | (0.4) | |
| 3 | 21 | (0.0) | 14 | (0.1) | 7 | (0.0) | |
| 4 | 31 | (0.1) | 22 | (0.1) | 9 | (0.0) | |
| SOFA (IQR) | 15 | (2) | 15 | (2) | 16 | (2) | 0.019 |
| Vasopressor support | 375 | (0.5) | 242 | (0.5) | 133 | (0.5) | 0.376 |
Notes: SD: standard deviation, SBP: systolic blood pressure, DBP: diastolic blood pressure, MAP: mean arterial pressure, HR: heart rate, RR: respiratory rate, Hb: hemoglobin, SOFA: Sepsis-related Organ Failure Assessment, IQR: interquartile range.
The mean PaO2 was 83.7±27.5mmHg, SpO2 92.4±2.3%, PaCO2 33±2.9mmHg, and bicarbonate 21.7±1.6mEq/L, with no significant differences between subgroups. The pH was 7.35±0.11 and the base excess −1.3±1.4mEq/L. The ventilatory parameters were a tidal volume of 481±58.3ml, FIO2 of 50±19%, plateau pressure 19.3±4.3cmH2O, and driving pressure of 10.8cmH2O. The mean time of mechanical ventilation was 7.1 days, ICU length of stay was 10.4 days and overall mortality was 18%. (Supplementary Material)
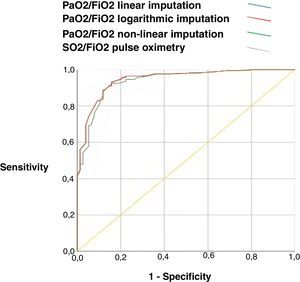
Correlation and validity resultsThe correlation between PaO2/FiO2 obtained from arterial gases, PaO2/FiO2 derived from one of the proposed methods (linear, non-linear, and logarithmic formula), and SpO2/FiO2 measured by the intraclass correlation coefficient was high (greater than 0.77, p<0.001). The different imputation methods and SpO2/FiO2 have a similar diagnostic performance in patients with severe hypoxemia (PaO2/FiO2 <150), with an area under the ROC curve greater than 0.8 with a similar sensitivity and specificity, PaO2/FiO2 linear imputation AUC ROC 0.84 (IC 0.81–0.87, p<0.001), PaO2/FiO2 logarithmic imputation AUC ROC 0.84 (IC 0.80–0.87, p<0.001), PaO2/FiO2 non-linear imputation AUC ROC 0.82 (IC 0.79–0.85, p<0.001), SpO2/FiO2 oximetry AUC ROC 0.84 (IC 0.81–0.87, p<0.001). Table 2 and Fig. 1.
Results of validity between the formulas of imputation of PaO2, PaO2/FiO2, SpO2/FiO2 and PaO2, PaO2/FiO2 of arterial blood gases and severe Hypoxemia PaO2/FiO2<=150.
| Method | AUC-ROC | CI 95% | Cut-off point | SENS | SPEC | PPV | NPV | LR+ | LR- | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| PaO2 linear imputation | 0.83 | (0.80, 0.86) | 73.1 | 0.65 | 0.83 | 0.19 | 0.81 | 3.88 | 0.20 | <0.001 |
| PaO2 logarithmic imputation | 0.84 | (0.81, 0.87) | 50.2 | 0.67 | 0.82 | 0.20 | 0.82 | 3.71 | 0.22 | <0.001 |
| PaO2 non-linear imputation | 0.60 | (0.55, 0.64) | 70.8 | 0.40 | 0.79 | 0.13 | 0.71 | 1.86 | 0.27 | <0.001 |
| PaO2/FiO2 linear imputation | 0.84 | (0.81, 0.87) | 147.6 | 0.67 | 0.82 | 0.20 | 0.82 | 3.71 | 0.22 | <0.001 |
| PaO2/FiO2 logarithmic imputation | 0.84 | (0.80, 0.87) | 119.1 | 0.62 | 0.87 | 0.19 | 0.81 | 4.64 | 0.15 | <0.001 |
| PaO2/FiO2 non-linear imputation | 0.82 | (0.79, 0.85) | 141.3 | 0.65 | 0.83 | 0.19 | 0.81 | 3.87 | 0.20 | <0.001 |
| SpO2/FiO2 oximetry | 0.84 | (0.81, 0.87) | 204.4 | 0.61 | 0.87 | 0.18 | 0.81 | 4.62 | 0.15 | <0.001 |
Notes: SENS: sensitivity; SPEC: specificity; AUC-ROC: area under the ROC curve; PPV: positive predictive values; NPV: negative predictive values; LR: likelihood ratio.
ROC curve with yield of PaO2/FiO2 imputed by the three methods of imputation and SpO2/FiO2.
Notes: AUC-ROC: area under the ROC-curve, PaO2/FiO2 linear imputation 0.84 (95%CI: 0.81–0.87); PaO2/FiO2 logarithmic imputation 0.84 (95%CI: 0.80–0.87); PaO2/FiO2 non-linear 0.82 (95%CI: 0.79–0.85); SO2/FiO2 pulse oximetry 0.84 (95%CI: 0.81–0.87).
We found that the correlation of the different methods applied (imputation and SpO2/FIO2) decreased with hemoglobin levels equal to or less than 7g/dl (0.58 p<0.001), as well as with total bilirubin values equal to or greater than 3mg/dL (0.68 p<0.001), the results above concordance between imputed PaO2/FiO2 and SpO2/FiO2 with Bland–Altman graph, and the validity for physiologic variable, mechanical ventilation and radiological findings between SpO2/FiO2 and severe Hypoxemia PaO2/FiO2<=150 showed in Supplementary Material.
In a practical way and to establish the usefulness of SpO2/FiO2 measurement, we present the ranges of values between SpO2/FiO2 and PaO2/FiO2 imputed by the three methods and PaO2/FiO2 measured by arterial gases in Table 3.
Values of correlation of SaO2/FiO2 by pulse oximetry and PaO2/FiO2 by three imputation techniques and arterial blood gases in patients under mechanical ventilation by ROC curve.
| SpO2/FiO2 | PaO2/FiO2 linear imputation | PaO2/FiO2 logarithmic imputation | PaO2/FiO2 non-linear imputation | PaO2/FiO2 arterial blood gases | |||||
|---|---|---|---|---|---|---|---|---|---|
| >300 | 297 | 328 | 247 | 284 | 203 | 323 | 246 | 324 | |
| 250 | 299 | 250 | 297 | 149 | 247 | 149 | 203 | 225 | 246 |
| 200 | 249 | 148 | 250 | 100 | 149 | 129 | 149 | 182 | 225 |
| 150 | 199 | 92 | 148 | 64 | 149 | 98 | 129 | 137 | 182 |
| 100 | 149 | 37 | 92 | 30 | 64 | 68 | 98 | 100 | 137 |
| <100 | 26 | 37 | 27 | 30 | 51 | 68 | 62 | 100 | |
This study found an adequate correlation between PaO2/FiO2 obtained by the three proposed imputation methods, SpO2/FiO2, and PaO2/FiO2 values obtained by arterial blood gases, considering the usefulness of these tools as non-invasive methods for the assessment of the oxygenation status in patients with invasive ventilatory support. We also found that the three imputation methods evaluated and the SpO2/FiO2 have an adequate diagnostic performance for severe hypoxemia (PaO2/FiO2<=150mmHg by arterial gases) in patients living at high altitude.
It was previously determined that the concordance between the different non-invasive oxygenation indexes and the PaO2/FiO2 obtained by arterial gases is high7,16,23,24 Cineci and Gomez found an overall correlation index of 0.745 between SpO2/FiO2 and PaO2/FiO2 in patients with ARF11; Rice et al. using imputation formulas, report a correspondence range between 0.73 and 0.8812,14,15 that are similar to those observed in our study (greater than 0.77). This accuracy can be influenced by the use of PEEP, hemoglobin levels, total bilirubin values, by the determinants of the affinity of oxygen for hemoglobin (pH, temperature, CO2, 2.3-diphosphoglycerate, and fetal hemoglobin)12,15 and extreme values of arterial saturation,6,25 which can vary in critically-ill ventilated patients. Our results show an important decrease in the correlation in subjects with hyperbilirubinemia (total bilirubin ≥3mg/dL) and hemoglobin less than ≤7mg/dL, without it being significantly affected by temperature, vasoactive support, and severity of multiorgan involvement.
Theoretically, imputation techniques that simulate the hemoglobin dissociation curve have a better diagnostic yield with the patient's oxygenation status. Brown's12,13 and Gadrey's24 studies found that the diagnosis of moderate-to-severe hypoxemia is better when non-linear formulas are used. Despite this, our data show similar performance among the different imputation methods, without important changes in their diagnostic capacity when patients with arterial oxygen saturation greater than 97% are included. This difference is probably explained by the type of population studied and the barometric pressure to which our patients are exposed. It is important to emphasize that our results are aimed at the recognition of severe hypoxemia and not hyperoxemia, where the subrogated SpO2 values may be more limited.25
Although mechanical ventilators represent a closed circuit, there are not pressurized; therefore, patients ventilated at high altitude may have lower PaO2 and SaO2 values than those obtained at sea level.6,26,27 Previous observations show that the corresponding values of SaO2/FiO2 from 214 to 235 are equivalent to a PaO2/FiO2 (for arterial gases) of 200 in inhabitants of low-altitude areas.11,15 However, there are no studies in patients under invasive mechanical ventilation living at more than 1500m a.s.l. that establish a single SpO2/FiO2 value corresponding to a given PaO2/FiO2. This limitation also affects the values obtained by imputation methods, since it is not possible to establish values that correspond to these measurements without any margin of error.13 Considering these difficulties, we propose a table of equivalence between SpO2/FiO2 ranges and PaO2/FiO2 values imputed by the three methods and that obtained by arterial gases, to establish values for the diagnosis and follow-up of patients with different degrees of hypoxemia that may be useful in clinical practice. We must not forget to evaluate these considering medical criteria and the condition of the patient.
We acknowledge that our study has certain limitations. First, a single arterial gas sampling was performed, which can affect the reliability of the measured values of the quantitative variables for the diagnosis of hypoxemia and the appearance of random errors. However, the samples were taken by trained personnel and keeping all the recommendations for adequate processing in the laboratory. Second, some data were collected retrospectively, which can generate an information bias; however, we verified that the values obtained from the clinical histories corresponded to the data reported directly by the laboratory. Third, despite having a large population living at high altitude, our sample does not include all possible altitudes; but we consider that it is unlikely that there are significant differences in the reliability of the formulas. Fourth, SpO2 values can be affected by methemoglobin and carboxyhemoglobin levels, which were not measured in the study. Despite this, none of the patients were suspected to have these disorders.
In conclusion, the present study found that at high altitude SaO2/FiO2 and imputed PaO2/FiO2 have a similar diagnostic yield in patients with severe hypoxemia, ventilated for various pathological conditions. Equivalence ranges between SaO2/FiO2 and PaO2/FiO2 values can be a non-invasive option in the follow-up of these subjects. It is necessary to develop additional prospective studies, where therapeutic goals of SpO2/FiO2 and imputed PaO2/FiO2 are explored to corroborate the safety ranges of these measurements.
FundingThe author received no financial support for the research, authorship, and/or publication of this article
Author contributionsGO, AB, MB, MGF and AL had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. GO, AB, MB, MGF, AL, GD, JO contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. GO, AB contributed substantially to data statistical analysis.
Conflict of interestsThe authors of this manuscript have no competing interests directly related to the manuscript's content.