To analyze the evolution of sepsis-related mortality in Spanish Intensive Care Units (ICUs) following introduction of the Surviving Sepsis Campaign (SSC) guidelines and the relationship with sepsis process-of-care.
DesignA prospective cohort study was carried out, with the inclusion of all consecutive patients presenting severe sepsis or septic shock admitted to 41 Spanish ICUs during two time periods: 2005 (Edusepsis study pre-intervention group) and 2011 (ABISS-Edusepsis study pre-intervention group).
ScopePatients with severe sepsis or septic shock admitted to Spanish ICUs.
PatientsAll ICU admissions from the emergency department or wards and all ICU patients with a diagnosis of severe sepsis or septic shock. A total of 1348 patients were included: 630 in the 2005 group and 718 in the 2011 group.
InterventionNone.
Primary endpointsICU mortality, 28-day mortality and Hospital mortality, hospital length of stay, ICU length of stay and compliance with the resuscitation bundle.
ResultsCompliance with the resuscitation bundle was significantly greater in the 2011 group (5.7% vs. 9.9%; p=0.005), and was associated to lower mortality (OR 0.602 [0.365–0.994]; p=0.048). The 2011 group had lower absolute in-hospital mortality (44.0% vs. 32.6%; p=0.01), 28-day mortality (36.5% vs. 23.0%; p=0.01), and adjusted mortality (OR 0.64 [0.49–0.83], p=0.001).
ConclusionsMortality related to severe sepsis or septic shock in Spain decreased between two patient cohorts in 2005 and 2011, and was attributable to earliness and improvement in sepsis care.
Analizar la evolución de la mortalidad relacionada con la sepsis en las unidades de cuidados intensivos (UCI) españolas desde la introducción de las directrices Surviving Sepsis Campaing y la relación con el proceso de atención de la sepsis.
DiseñoEstudio prospectivo de cohortes. Se incluyeron de manera consecutiva, todos los pacientes con sepsis grave o shock séptico ingresados en 41 UCI españolas durante 2 periodos de tiempo: en 2005 (grupo pre-intervención en el estudio Edusepsis) y en 2011 (grupo pre-intervención en el estudio ABISS-Edusepsis).
ÁmbitoPacientes con sepsis grave o shock séptico ingresados en las UCI españolas.
PacientesTodos los ingresos en UCI procedentes de Urgencias o planta y todos los pacientes de UCI con diagnóstico de sepsis grave/shock séptico. Se incluyeron 1348 pacientes: 630 del grupo de 2005 y 718 del grupo de 2011.
IntervenciónNinguna.
Variables de interés principalMortalidad en UCI, a 28 días y hospitalaria, estancia en la UCI y en el hospital y cumplimiento con el bundle de reanimación.
ResultadosEl cumplimiento del bundle de reanimación fue significativamente mayor en el grupo de 2011 (5,7 frente a 9,9%, p=0,005) y se asoció con una menor mortalidad (OR 0,602 [0,365 a 0,994], p=0,048). El grupo de 2011 tuvo una menor mortalidad absoluta hospitalaria (44,0 frente a 32,6%, p=0,01), mortalidad a los 28 días (36,5 frente a 23,0%, p=0,01) y mortalidad ajustada (OR 0,64 [0,49 a 0,83], p=0,001).
ConclusionesLa mortalidad relacionada con la sepsis grave y el shock séptico en España disminuyó entre las 2 cohortes de pacientes de 2005 y 2011, atribuible a la precocidad y las mejoras en la atención de la sepsis.
Severe sepsis and septic shock are major healthcare problems worldwide, with high mortality and increasing incidence. Before the start of the Surviving Sepsis Campaign (SSC) in 2002, in the USA, there were 300 cases of severe sepsis per 100,000 population and 2.26 cases per 100 hospital discharges; half of those received intensive care. The overall rate of sepsis mortality was 28.6%; mortality increased with age, from 10% in children to 38.4% in those >85 years old. At an average cost of $22,100 per case, the total annual cost was $16.7 billion. The incidence was projected to increase by 1.5% per year.1 An epidemiological study in Spain, that analyzed the 2006–2011 National Hospital Discharge Registry, reported that overall incidence per year of severe sepsis was 86.97 cases per 100,000 population (increasing from 63.91 cases/100,000 population in 2006 to 105.51 cases/100,000 population in 2011) representing 1.1% of all hospitalisations and 54% of hospitalisations with sepsis. The overall mortality rate during the study period was 37.1 cases per 100,000 population with a significant decrease in mortality rates with an overall annual percent change of −3.24%,2 the incidence of severe sepsis attended in the Spanish ICU was 12.4% with high ICU and hospital mortality rates (48.2 and 54.3% respectively),3 with treatment costing around 500 million euros annually.4
In the past decade many studies have demonstrated improved survival in septic patients with early administration of appropriate antibiotics,5–8 lactate levels measurements,9 early goal-directed hemodynamic resuscitation,10 management with replacement doses of corticosteroids,11 glycemic control,12 drotrecogin alfa (activated) administration,13 and protective mechanical ventilation.14 These therapeutic advances were collected in the first Surviving Sepsis Campaign (SSC) guidelines15,16 with the intention to reduce sepsis mortality by 25% in five years. The progressive implementation of these recommendations achieved a progressive fall in mortality.17,18 An Spanish study showed that compliance with the resuscitation bundle is associated with improvement in survival in patients with severe sepsis/septic shock,19 also, in 2010, a meta-analysis of all studies comparing outcomes in patients who received bundled care vs. non protocolized care demonstrated a clear association between the use of bundles and lower mortality,20 even though most of the original recommendations in bundled care were changed after randomized controlled trials failed to confirm the efficacy of specific sepsis treatments (most of which conform the management bundle),21–26 as is reflected in the recently updated SSC guidelines.27,28
Two recent studies concluded that there has been a secular decrease in severe sepsis mortality. One study analyzed patients pooled from the control groups of 36 randomized controlled trials investigating severe sepsis and compared the 28-day mortality with patients in the administrative Nationwide Inpatient Sample database.29 The other study analyzed hospital mortality due to severe sepsis from a large database of patients in Australian and New Zealand intensive care units (ICU).30 Both studies found 1–3% annual improvement in crude severe sepsis mortality. The mechanism underlying this decline is unclear, but is probably related to improved processes of care.
The Edusepsis study evaluated the impact of a nationwide quality improvement intervention in Spain based on the SSC guidelines, showing an improvement in compliance with treatment recommendations accompanied by a reduction in mortality.31 However, not all the effects of the intervention were sustained; for example, early use of antibiotics decreased in the long-term follow-up. This is especially relevant considering an analysis of the impact of individual components of the resuscitation bundle on mortality in the Edusepsis study found that early empirical antibiotic administration was the most important factor.4 As in other time-dependent pathologies, in sepsis the timeliness and appropriateness of treatments administered in the first hour after the onset of disease can influence outcomes. For all these reasons, a new study was designed to focus specifically on educational interventions about early administration of empirical antibiotics in severe sepsis and septic shock (ABISS-Edusepsis study). Both the original Edusepsis study and the ABISS-Edusepsis study employed a “control group” documented before the educational interventions.
The primary objective of the study was to analyze the evolution of sepsis-related mortality in Spanish ICUs since the introduction of the Surviving Sepsis Campaing (SSC) guidelines and the relationship with the improvement of sepsis process-of-care. The secondary objective was to analyze the evolution of sepsis process-of-care by using the sepsis resuscitation bundle.
Patients and methodsDesignWe designed a cohort study to compare two groups of patients with severe sepsis or septic shock treated in Spanish ICUs during two time periods: the first group (data collected between November and December 2005) was the pre-intervention group in the Edusepsis study, and the second group (data collected between April and June 2011) was the pre-intervention group in the ABISS-Edusepsis study (an ongoing study, in the data analysis phase. http://www.edusepsis.org/en/abiss-edusepsis.html). Only data from ICUs that participated in both studies were included in the present study.
Patients and process-of-care and outcome measurementsWe used the same inclusion-exclusion criteria and definitions of severe sepsis/septic shock, acute organ dysfunction, and onset of sepsis (time zero) as in the two Edusepsis studies.5,31 Briefly, in both studies, all ICU admissions from the emergency department or from wards and all ICU patients were actively screened daily for severe sepsis or septic shock. Time zero was determined according to the patient's location within the hospital when sepsis was diagnosed. Researchers recorded data related to ten items (tasks or targets), grouped in the sepsis resuscitation bundle (6 items that should begin immediately and be accomplished within 6h of time zero: lactate measurement, fluids and vasopressors, blood extraction for cultures, administration of broad spectrum antibiotics, achievement of central venous pressure ≥8mmHg, and achievement of central venous oxygen saturation ≥70%).
Bundle compliance and clinical outcomeThe primary outcome measure was hospital mortality and compliance with the individual items of the resuscitation bundle in the established time frames. Compliance was defined as evidence that bundle tasks were done and targets were achieved within the indicated time frame. Secondary outcome measures included 28-day mortality, hospital length of stay, and ICU length of stay.
Statistical analysisDescriptive statistics included frequencies and percentages for categorical variables and means, standard deviations, medians, and interquartile ranges for continuous variables. To compare categorical variables between the two study periods, we used chi-square analysis. To compare continuous variables during the two study periods, we used Student's t-test or the Mann–Whitney test, as appropriate. We constructed 3 multivariate logistic regression models, with hospital mortality as the dependent variable:
- •
Model I was constructed to assess the protective effect of bundles and includes as independent variables: resuscitation bundle, APACHE II score, age, patient location at sepsis diagnosis, site of infection, and baseline acute organ dysfunctions.
- •
Model II was constructed to assess the difference in mortality between the two study periods adjusted by APACHE II score, age, patient location at sepsis diagnosis, site of infection and baseline acute organ dysfunctions.
- •
Model III was constructed to assess the potential role of bundle compliance in the reduction of the adjusted mortality and includes the same independent variables than Model II adding the resuscitation bundle.
Statistical tests were two-tailed and significance was set at 0.05. We used SPSS version 17.0 (SPSS, Chicago, IL, USA) for all analyses.
Ethics committee approvalEach participating centers’ research and ethical review boards approved the study and patients remained anonymous. The need for informed consent was waived in view of the observational and anonymous nature of the study.
ResultsData from the 41 ICUs participating in both studies were included. All ICUs were medical-surgical and most (86%) were in teaching hospitals training residents. No patients were excluded.
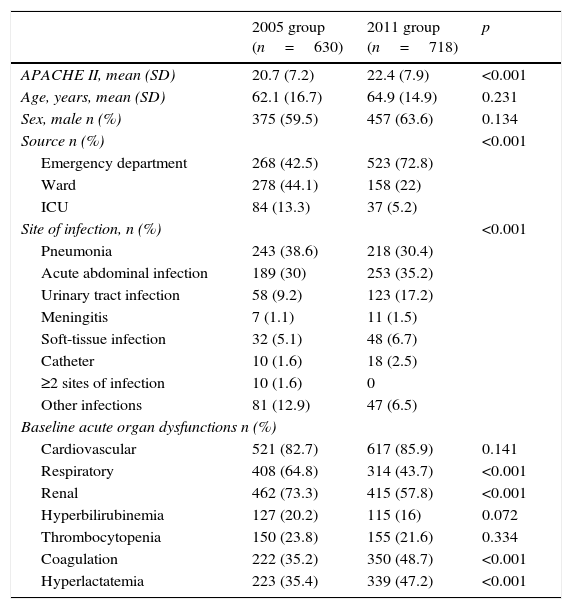
Patient characteristicsIn the two periods, 1348 patients fulfilled criteria for severe sepsis or septic shock (630 patients in the 2005 group and 718 in the 2011 group). Patients in the 2011 group were older and more severely ill. In 2005, sepsis was diagnosed predominantly in the ward, whereas in 2011 sepsis was diagnosed predominantly in the emergency department. Pneumonia was most common infection in 2005 while acute abdominal infection was the predominant infection in 2011. More than 80% of patients in both periods had septic shock. Table 1 shows patient characteristics in the two periods.
Demographic and clinical characteristics of patients, by group.
| 2005 group (n=630) | 2011 group (n=718) | p | |
|---|---|---|---|
| APACHE II, mean (SD) | 20.7 (7.2) | 22.4 (7.9) | <0.001 |
| Age, years, mean (SD) | 62.1 (16.7) | 64.9 (14.9) | 0.231 |
| Sex, male n (%) | 375 (59.5) | 457 (63.6) | 0.134 |
| Source n (%) | <0.001 | ||
| Emergency department | 268 (42.5) | 523 (72.8) | |
| Ward | 278 (44.1) | 158 (22) | |
| ICU | 84 (13.3) | 37 (5.2) | |
| Site of infection, n (%) | <0.001 | ||
| Pneumonia | 243 (38.6) | 218 (30.4) | |
| Acute abdominal infection | 189 (30) | 253 (35.2) | |
| Urinary tract infection | 58 (9.2) | 123 (17.2) | |
| Meningitis | 7 (1.1) | 11 (1.5) | |
| Soft-tissue infection | 32 (5.1) | 48 (6.7) | |
| Catheter | 10 (1.6) | 18 (2.5) | |
| ≥2 sites of infection | 10 (1.6) | 0 | |
| Other infections | 81 (12.9) | 47 (6.5) | |
| Baseline acute organ dysfunctions n (%) | |||
| Cardiovascular | 521 (82.7) | 617 (85.9) | 0.141 |
| Respiratory | 408 (64.8) | 314 (43.7) | <0.001 |
| Renal | 462 (73.3) | 415 (57.8) | <0.001 |
| Hyperbilirubinemia | 127 (20.2) | 115 (16) | 0.072 |
| Thrombocytopenia | 150 (23.8) | 155 (21.6) | 0.334 |
| Coagulation | 222 (35.2) | 350 (48.7) | <0.001 |
| Hyperlactatemia | 223 (35.4) | 339 (47.2) | <0.001 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SD, standard deviation; ICU, intensive care unit.
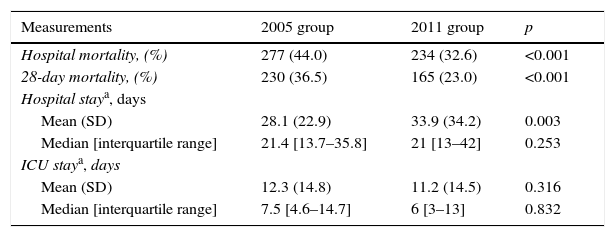
Table 2 reports the outcome data. Patients in the 2011 group had lower hospital mortality (32.6% vs. 44.0%; p<0.001) and 28-day mortality (23.0% vs. 36.5%; p<0.001). No differences were observed in the ICU stay in the surviving populations, but the mean hospital stay was higher in the 2011 group (28.1±22.9 vs. 33.9±34.2 days; p=0.003).
Outcomes measurements by group.
| Measurements | 2005 group | 2011 group | p |
|---|---|---|---|
| Hospital mortality, (%) | 277 (44.0) | 234 (32.6) | <0.001 |
| 28-day mortality, (%) | 230 (36.5) | 165 (23.0) | <0.001 |
| Hospital staya, days | |||
| Mean (SD) | 28.1 (22.9) | 33.9 (34.2) | 0.003 |
| Median [interquartile range] | 21.4 [13.7–35.8] | 21 [13–42] | 0.253 |
| ICU staya, days | |||
| Mean (SD) | 12.3 (14.8) | 11.2 (14.5) | 0.316 |
| Median [interquartile range] | 7.5 [4.6–14.7] | 6 [3–13] | 0.832 |
Abbreviations: SD, standard deviation; ICU, intensive care unit.
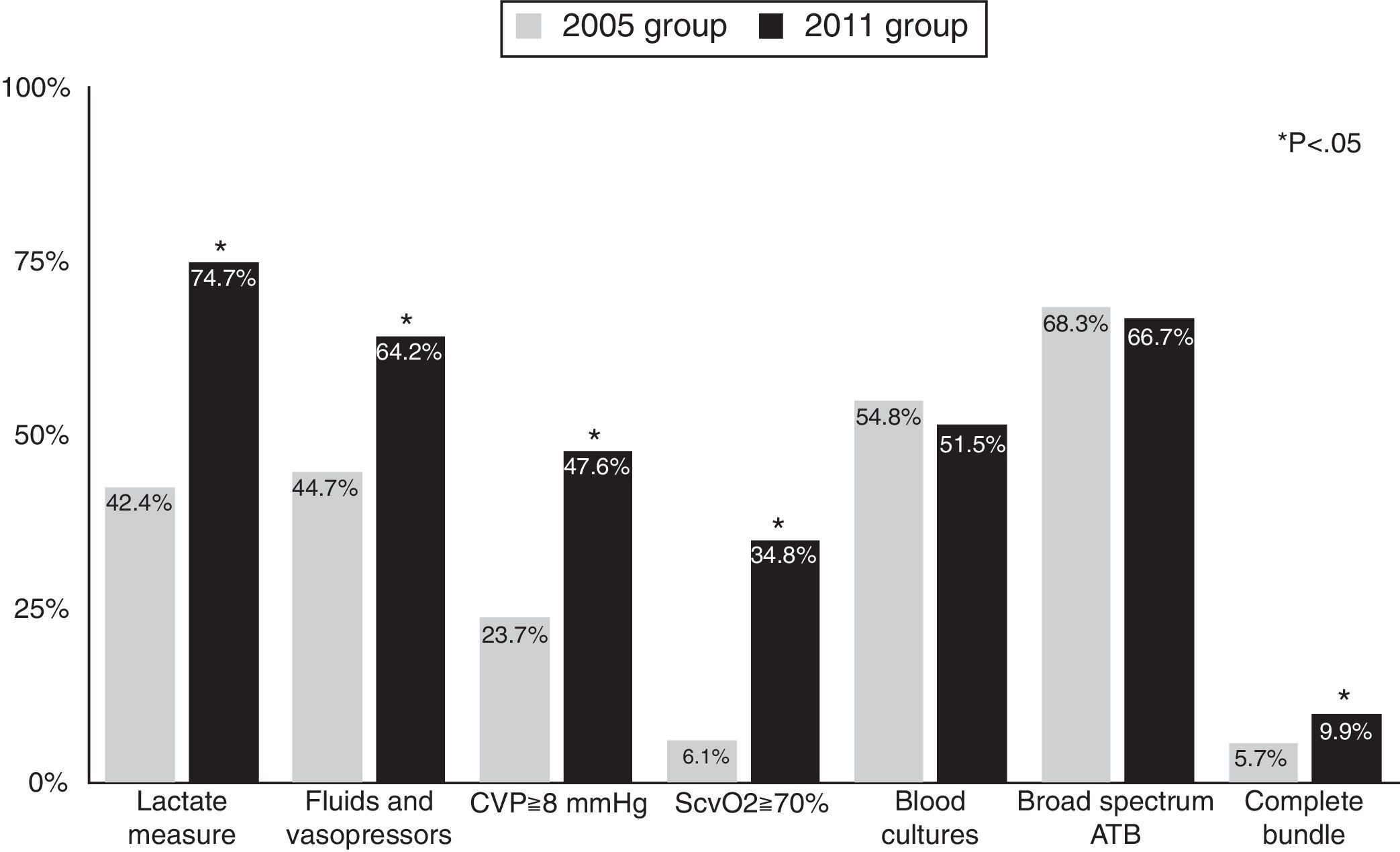
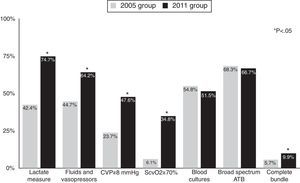
Rates of compliance with resuscitation bundle items increased between the two periods. Compliance with the resuscitation bundle increased from 5.7% in the 2005 group to 9.9% in the 2011 group (p=0.005). Fig. 1 shows compliance with the items in the resuscitation bundle. In 2005, the only two items in the resuscitation bundle for which compliance was higher than 50% were blood extraction for cultures before antibiotic administration (54.8%) and early administration of broad spectrum antibiotics (68.3%); compliance with these two items was similar in 2011. Compliance with the other 4 items was lower than 50% in 2005 and improved significantly in 2011.
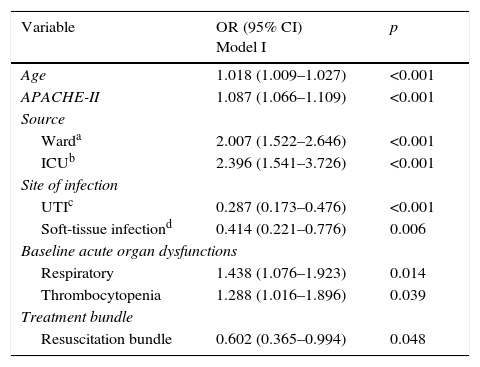
Multivariate logistic regressionTable 3 (Model I) showed, after to adjust for possible confounders, that compliance with the resuscitation bundle are both associated with lower mortality. In addition, the diagnosis of sepsis when the patient is located at ward or at ICU was independently associated with an increase in hospital mortality compared with sepsis identification in the Emergency Department. Two sites of infection (urinary-tract and soft-tissue) were associated to lower mortality than pneumonia, and two baseline acute organ dysfunctions (respiratory and trombocytopenia) were associated to higher mortality.
Multivariate analysis of factors associated with mortality, Model I; only significant variables are shown.
| Variable | OR (95% CI) Model I | p |
|---|---|---|
| Age | 1.018 (1.009–1.027) | <0.001 |
| APACHE-II | 1.087 (1.066–1.109) | <0.001 |
| Source | ||
| Warda | 2.007 (1.522–2.646) | <0.001 |
| ICUb | 2.396 (1.541–3.726) | <0.001 |
| Site of infection | ||
| UTIc | 0.287 (0.173–0.476) | <0.001 |
| Soft-tissue infectiond | 0.414 (0.221–0.776) | 0.006 |
| Baseline acute organ dysfunctions | ||
| Respiratory | 1.438 (1.076–1.923) | 0.014 |
| Thrombocytopenia | 1.288 (1.016–1.896) | 0.039 |
| Treatment bundle | ||
| Resuscitation bundle | 0.602 (0.365–0.994) | 0.048 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; OR, odd ratio; CI, confidence interval; ICU, intensive care unit; UTI, urinary tract infection.
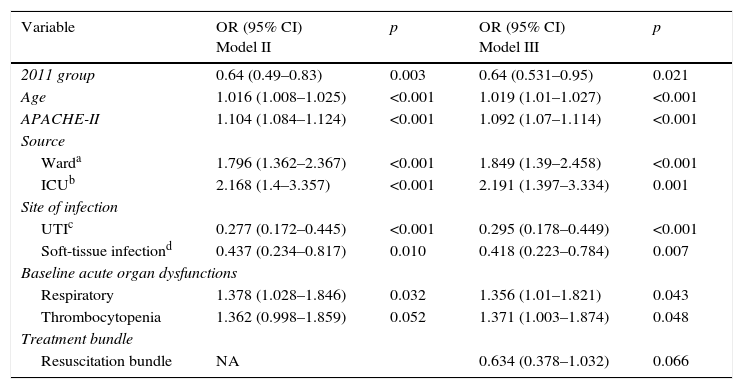
After adjusting for possible confounders (Table 4, Model II), the 2011 cohort was independently associated with lower hospital mortality (OR 0.64 [0.49–0.83], p=0.003). When we included in the previous model the compliance with the resuscitation bundle (Table 4, Model III), the 2011 cohort kept a significant lower adjusted mortality (OR 0.64 [0.531–0.95], p=0.021).
Multivariate analysis of factors associated with mortality, Models II and III; only significant variables are shown.
| Variable | OR (95% CI) Model II | p | OR (95% CI) Model III | p |
|---|---|---|---|---|
| 2011 group | 0.64 (0.49–0.83) | 0.003 | 0.64 (0.531–0.95) | 0.021 |
| Age | 1.016 (1.008–1.025) | <0.001 | 1.019 (1.01–1.027) | <0.001 |
| APACHE-II | 1.104 (1.084–1.124) | <0.001 | 1.092 (1.07–1.114) | <0.001 |
| Source | ||||
| Warda | 1.796 (1.362–2.367) | <0.001 | 1.849 (1.39–2.458) | <0.001 |
| ICUb | 2.168 (1.4–3.357) | <0.001 | 2.191 (1.397–3.334) | 0.001 |
| Site of infection | ||||
| UTIc | 0.277 (0.172–0.445) | <0.001 | 0.295 (0.178–0.449) | <0.001 |
| Soft-tissue infectiond | 0.437 (0.234–0.817) | 0.010 | 0.418 (0.223–0.784) | 0.007 |
| Baseline acute organ dysfunctions | ||||
| Respiratory | 1.378 (1.028–1.846) | 0.032 | 1.356 (1.01–1.821) | 0.043 |
| Thrombocytopenia | 1.362 (0.998–1.859) | 0.052 | 1.371 (1.003–1.874) | 0.048 |
| Treatment bundle | ||||
| Resuscitation bundle | NA | 0.634 (0.378–1.032) | 0.066 | |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; OR, odd ratio; CI, confidence interval; ICU, intensive care unit; UTI, urinary tract infection; NA, not applicable.
We assessed whether sepsis-related mortality in Spanish ICUs has decreased since the introduction of the Surviving Sepsis Campaign (SSC) guidelines and whether decreases are attributable to bundle compliances and other improvements in sepsis care. We found that compliance with the 6-h and 24-h bundles improved and that 28-day and hospital mortality decreased in this 6 years period, suggesting a sustained effect of the SSC moreover, this reduction in hospital mortality remained significant after adjustments. We also found that compliance with the resuscitation bundle are independent protective factors for mortality. Our results are consistent with recent clinical trials, where the mortality due to septic shock was around 24–26%,32 and with epidemiological studies that show a declining trend in severe sepsis mortality over time.2,33 We found that 28-day mortality and hospital mortality decreased despite an increase in predicted mortality as evidenced by higher APACHE II scores; these results corroborate those reported in Stevensons et al.’s29 meta-analysis and Kaukonen et al.’s30 large epidemiological study. A recently published study of a collaborative change intervention aimed at facilitating adoption of SCC bundles in 218 hospitals over 7.5 years found compliance improved over time and increased compliance is associated with decreased mortality.34
The dramatic decrease in sepsis mortality between 2011 and 2005 probably is multifactorial. Increased bundled care is playing a role but the lack of change in adjusted mortality between Model II and III is suggesting that other uncontrolled factors are also important. Probably, implementing the SSC guidelines beginning in 2005 resulted in earlier diagnosis (reflected in the increased proportion of sepsis diagnosed in the emergency department), and lower treatment variability both among clinicians and within clinicians between patients.35 According to Kaukonen et al.30 perhaps there are other reasons we have not controlled in our study (better source control, more adequate empirical antibiotic therapy, earlier transfer to ICU, better overall management of the septic patient, dissemination of protocols, greater expertise of health workers, increased sensitivity for this disease, greater involvement of health institutions, etc.), that also affect the drop in mortality over time. In addition, in a recently published study36 was described a number of factors associated with in-hospital mortality among patients with severe sepsis or septic shock (age, active cancer, diabetes, DNR status on ED arrival, lack of fever, hypoglycemia, and intubation) despite receipt of early protocolized resuscitation in the ED, providing insights into aspects of early sepsis care that can be targets for future intervention.
Most process-of-care indicators improved over time, but compliance with two recommended tasks (acquiring blood cultures before antibiotic administration and broad-spectrum antibiotics administration before 3h) did not change. In the 2005 group, these were the two items in the resuscitation bundle with the highest compliance (54.8% and 68.3%, respectively), improved compliance with these tasks should have an important impact on mortality.9,13 Miller et al.35 underlined the importance of these early interventions when they reported that compliance with early resuscitation bundle items was associated with a lower probability of being eligible for later resuscitation and maintenance bundle items, probably reflecting lesser severity due to the improvements brought about by the early treatments.
A recent multicenter cohort study conducted in Holland37 showed that the implementation of a national program sepsis resulted in improved adherence to sepsis bundles in severe sepsis and septic shock patients and was associated with reduced adjusted in-hospital mortality only in participating ICUs, suggesting direct impact of sepsis screening and application bundle on in-hospital mortality. Our study demonstrated improvements in hemodynamic resuscitation over time. However, recent trials in patients with septic shock failed to demonstrate any benefit of protocolized resuscitation when compared with “usual care”.38–41 Although no consensus exists among clinicians regarding optimal hemodynamic monitoring and to date no method has proven superior, the “usual care” in these trials includes early identification of septic patients, early antibiotic treatment, and early volume resuscitation measures. The improvement in hemodynamic resuscitation between the two periods in our study is probably due to earlier resuscitation more than greater protocolized resuscitation. One of the most important changes between the two periods was the place where sepsis was diagnosed. The proportion of cases diagnosed in the emergency department increased from 42.5% in 2005 to 72.3% in 2011, and the proportion of cases diagnosed in the wards decreased from 44.1% in 2005 to 22% in 2011. These findings indicate earlier detection of sepsis and hence earlier initiation of treatment. Importantly, in the above-mentioned studies38–41 comparing “usual care” with protocolized resuscitation, all cases of septic shock benefited from early detection and initiation of treatment, so perhaps earlier diagnosis and treatment rather than differences in how treatment is administered is what determines prognosis. We agree with Levy42 that the priority should be to establish systems to identify and treat septic patients early.
On the other side, there are several differences between the 2 cohorts: the 2011 group had a higher rate of urinary tract infection and lower rate of respiratory dysfunction (associated to lower mortality), but also those patients were older and had higher APACHE II score (associated to higher mortality); we cannot discard that, despite the adjustments, those differences in the case-mix also influences the difference in mortality.
Our study shows several limitations, the participation in both studies was entirely voluntary, and the hospitals that participated are not necessarily representative of those that did not participate; therefore, our findings may not be generalizable. The length of study periods, its nonrandomized design and the lack of control group precludes establishing a causal connection between the improvements in process-of-care variables and outcomes. Thus, although we observed a better compliance with most of the resuscitation bundle in 2011, related with a decrease in mortality, these findings do not necessarily imply a causal relationship between the compliance with sepsis bundles and outcomes.
Moreover, our study was limited to patients admitted to the ICU, and we cannot know how possible improvement in process-of-care variables in other areas of the hospital affected outcomes.
Finally, the latest SSC guidelines reflect some changes in the standard of care at the time of our study, such as the use of hydroxyethyl starch or drotrecogin alfa (activated). Considering these changes actually strengthens conclusions drawn from our results.
In conclusion, the mortality related to severe sepsis/septic shock in Spain, between two cohorts of patients in 2005 and 2011, decreased dramatically attributable to earliness and improvements in sepsis care, including higher compliance with resuscitation bundle. Nevertheless, compliance with some important items of the resuscitation bundle have not improved enough; early administration of effective antimicrobials could further improve outcomes.
Authors’ contributionsBaltasar Sánchez: data analysis, drafted, translated and corrected the manuscript.
Ricard Ferrer: national coordinator, study design, data analysis, drafted, translated and corrected the manuscript.
David Suárez: database development, data analysis, corrected the manuscript.
Eduardo Romay: data collection, corrected the manuscript.
Enrique Piacentini: site coordinator, data collection and corrected the manuscript.
Gemma Gomá: data collection and corrected the manuscript.
María Luisa Martínez: data collection and corrected the manuscript.
Antonio Artigas: project coordinator, drafted and corrected de manuscript.
Ethical responsibilitiesProtection of people and animalsThe authors declare that in this research have not been performed experiments on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace about publication of patient data.
Right to privacy and informed consentThe authors declare that in this paper does not appear patient data.
FundingThis work was supported by Instituto de Salud Carlos III: PI10/01497 and CM12/00066.
Conflicts of interestThe authors declare no conflict of interest.
We thank John Giba for help with English.
Edusepsis Study Group: Mª Mar Cruz (Virgen de la Salud Toledo-Acquaroni); Carmen Fernández González (Complejo Hospitalario de Ferrol. Arquitecto Marcide), Paz Merino, Elena Bustamante (Hospital Can Misses); Sandra Barbadillo (Hospital General de Cataluña); María de la Cruz Martín, Joaquin Ramon (Centro Médico Delfos); Luis Alvarez Rocha (Complexo Hospitalario Universitario de A Coruña); Nestor Bacelar (Clínica Corachan); Belén Jimenez Bartolomé(Hospital Clínico Universitario Lozano Blesa Zaragoza); Juan Diego Jiménez Delgado (Hospital Comarcal Don Benito-Villanueva) Demetrio Carriedo Ule, Ana María Dominguez Berrot, Francisco Javier Díaz Dominguez (Complejo Asistencial Universitario de León); Juan Machado Casas(Complejo Hospitalario de Jaén); Clara Laplaza Santos; Manuel García-Montesinos; Enrique Maraví Poma (Complejo Hospitalario de Navarra, Pamplona); Victor Lòpez Ciudad; Pablo Vidal cortes (Complejo Hospitalario de Ourense); Ana Navas, Gemma Gomà, María Luisa Martínez, Antonio Artigas (Hospital de Sabadell, Consorci Hospitalari Parc Tauli); Manuel Castellano Hernandez; Rafael Domínguez (Hospital Alto Guadalquivir, Andújar); Miguel Martínez, Jose Antonio Fernández, Fernando Callejo, María Jesús López Pueyo (Hospital General Yagüe); Alec Tallet (Hospital General de Segovia); Pau Torrabadella, Alvaro Salcedo, Claudio Durán (Hospital Universitari Germans Trias i Pujol); Iratxe Seijas (Hospital de Cruces); Teresa Recio Gómez, Abilio Arrascaeta (San Pedro de Alcántara, Cáceres); Angel Arenaza, Ana Morillo, Daniel Del Toro, Tomá s Guzman (Hospital Virgen de la Macarena); Pilar Marco, Izaskun Azkarate (Hospital de Donostia); Isabel Rodríguez (Hospital General de Baza); Eduardo Palencia, Pablo García Olivares, Patricia Santa Teresa Zamarro (Hospital Gregorio Marañón Madrid); Eugenia Yuste (Hospital Universitario San Cecilio); Jordi Solé Violán (Hospital Dr Negrín. Las Palmas); José Blanquer, Mónica García (Hospital Clínico Valencia); Juan Carlos Ballesteros (Hospital Universitario de Salamanca); Antonio Blesa, Fernando Martínez, Alejandro Moneo (Hospital San Carlos); Carlos Pérez (Hospital Santiago Apóstol); Jose Ángel Berezo, Jesús Blanco (Hospital Río Hortega Valladolid); Francisco Javier Martín López (Hospital comarcal Santa Ana, Motril); Ramón Vegas Pinto (Hospital de Antequera); Pilar Martinez Trivez (Hospital de Barbastro Huesca); Antonio Reyes Garcia (Hospital de la Princesa de Madrid); Lluís Zapata, Paula Vera (Hospital de la Santa Creu i Sant Pau); Eduardo Antón (Hospital de Manacor); Juan Carlos Yebenes (Hospital de Mataró); María de las Olas Cerezo Arias (Hospital de Mérida); Francisco García delgado (Hospital de Montilla); Javier Fierro Rosón, Josefa Peinado Rodriguez (Hospital del Poniente, El Ejido); Mª Jesús Broch Porcar (Hospital de Sagunto); María Álvarez (Hospital de Terrassa); Francisco Álvarez, Mª Pilar Gracia Arnillas (Hospital del Mar); Francisco Valenzuela (Hospital de Jerez); Patricia Albert de la Cruz (Hospital del Sureste); Rafael Blancas Casero, Blanca López Matamala (Hospital del Tajo); Monserrat Sisón Heredia (Hospital Dr. José Molina Orosa); Pedro Olaechea, Celia Sañudo (Hospital Galdakao-Usansolo); Jose Manuel Gutierrez Rubio (Hospital General de Albacete); Roberto Reig (Hospital General de Castellón); Alfonso Ambrós, Julian Ortega (Hospital General de Ciudad Real); Leandro Fajardo Feo (Hospital General de Fuerteventura); Pau Garro (Hospital General de Granollers); Francisco Navarro Pellejero (Hospital General de la Defensa en Zaragoza); Ana Trujillo Alonso (Hospital general de La Palma); Rosa Catalán (Hospital General de Vic); Assumpta Rovira, Nicolas Rico (Hospital General Hospitalet de LLobregat); Jose Manuel Allegue Gallego, Luis Herrera Para, Josefa Murcia Paya (Hospital General Universitario Santa Lucía, Cartagena); José Córdoba Alonso, Dolores Ocaña (Hospital La Inmaculada de Huercal-Overa); Jose Francisco Olea Parejo (Hospital Lucus Augusti, Lugo); Pedro Galdos Anuncibay (Hospital Puerta del Hierro); Manuel Salido Mota (Hospital Regional Universitario Carlos Haya); María Jesús Gómez (Hospital General Universitario Reina Sofía de Murcia); Ana Isabel Ezpeleta Galindo, Paloma Dorado (Hospital Royo Villanova, Zaragoza); Arantxa Lander Azcona, Rosario Elbaile (Hospital San Jorge, Huesca); Diego Mendoza (Hospital Sant Joan Despí Moisès Broggi); Francisca Prieto (Hospital de Sta. Bárbara, Puertollano’; Luis Vallejo (Hospital SAS La Línea); Jose Ignacio Ayestarán Rota (Hospital Son Espasses); Marcio Borges (Hospital Son Llatzer); Enrique Piacentini, Ricard Ferrer (Hospital Univerisari Mútua Terrassa); Josep Maria Sirvent, Sara Herranz Ulldemolins (Hospital Universitari Josep Trueta de Girona); Fernando Iglesias Llaca, Lorena Forcelledo Espina, Francisco Taboada Costa, José Antonio Gonzalo Guerra (Hospital Universitario Central de Asturias); Leonardo Lorente Ramos (Hospital Universitario Canarias. Tenerife); Helena Yañez (Hospital universitario de Guadalajara); Ana Loza (Hospital Universitario de Valme); Jose ¿ Miguel Soto, Constantino Tormo (Hospital Universitario Dr. Peset); Borja Suberbiola (Hospital Universitario Marqués de Valdecilla); Domingo Ruiz de la Cuesta Martin, Ignacio Tomás Marsilla (Hospital Universitario Miguel Servet Zaragoza); Mar Martín Velasco (Hospital Universitario Nuestra Señora de Candelaria); Rafael León López, Juan Carlos Pozo (Hospital Universitario Reina Sofia de Córdoba); Jose Ángel Berezo, Jesús Blanco (Hospital Río Hortega Valladolid); Paula Ramírez (Hospital Universitario y Politecnico la Fe); Juan Carlos Ruiz Rodriguez, Jesus Caballero, Adolf Ruiz, Alejandra García, Jordi Riera, Javier Sarrapio, (Hospital Universitari Vall d’ Hebron); Carola Giménez Esparza (Hospital Vega Baja orihuela); Ana Carolina Caballero (Hospital Virgen de la Concha, Zamora); María Victoria de la Torre, Cristina Salazar (Hospital Virgen Victoria de Málaga); Carlos Ortiz (Hospital Virgen del Rocio); Eduardo Palencia Herrejón, Begoña Bueno (Hospital Infanta Leonor, Madrid); Gumersindo González, Díaz; Andres Carrillo (Hospital General Universitario Morales Meseguer, Murcia); Manuel Rodríguez (Hospital Juan Ramon Jiménez); Raquel Valero Gracia (MAZ MATEPSS SUMA Intermutual Zaragoza); Ruth Jorge García (Hospital Nuestra Señora de Gracia Zaragoza); Manuel Quintana, Miguel Ángel Taberna (Hospital Nuestra Sra del Prado); José Carlos Torralba Allué (Hospital General Obispo Polanco Teruel); Isidro Prieto del Portillo (Hospital Universitario Ramón y Cajal); José Luis Monzón, Adolfo Calvo Martínez (Hospital de Logroño); Ricardo Diaz Abad, Miguel Ángel Blasco Navalpotro, Frutos Del Nogal Sáez, Jesús Rebollo Ferreiro, José Suarez Saiz (Hospital Universitario Severo Ochoa), Mar Gobernado, Mª José Fernandez Calavia (Hospital de Sta. Bárbara, Soria); Francisco José Guerrero, Felipe Cañada, Milagros Balaguer, Isabel Mertín, Carmen López, Daniel Sánchez (Hospital Torrecárdenas); Jose María Bonell (USP H Clínica Palma Planas); José Castaño (Hospital Universitario Virgen de las Nieves); Hospital Virgen del Puerto, Plasencia).