INTRODUCTION
Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) have been shown to occur frequently in Intensive Care Units (ICU) patients and have been independently associated with mortality1,2. Unlike many commonly encountered disease processes which remain within the purview of a given discipline, IAH and the ACS readily cross the usual barriers and may occur in any patient population regardless of age, illness, or injury. As a result, no one specific speciality can represent the wide variety of health care workers who might encounter patients with IAH and/or ACS in their daily practice. For this reason, a multidisciplinary, international organisation, the World Society of the Abdominal Compartment Syndrome (WSACS www.wsacs.org) was founded in 2004 with the aim to foster education, to facilitate research and to improve outcome for these patients. As an introduction to the Third World Congress on Abdominal Compartment Syndrome (www.wcacs.org), organised by the WSACS, which will be held in March 2007 in Antwerp, Belgium, this review will try to give a concise overview of the epidemiologic data, etiology, diagnosis, IAP measurement, organ dysfunction, prevention and treatment options related to ACS, focusing on recent developments and possibilities for the future. It reflects the most important evolutions in literature as well as well as the personal experience of the authors.
ETIOLOGY AND EPIDEMIOLOGY
The ACS is diagnosed when there is increased intra-abdominal pressure (IAP) along with evidence of end-organ dysfunction3. IAH is diagnosed when IAP is moderately increased, but there is no evidence of organ dysfunction yet, although subtle forms of organ dysfunction may be present at levels of IAP previously deemed to be safe4. There may even be a «dose-dependent» association between IAP and organ dysfunction. Where etiology is concerned, there are two major factors to discuss: first, the origin of the increased IAP itself and secondly, the etiology of organ dysfunction in the presence of IAH.
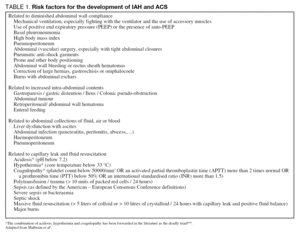
Why and when does the pressure within the abdominal cavity rise? In analogy to the situation in the brain, there are essentially two parts in the abdominal pressure-volume curve. At low intra-abdominal volumes (and pressures) the abdominal wall is very compliant and relatively large increases in volume will lead to minor changes in IAP only5. However, at higher volumes the abdominal wall compliance reaches its compensatory limits and small volume changes can lead to large changes in IAP, which means that a small increase in intra-abdominal volume can lead to clinically important IAH. This abdominal pressure-volume curve is shifted to the left in situations where the abdominal wall compliance is decreased due to haematoma, voluntary muscle activity, edema or other factors. Therefore, IAH is usually associated with a situation that leads to increased abdominal volume, decreased abdominal compliance or a combination of both. The WSACS published a list of risk factors associated with these situations4. They are summarized in table 1.
The mechanisms that link IAH with organ dysfunction are not yet completely understood. There is certainly a direct mechanical effect of the increased IAP on the blood supply of the intra-abdominal organs, which is most convincingly seen in the kidney6,7. Some of the deleterious effects may be associated with direct compression of the organ involved and hormonal changes have been implicated as well. However, IAH also has an impact on distant organ function. Ischemia-reperfusion injury may be involved in this complex pathophysiology as a «second-hit» phenomenon after shock resuscitation8,9. The mechanisms involved will be clarified when discussing the effect of IAH on the different organ systems.
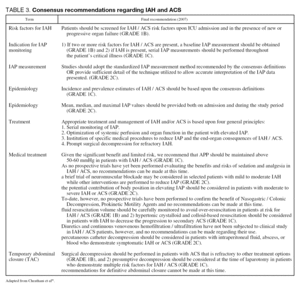
DEFINITIONS AND DIAGNOSIS OF IAH AND ACS
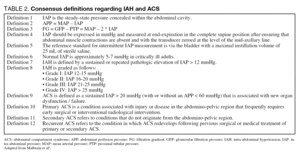
The first consensus paper by the WSACS, published in 2006, contains a list of definitions related to IAH and ACS4. The definitions are listed in table 2 and the recommendations in table 3. These definitions are based on the best available scientific data today, but they are likely to undergo some minor changes in the future. The different methods used for diagnosis of ACS will be discussed here.
Clinical examination and medical imaging
Several surveys among clinicians show that many of them use clinical examination for the diagnosis of ACS. This has been shown to be unreliable with a sensitivity and positive predictive value of around 40-60%10,11. The use of abdominal perimeter is equally inaccurate. Radiologic investigation with plain radiography of the chest or abdomen, abdominal ultrasound or CT-scan are also insensitive to the presence of increased IAP. However, they can be indicated to illustrate the cause of IAH (bleeding, hematoma, ascites, abscess...) and may offer clues for management (paracenthesis, drainage of collections...).
IAP measurement
The most important prerequisite for the diagnosis and treatment of ACS is IAP measurement. Since the abdominal contents are primarily non compressive in nature and predominantly fluid-based, they can be assumed to behave according to Pascal's law. Therefore, the IAP measured at one point can be assumed to be the pressure throughout the abdominal cavity.
The IAP can be measured directly or indirectly, either intermittently or continuously6. The gold standard is direct intraperitoneal measurement, but this is rarely available in clinical situations. Today, it is mostly used for animal research or in clinical studies during laparoscopy where IAP can be measured directly through a Verres needle or a laparoscopy port. The most frequently used routes for indirect IAP measurement are the bladder and the stomach.
Transvesical IAP measurement
The previously mentioned surveys indicate that the transvesical technique is the most frequently used method for IAP measurement today, due to simplicity and minimal cost5. It was also put forward as the gold standard in the WSACS consensus paper by Malbrain et al4. There is still some debate about the optimal amount of instillation volume for measurement. Initially, up to 200 mL of instillation volume was used, but this has been shown to lead to overestimation of IAP12 and thus to false classification and possibly treatment of IAH. There is a trend towards using smaller instillation volumes12-14. The WSACS consensus paper mentions an instillation volume of «no more than 25 mL». It is possible that even lower instillation volumes may be advocated in the future. Several commercially available tools have been developed for transvesical IAP measurement e.g. the FoleyManometer (Holtech Medical, Kopenhagen, Denmark www.holtech-medical.com) or the AbViser valve (Wolfe Tory, Salt Lake City, Utah, USA www.wolfetory.com). A transvesical technique for continuous measurement has been described, but this technique is not widely used today15.
Transgastric measurement
Measurement through the stomach has some advantages: it avoids the problems associated with creating a hydrostatic fluid column in the bladder and it is easier to use for continuous measurement. Several balloon tipped catheters have been developed for IAP measurement. The Spiegelberg monitor (Spiegelberg, Hamburg, Germany www.spiegelberg.de) was developed for intracranial pressure measurement but can be used also for continuous IAP measurement16,17. Pulsion (Pulsion Medical Systems, Munich, Germany www.pulsion.com) developed a balloon tipped catheter for continuous measurement. The CiMON monitor attached to it will offer continuous IAP and abdominal perfusion pressure (APP) measurement, along with respiratory variations in IAP. This monitor is yet to be clinically validated, but it may offer more possibilities for real-time dynamic evaluation of IAH in the future.
APP measurement
Analogous to the widely accepted and clinically utilized concept of cerebral perfusion pressure, calculated as mean arterial pressure (MAP) minus intracranial pressure (ICP), APP, calculated as MAP minus IAP, has been proposed as a more accurate predictor of visceral perfusion and a potential endpoint for resuscitation18-21. APP, by considering both arterial inflow (MAP) and restrictions to venous outflow (IAP), has been demonstrated to be statistically superior to either parameter alone in predicting patient survival from IAH and ACS21. A target APP of at least 60 mmHg has been demonstrated to correlate with improved survival from IAH and ACS.
THE EFFECT OF IAH ON ORGAN FUNCTION
It is beyond the scope of this text to give a complete overview of all pathophysiologic mechanisms involved. We have focused on those pathologic observations that have direct implications on the clinical management of critically ill patients
Effect on the cardiovascular system
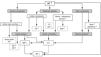
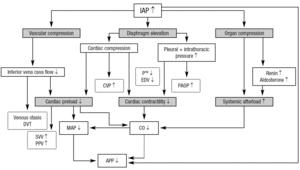
IAH is associated with a number of effects on the cardiovascular system that are caused by multiple factors22. A very important concept is the abdominothoracic transmission, which means that the intrathoracic pressure increases during IAH due to the cephalad movement of the diaphragm. Animal and human experiments have shown that 20-80% of the IAP is transmitted to the thorax. This phenomenon accounts for most of the cardiovascular as well as the pulmonary and neurologic consequences of IAH. Figure 1 illustrates the cardiovascular effects of IAH.
Figura 1. The cardiovascular effects of IAH. APP: abdominal perfusion pressure; CO: cardiac output; CVP: central venous pressure: DVT: deep vein thrombosis; EDV: end diastolic volume; IAP: intra-abdominal pressure; MAP: mean arterial pressure; PAOP: pulmonary artery occlusion pressure; PE: pulmonary embolism; PPV: pulse pressure variation. P: transmural pressure; SVV: stroke volume variation.
A very important issue in the management of patients with IAH is the interpretation of haemodynamic monitoring parameters. Due to the abdominothoracic transmission of pressure, traditional filling pressures (central venous pressure [CVP], and pulmonary artery occlusion pressure [PAOP]) are «falsely» elevated in the presence of IAH, and do not reflect true cardiac filling. Therefore, it may be more useful to use volumetric monitoring parameters such as right ventricular end diastolic volume index (RVEDVI) or global end diastolic volume index (GEDVI)23-27. Preload responsiveness can best be evaluated using dynamic parameters such as pulse pressure variation (PPV) or stroke volume variation (SVV)28,29, provided that patients have a regular sinus rhythm, are completely sedated and do not exhibit spontaneous breathing movements. If these volumetric or dynamic parameters are not available and filling pressures have to be used for haemodynamic monitoring, they should be corrected for intrathoracic pressure. This means that transmural CVP (CVPTM) is equal to CVP minus intrathoracic pressure (ITP) and PAOPTM = PAOP ITP. Since the abdominothoracic transmission amounts to 20-80%, ITP can be assumed to be IAP/2 and transmural filling pressures can be estimated as:
CVPTM = CVP IAP/2
PAOPTM = PAOP IAP/2
The surviving sepsis campaign guidelines targeting initial and ongoing resuscitation towards a CVP of 8 to 12 mmHg30 and other studies targeting a MAP of 65 mmHg31 should be interpreted and adjusted according to these findings.
Effects of IAH on the respiratory system
The transmission of IAP to the thorax also has an impact on the respiratory system. The major problem lies in the reduction of the functional residual capacity (FRC). Together with the alterations caused by secondary adult respiratory distress syndrome (ARDS) this will lead to the so-called «baby-lungs». The chest wall compliance is reduced during IAH while lung compliance remains virtually unchanged, which leads to decreased overall compliance of the respiratory system32,33. Some recommendations can be made in terms of ventilation strategy for patients with IAH:
1) Best PEEP should be set to counteract IAP whilst in the same time avoiding over-inflation of already well-aerated lung regions.
Best PEEP = IAP
2) In analogy to the cardiovascular system, ARDS consensus definitions and recommendations should be adapted to take into account the influence of IAP on intrathoracic pressure. During lung protective ventilation, the plateau pressures should be limited to transmural plateau pressures below 35 cmH2O
Pplat = Pplat IAP/2
3) Monitoring of extravascular lung water index (EVLWI) seems warranted in risk patients since IAH is associated with increased risk of lung edema34. Capillary leak syndrome and IAH have a synergistic effect on the generation of lung edema.
4) The presence of IAH will lead to pulmonary hypertension via increased ITP with direct compression on lung parenchyma and vessels and via the diminished left and right ventricular compliance. In this case the administration of inhaled NO or ilomedine (prostacyclin) may be justified.
The effect of IAH on the central nervous system
A direct relationship between IAP and ICP has been observed in both animal and human studies20,35-38. Several authors hypothesized that the increase in ICP secondary to IAH was caused by increased ITP, leading to increased CVP and decreased venous return from the brain and thus, venous congestion and brain edema. This hypothesis gained acceptance when Bloomfield et al demonstrated that the association between IAP and ICP could be abolished by performing a sternotomy and bilateral pleuropericardotomy in pigs37. The reduced systemic blood pressure associated with decreased cardiac preload and the increase in ICP also leads to a decrease in cerebral perfusion pressure (CPP). Some authors have demonstrated successful treatment of refractory intracranial hypertension with abdominal decompression or neuromuscular blockers20,35.
Some recommendations:
1) IAP monitoring is essential for all traumatic or nontraumatic patients at risk for intracranial hypertension (ICH) or IAH (according to the risk factors published by the WSACS).
2) In all patients with ICH, preventive measures should be undertaken to avoid increase in IAP, and conversely, in all patients with IAH, a possible association with ICH should be considered and preventive measures should be taken (head of bed elevation, avoid hypervolemia, hypernatriemia and hyperthermia...).
3) Avoid hypervolemia in patients with IAH to prevent further increase in ICP.
4) Consider using APP (abdominal perfusion pressure; APP = MAP-IAP) as a resuscitation target in patients where ICP is not available and CPP can not be used as a target.
5) Avoid laparoscopy in patients at risk for ICH. The pneumoperitoneum used for laparoscopy creates a situation analogous to experimental settings of IAH and ICH in which detrimental effects on ICP have been observed. This is especially important in trauma patients with associated brain and abdominal injuries.
The effect of IAH on renal function
Renal dysfunction is one of the most consistently described organ dysfunctions associated with IAH. The etiology is multifactorial and offers a unique insight into the deleterious and sometimes cumulative effects of IAH on organ function.
The most important effect of IAH on the kidney is related to renal blood flow. IAH has been shown to lead to renal venous compression and increased renal venous pressure39,40. Also, renal arterial blood flow and microcirculatory flow in the renal cortex are decreased.. Direct compression of the renal cortex may be a contributing factor39,41. The changes in renal blood flow lead to activation of the renin-angiotensin-aldosteron pathway and also, ADH secretion is increased in IAH42,43. The clinical importance of these changes is still unclear.
Biancofiore and Sugrue showed that renal dysfunction is rather common in IAH6,7,44-46. Ulyatt suggested that filtration gradient (FG) is an important factor in explaining renal failure associated with IAH47. The FG is the mechanical force across the glomerulus and is equal to the difference between glomerular filtration pressure and the proximal tubular pressure. Glomerular filtration pressure is equal to RPF and thus to MAP IAP. In the presence of IAH, proximal tubular pressure can be equated with IAP. The FG can therefore be calculated as FG = MAP (2x IAP). This explains why the kidney seems to be more vulnerable to IAH than other surrounding organs and is probably one of the key factors in the development of IAH-induced renal failure7,21.
The effect of IAH on other organ functions
Animal and human studies have shown decreased hepatic arterial flow as well as decreased portal flow and increased portacollateral flow. Furthermore, even moderate levels of IAP have been associated with impaired hepatocellular function48,49. IAH is inversely correlated with indocyanine green clearance50. Biancofiore et al demonstrated that liver disease and especially liver surgery and transplantation are often associated with IAH and ACS51. Cytochrome P450 function and other liver functions may be impaired in IAH. Therefore, it seems wise to avoid hepatotoxic medications, increase the attention towards therapeutic interactions, treat IAH aggressively and maximize supportive treatment for patients with IAH and liver dysfunction.
Where the digestive tract is concerned, intra-abdominal hypertension causes diminished perfusion and mucosal acidosis and sets the stage for multiple organ failure52. The ischemia and reperfusion injury to the gut serves as a second insult in a two hit model of MOF where the lymph flow conducts gut-derived pro-inflammatory cytokines to remote organs9,52. These complex mechanisms are not yet completely understood, but they will undoubtedly be the subject of further study in the next few years.
TREATMENT OF IAH
In analogy to other compartment syndromes in the human body, decompressive laparotomy (DL) seems the most logical treatment option. It is also the most widely used and best described treatment modality today. However, DL leaves the patient with an open abdomen which can lead to extensive fluid losses, infection, enterocutaneous fistulae, ventral hernia and cosmetic dysfunction. Therefore, DL is mostly used today as a rescue therapy for patients with overt ACS, who have not responded to medical treatment. Indications and results for different treatment modalities will be discussed here.
Decompressive laparotomy
A recent systematic review on decompressive laparotomy, based on 18 studies, was published by De Waele et al53. This review illustrated that DL is successful in lowering IAP in all studies. Concerning the results on organ function, results are variable. Regarding the cardiovascular function, heart rate en MAP remained unchanged in most studies. CVP and PAOP decreased significantly, which is to be expected in view of the abdominothoracic transmission. This probably does not reflect a true improvement in cardiac function. However, cardiac index was also improved. In analogy, peak inspiratory pressures decreased after decompression, but also PaO2/FiO2 improved. The effect on renal function is less clear. In most studies, urine output was significantly improved after DL, but interestingly, in the two largest series54,55 urine output was not affected. Sugrue suggests that acute tubular necrosis might be involved which takes longer to recuperate and does not appear in short-term outcome analyses. In general, DL seems to have a beneficial effect on organ function. Overall mortality remains high (49.2%). Although most authors agree that DL should be performed in patients with IAP > 20 mmHg and new or progressive organ failure, there is some reluctance to perform DL because of the practical consequences in terms of fluid loss through the open abdomen, difficult wound dressings, risk of infection or fistula, re-interventions, cost and longer hospital stay. However, a well performed study by Cheatham et al56 demonstrated that physical, social and mental health after DL is restored to the level of the general population after abdominal wall reconstruction and DL does not lead to permanent disability or unemployment.
Since the goal of DL is to decompress and thereby increase intra-abdominal volume it is usually not possible to close the abdomen primarily, which means that some form of temporary abdominal closure (TAC) has to be performed to protect the abdominal contents and to allow healing, followed by an abdominal wall reconstruction which is usually planned after several months. The most widely used techniques for TAC are a Bogota bag (a plastic sheet cut from a sterile bag of infusion fluids sewn to the fascia or skin edges), resorbable surgical mesh with or without split thickness skin grafting, towel clip closure (in situations where other TAC methods are not available or too time consuming), commercially available devices such as zippers or Wittman patches or vacuum assisted closure (VAC). The detailed description of these techniques, their indications and results are beyond the scope of this text. There is certainly a vast body of scientific data on this subject.
Minimally invasive surgical decompression
Because of the complications associated with full DL, surgeons have been searching for less invasive techniques to decompress the abdomen. Endoscopic techniques based on the components separation concept described by Ramirez, Voss and others57,58, like the subcutaneous anterior abdominal fasciotomy59 are being developed and might replace DL in selected cases in the future.
Non surgical management
Most non-surgical treatment strategies are aimed at either decreasing abdominal volume or increasing wall compliance. An overview of possible treatment strategies is given in table 4. Some of these will be highlighted here in detail.
Evacuation of intraluminal contents
Non-invasive removal of intraluminal contents by gastric tube placement and suctioning, rectal tube placement, enemas and, if indicated, endoscopic decompression should be attempted60-62.
Also, gastroprokinetics (such as metoclopramide or erythromycin) and/or colonoprokinetics (neostygmine or prostygmine) can be used63-67. In patients with gross dilatation of the stomach or the colon, this alone may be sufficient to lower IAP to harmless levels, but in most general ICU patients, other measures will have to be considered.
Evacuation of extraluminal contents
Drainage of tense ascites may result in a decrease in IAP68-72. Paracenthesis is the treatment of choice in burn patients with secondary ACS73-75 or any other patients who develop ascites after massive (usually crystalloid) fluid resuscitation. If intra-abdominal abscesses, hematomas or fluid collections are present, they should be drained also.
Use of sedation and neuromuscular blockers
Increased muscle tone in the rectus abdominal wall muscles due to voluntary muscle contraction, pain or agitation, causes decreased abdominal wall compliance and thus IAH. Therefore, it is important to titrate analgesia and sedation to allow for maximal relaxation of the abdominal wall muscles. However, in critically ill patients with capillary leak and abdominal wall edema, control of pain and agitation are often not sufficient and the use of neuromuscular blockers has to be considered. In a single case report, single dose administraton of cisatracurium, followed later by continuous infusion of cisatracurium, was successful in lowering IAP to safe levels and was also associated with an increase in urine output76. Other authors have confirmed these findings20,77. However, neuromuscular blockers have been associated with increased incidence of ventilator-associated pneumonia and ICU muscular weakness and their use has been restricted in the last few years to avoid these and other complications. The possible benefit of reducing IAP has to be balanced against the risk of complications at the individual patient level.
Correction of capillary leak and positive fluid balance
Most patients with IAH, due to the nature of their illness or trauma, present with capillary leak syndrome. In the early stages of their illness it is important to resuscitate these patients towards euvolemia and adequate intravascular fluid status, both in terms of their general condition and in terms of their IAH, since hypovolemia in patients with IAH can lead to splanchnic hypoperfusion and aggravation of the organ dysfunction78,79. Dobutamine may help to counteract this splanchnic hypoperfusion80.
However, fluid resuscitation will lead also to increased edema formation, third spacing and possibly to a vicious cycle of ongoing IAH. After hemodynamic stabilisation, correction of the fluid balance and decreasing edema formation becomes important. If renal function is only minimally to mildly compromised and the patient is hemodynamically stable, mobilisation of edema by administration of colloids e.g. albumin (to increase colloid osmotic pressure) and diuretics can be attempted. However, as renal function deteriorates further, patients often do not respond to diuretic therapy. Fluid removal by means of ultrafiltration has been demonstrated to have a beneficial effect on IAP and possibly on organ function81,82. The institution of renal replacement therapy with fluid removal, if hemodynamically tolerated, should not be delayed. In patients with borderline hemodynamic status, continuous forms of RRT may be preferred over intermittent RRT to avoid hemodynamic instability.
Octreotide
Kacmaz et al found evidence of ischemia-reperfusion injury in animals after decompressive laparotomy83. They hypothesize that the organ dysfunction associated with IAH is caused by ischemic damage, and decompressive laparotomy leads to reperfusion injury causing a second-hit phenomenon. This reperfusion injury seems to be counteracted by the administration of octreotide, a long acting somatostatin analogue, before decompression. Further research in humans is necessary to confirm these findings.
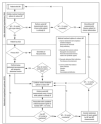
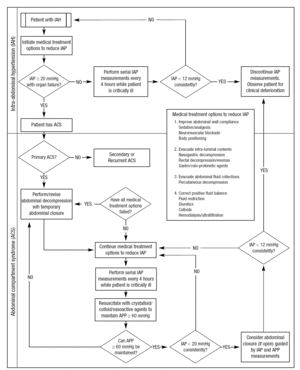
The most difficult issue is to decide what to do to whom and when. Few scientific data are available at this moment to guide treatment. A possible treatment algorithm is shown in figure 2. Large interventional studies will have to be conducted to further elucidate this complex issue.
Figura 2. Possible treatment algorithm. Adapted from Cheatham ML, et al86.
CONCLUSION
Intra-abdominal hypertension and abdominal compartment syndrome occur frequently in ICU patients and are independently associated with mortality. Since diagnosis relies entirely on the knowledge of IAP, the different techniques for accurate IAP measurement are mentioned in this paper. The effect of IAH on different organ systems is described, along with recommendations to compensate for these effects. The ultimate goal of treatment is not only to decrease IAP, but also to improve organ function and to decrease mortality. Decompressive laparotomy is the only treatment option that has been shown to reach most of these goals today. However, some less invasive techniques and some medical treatment strategies have shown promise in achieving IAP reduction as well as organ function improvement. Indications for these techniques and implementation of a treatment algorithm will require additional clinical research, which is likely to be fostered by the WSACS. «It is time to pay attention!», this was the title of a recent review 4 and the slogan of the 3rd World Congress on Abdominal Compartment Syndrome (WCACS2007) held in Antwerp, Belgium in 2007, march 22-24 (www. wcacs.org).
Correspondence:
Dr. M. Malbrain.
e-mail: manu.malbrain@skynet.be
Manuscrito aceptado el 30-I-2007.