To evaluate the association between angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) use prior to a septic shock episode and the development, prognosis and long-term recovery from acute kidney injury (AKI).
DesignA single-centre, prospective observational study was carried out between September 2005 and August 2010.
ScopePatients admitted to the ICU of a third level hospital.
PatientsA total of 386 septic shock patients were studied.
InterventionsNone.
Variables of interestUse of ACEIs/ARBs, AKI development, recovery of previous creatinine levels and time to recovery.
ResultsA total of 386 patients were included, of which 312 (80.8%) developed AKI during ICU stay and 23% were receiving ACEIs/ARBs. The percentage of patients on ACEIs/ARBs increased significantly in relation to more severe stages of AKI irrespective of the kind of AKI score. After adjusting for confounders, the development of AKI was independently associated to the use of ACEIs/ARBs (OR 2.19; 95%CI 1.21–3.84; p=.04). With respect to the recovery of kidney function, the group of patients on ACEIs/ARBs had significantly higher creatinine levels at ICU discharge and needed hemodialysis more frequently thereafter. However, use of ACEIs/ARBs affected neither recovery of previous creatinine levels nor significantly delayed recovery.
ConclusionsThe use of ACEIs/ARBs before septic shock episodes was correlated to AKI development and severity, but did not affect the recovery of kidney function after sepsis resolution.
Evaluar la asociación entre la administración previa a un episodio de shock séptico de inhibidores de la enzima conversora de la angiotensina (IECA) y antagonistas de los receptores de la angiotensina 2 (ARAII) con el desarrollo, pronóstico y recuperación a largo plazo del fracaso renal agudo (FRA).
DiseñoEstudio unicéntrico prospectivo observacional desarrollado entre septiembre de 2005 y agosto de 2010.
ÁmbitoPacientes ingresados en la UCI de un hospital de tercer nivel.
PacientesUn total de 386 pacientes en shock séptico.
IntervencionesNinguna.
Variables de interés principalesUso de IECA/ARAII, desarrollo de FRA, recuperación de niveles de creatinina previos y retraso hasta la recuperación.
ResultadosSe incluyeron 386 pacientes, 312 (80,8%) desarrollaron FRA durante su estancia en la UCI, el 23% se encontraban en tratamiento previo con IECA-ARAII. El porcentaje de pacientes en tratamiento con IECA/ARAII se incrementó en relación con el incremento de la gravedad del FRA independientemente de la escala de gravedad utilizada. Tras ajustar por los factores de confusión el tratamiento con IECA-ARAII se asoció de forma independiente al desarrollo de FRA (OR 2,19; IC 95% 1,21-3,84; p=0,04). El grupo de pacientes en tratamiento con IECA/ARAII tuvo niveles significativamente más altos de creatinina al alta de la UCI y necesitó con mayor frecuencia de hemodiálisis. Sin embargo, su uso no afectó a la recuperación de la creatinina previa ni la retrasó.
ConclusionesEl uso de IECA-ARAII previo al desarrollo de un shock séptico se asoció con el desarrollo de FRA y su gravedad, pero no con su recuperación.
Patients with acute kidney injury (AKI) have a higher risk of short-term and long-term mortality and of chronic kidney injury and are also a significant healthcare burden.1–4 Sepsis is the trigger for AKI development in approximately 50% of critically ill patients.5 Sepsis-induced AKI increases mortality from sepsis by 50–60% and also extends intensive care unit (ICU) stay.6 The pathophysiology of septic AKI, although not as yet firmly established, is considered to be different from AKI of other aetiologies. In response to a drop in blood pressure, circulating angiotensin I, under the action of the angiotensin-converting enzyme (ACE), is hydrolysed to angiotensin II, which causes systemic micro-artery contraction, increased peripheral resistance and raised blood pressure. Thus, the renin-angiotensin-aldosterone system could play a key role in sepsis-related AKI.7
Renin-angiotensin system blockers, including angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are frequently used in clinical practice.8 Since angiotensin II constricts efferent arterioles in glomeruli, ACEIs cause efferent arteriolar dilatation. This action mechanism might be hazardous in clinical situations where kidney blood flow is prone to decrease, resulting in further drops in intra-glomerular pressure and the glomerular filtration rate. While ACEIs are known to slow down the progression of chronic kidney disease, their role in AKI remains controversial. Studies of ACEIs and ARBs as used by surgery patients have reported disparate findings.9,10 Some have demonstrated an increased risk for postoperative AKI, others a decreased risk and others no impact on the risk. In sepsis in particular, the use of ACEIs and ARBs has been identified as contributing to the development of AKI.11 Thus, National Clinical Guideline Centre recommendations for AKI include temporarily suspending ACEIs and ARBs in adults with sepsis until clinical condition has improved and stabilised.12 However, the role played by ACEIs and ARBs in long term kidney function recovery has not been extensively evaluated.
The aim of this study was to assess the association of ACEI and ARB use prior to a septic shock episode with development, prognosis and long-term recovery from AKI.
Patients and methodsStudy design and settingThis study is based on the results of a prospectively documented database which results have been previously published.13 We conducted a single-centre, prospective, observational study in a 30-bed adult intensive care department at Marques de Valdecilla University Hospital in Spain between September 2005 and August 2010. Eligible patients were adults (aged 18 years and older) admitted consecutively to the ICU with septic shock according to the 2001 International Sepsis Definitions Conference.14 Exclusions were as follows: patients with end-stage kidney disease receiving chronic renal replacement therapy (RRT) or whom care was withdrawn within 6h of septic shock onset or whom septic shock onset could not be accurately determined. The Ethics Committee of the hospital approved the study and waived the need for patients’ written informed consent.
Demographic and clinical characteristics recorded were as follows: age and sex; immunosuppression details (acquired immune deficiency syndrome [AIDS], neutropenia [neutrophil count <1×109/L], exposure to glucocorticoids [>0.5mg/kg for >30 days] and/or immunosuppressive or cytotoxic medications, solid organ transplantation, allogeneic or autologous stem cell transplantation, haematological malignancy, or solid tumour); and use of ACEIs and ARBs. Also recorded were their Acute Physiology and Chronic Health Evaluation II (APACHE II) score at 24h and Sequential organ failure assessment (SOFA) score on ICU admission. Early goal-directed resuscitation therapy (EGDT) protocol achievement15 was recorded, defined as adequate for the following values: central venous oxygen saturation (ScVO2) ≥70%; central venous pressure ≥8mmHg; and mean arterial pressure ≥65mmHg within 6h of septic shock onset.
Exposure to ACEIs/ARBs was defined as confirmed administration in the 24h before ICU admission. In all cases, ACEI/ARB administration was stopped as soon as sepsis was diagnosed. AKI was determined according to kidney disease: Improving Global Outcomes (KDIGO) criteria.16 KDIGO score was calculated retrospectively. Baseline creatinine was defined as the last creatinine measurement in 7–365 days before ICU admission.17,18 For patients for whom this data was unavailable (4%), baseline creatinine values were calculated by the Modification of Diet in Renal Disease (MDRD) equation as recommended by the Acute Dialysis Quality Initiative (ADQI) Working Group (assuming a lower baseline glomerular filtration rate (GFR) limit of 75mL/min).19 Creatinine was measured daily during ICU stay. Patients were classified retrospectively according to the maximum KDIGO class reached during their ICU stay: stage 1, increase in serum creatinine ≥0.3mg/dL within 48h or to 1.5–1.9 times baseline; stage 2 increase in serum creatinine to 2.0–2.9 times baseline; and stage 3, increase in serum creatinine to 3.0 times baseline or to at least 4.0mg/dL or initiation of RRT. All patients were followed up during 1 year period and kidney function was evaluated on first month, third month and one year after ICU discharge.
Statistical analysisDiscrete variables were expressed as numbers (percentages) and continuous variables as mean and standard deviation (SD), except for biomarker levels, which were expressed as medians and quartiles. For categorical variables, statistical differences between groups were assessed using the chi-square test and Yates’ correction or Fisher's exact test, as appropriate. The Student's t-test was used for continuous variables. Multivariable logistic regression was used to assess risk factors for the development of septic AKI. The model included only factors that were found to be significantly predictive in the preceding univariate analysis. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. A p value of <0.05 was considered statistically significant. For survival analyses, we generated Kaplan–Meier estimates, assessed between-group differences using the log-rank test and expressed the data as cumulative mortality curves. SPSS statistical software package 15.0 (SPSS, Inc., Chicago, IL) was used for all statistical analyses.
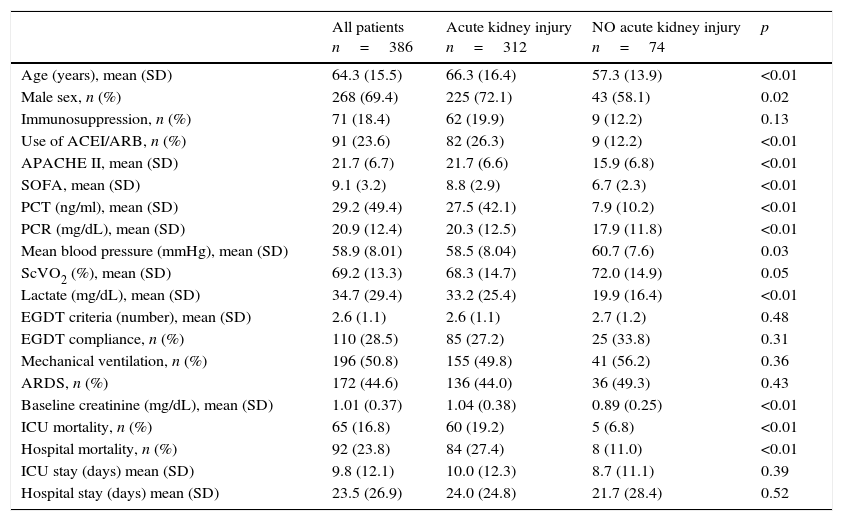
ResultsPatient characteristicsDuring the study period a total of 663 septic shock patients were admitted on the ICU. 277 patients were not finally included because of the lack of follow up information. Thus, 386 patients were included in the study, 91 (23%) receiving ACEIs/ARBs. The mean (SD) age of the cohort was 64.3 (15.5) years and 268 patients (69%) were male. Mean (SD) APACHE II and SOFA scores were 21.7 (6.7) and 9.1 (3.2), respectively. Resuscitation goals within the first 6h of septic shock were successfully achieved in 189 patients (48.5%). Patient characteristics are summarized in Table 1.
Characteristics of study population.
| All patients n=386 | Acute kidney injury n=312 | NO acute kidney injury n=74 | p | |
|---|---|---|---|---|
| Age (years), mean (SD) | 64.3 (15.5) | 66.3 (16.4) | 57.3 (13.9) | <0.01 |
| Male sex, n (%) | 268 (69.4) | 225 (72.1) | 43 (58.1) | 0.02 |
| Immunosuppression, n (%) | 71 (18.4) | 62 (19.9) | 9 (12.2) | 0.13 |
| Use of ACEI/ARB, n (%) | 91 (23.6) | 82 (26.3) | 9 (12.2) | <0.01 |
| APACHE II, mean (SD) | 21.7 (6.7) | 21.7 (6.6) | 15.9 (6.8) | <0.01 |
| SOFA, mean (SD) | 9.1 (3.2) | 8.8 (2.9) | 6.7 (2.3) | <0.01 |
| PCT (ng/ml), mean (SD) | 29.2 (49.4) | 27.5 (42.1) | 7.9 (10.2) | <0.01 |
| PCR (mg/dL), mean (SD) | 20.9 (12.4) | 20.3 (12.5) | 17.9 (11.8) | <0.01 |
| Mean blood pressure (mmHg), mean (SD) | 58.9 (8.01) | 58.5 (8.04) | 60.7 (7.6) | 0.03 |
| ScVO2 (%), mean (SD) | 69.2 (13.3) | 68.3 (14.7) | 72.0 (14.9) | 0.05 |
| Lactate (mg/dL), mean (SD) | 34.7 (29.4) | 33.2 (25.4) | 19.9 (16.4) | <0.01 |
| EGDT criteria (number), mean (SD) | 2.6 (1.1) | 2.6 (1.1) | 2.7 (1.2) | 0.48 |
| EGDT compliance, n (%) | 110 (28.5) | 85 (27.2) | 25 (33.8) | 0.31 |
| Mechanical ventilation, n (%) | 196 (50.8) | 155 (49.8) | 41 (56.2) | 0.36 |
| ARDS, n (%) | 172 (44.6) | 136 (44.0) | 36 (49.3) | 0.43 |
| Baseline creatinine (mg/dL), mean (SD) | 1.01 (0.37) | 1.04 (0.38) | 0.89 (0.25) | <0.01 |
| ICU mortality, n (%) | 65 (16.8) | 60 (19.2) | 5 (6.8) | <0.01 |
| Hospital mortality, n (%) | 92 (23.8) | 84 (27.4) | 8 (11.0) | <0.01 |
| ICU stay (days) mean (SD) | 9.8 (12.1) | 10.0 (12.3) | 8.7 (11.1) | 0.39 |
| Hospital stay (days) mean (SD) | 23.5 (26.9) | 24.0 (24.8) | 21.7 (28.4) | 0.52 |
ACEI/ARB: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; APACHE: Acute Physiology and Chronic Health Evaluation; ARDS: acute respiratory distress syndrome; CRP: C reactive protein; EGDT: early goal directed therapy; PCT: procalcitonin; SOFA: sepsis related organ failure assessment; SvcO2: central venous oxygen saturation.
Of the 386 patients in septic shock, 312 (80.8%) developed AKI during their ICU stay. According to KDIGO criteria, 98 patients (25.4%) were stage 1, 115 (29.8%) were stage 2 and 99 (25.6%) were stage 3. Table 1 shows univariate comparisons of demographic characteristics, severity scores, haemodynamic parameters and analytical parameters for patients with septic shock who developed and who did not develop AKI. Patients who developed AKI were older and more frequently male, were more severely ill (as indicated by higher APACHE II and SOFA scores), had poorer haemodynamic parameters on ICU admission and were more likely to have been receiving ACEIs or ARBs. The group of patients with septic AKI had significantly higher ICU mortality (19.2% vs. 6.8%; p<0.01) and hospital mortality (27.4% vs. 11%; p<0.01).
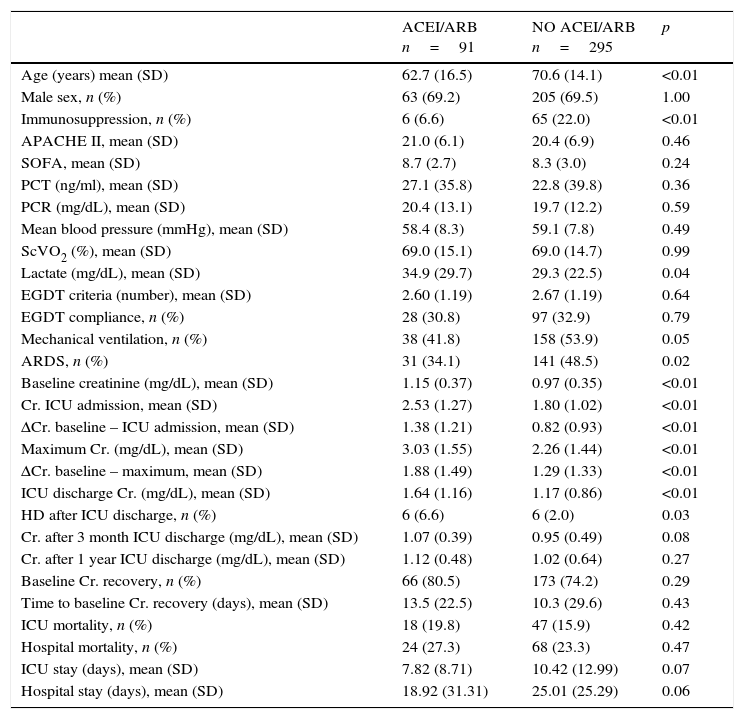
ACEI/ARB use and AKI developmentOf the 386 patients in the study, 91 (23.6%) were receiving ACEI/ARB treatment on septic shock onset. Creatinine levels on ICU admission, maximum creatinine levels and the increase from baseline to maximum levels [1.9 (1.5)mg/dL vs. 1.3 (1.3)mg/dL; p<0.001] and to ICU admission levels [1.4 (1.2)mg/dL vs. 0.8 (0.9)mg/dL; p<0.001] were significantly higher in the group of patients on ACEIs/ARBs (Table 2).
Differences between groups depending on ACEI/ARB exposure.
| ACEI/ARB n=91 | NO ACEI/ARB n=295 | p | |
|---|---|---|---|
| Age (years) mean (SD) | 62.7 (16.5) | 70.6 (14.1) | <0.01 |
| Male sex, n (%) | 63 (69.2) | 205 (69.5) | 1.00 |
| Immunosuppression, n (%) | 6 (6.6) | 65 (22.0) | <0.01 |
| APACHE II, mean (SD) | 21.0 (6.1) | 20.4 (6.9) | 0.46 |
| SOFA, mean (SD) | 8.7 (2.7) | 8.3 (3.0) | 0.24 |
| PCT (ng/ml), mean (SD) | 27.1 (35.8) | 22.8 (39.8) | 0.36 |
| PCR (mg/dL), mean (SD) | 20.4 (13.1) | 19.7 (12.2) | 0.59 |
| Mean blood pressure (mmHg), mean (SD) | 58.4 (8.3) | 59.1 (7.8) | 0.49 |
| ScVO2 (%), mean (SD) | 69.0 (15.1) | 69.0 (14.7) | 0.99 |
| Lactate (mg/dL), mean (SD) | 34.9 (29.7) | 29.3 (22.5) | 0.04 |
| EGDT criteria (number), mean (SD) | 2.60 (1.19) | 2.67 (1.19) | 0.64 |
| EGDT compliance, n (%) | 28 (30.8) | 97 (32.9) | 0.79 |
| Mechanical ventilation, n (%) | 38 (41.8) | 158 (53.9) | 0.05 |
| ARDS, n (%) | 31 (34.1) | 141 (48.5) | 0.02 |
| Baseline creatinine (mg/dL), mean (SD) | 1.15 (0.37) | 0.97 (0.35) | <0.01 |
| Cr. ICU admission, mean (SD) | 2.53 (1.27) | 1.80 (1.02) | <0.01 |
| ΔCr. baseline – ICU admission, mean (SD) | 1.38 (1.21) | 0.82 (0.93) | <0.01 |
| Maximum Cr. (mg/dL), mean (SD) | 3.03 (1.55) | 2.26 (1.44) | <0.01 |
| ΔCr. baseline – maximum, mean (SD) | 1.88 (1.49) | 1.29 (1.33) | <0.01 |
| ICU discharge Cr. (mg/dL), mean (SD) | 1.64 (1.16) | 1.17 (0.86) | <0.01 |
| HD after ICU discharge, n (%) | 6 (6.6) | 6 (2.0) | 0.03 |
| Cr. after 3 month ICU discharge (mg/dL), mean (SD) | 1.07 (0.39) | 0.95 (0.49) | 0.08 |
| Cr. after 1 year ICU discharge (mg/dL), mean (SD) | 1.12 (0.48) | 1.02 (0.64) | 0.27 |
| Baseline Cr. recovery, n (%) | 66 (80.5) | 173 (74.2) | 0.29 |
| Time to baseline Cr. recovery (days), mean (SD) | 13.5 (22.5) | 10.3 (29.6) | 0.43 |
| ICU mortality, n (%) | 18 (19.8) | 47 (15.9) | 0.42 |
| Hospital mortality, n (%) | 24 (27.3) | 68 (23.3) | 0.47 |
| ICU stay (days), mean (SD) | 7.82 (8.71) | 10.42 (12.99) | 0.07 |
| Hospital stay (days), mean (SD) | 18.92 (31.31) | 25.01 (25.29) | 0.06 |
ACEI/ARB: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; APACHE: Acute Physiology and Chronic Health Evaluation; ARDS: acute respiratory distress syndrome; CRP: C reactive protein; EGDT: early goal directed therapy; PCT: procalcitonin; SOFA: sepsis related organ failure assessment; SvcO2: central venous oxigen saturation.
After adjustment for confounders (age, sex, severity, baseline creatinine, immunosuppression and mean blood pressure and lactate levels on ICU admission), the development of AKI was independently associated with the use of ACEIs/ARBs (OR 2.19; 95%CI 1.21–3.84, p=0.04).
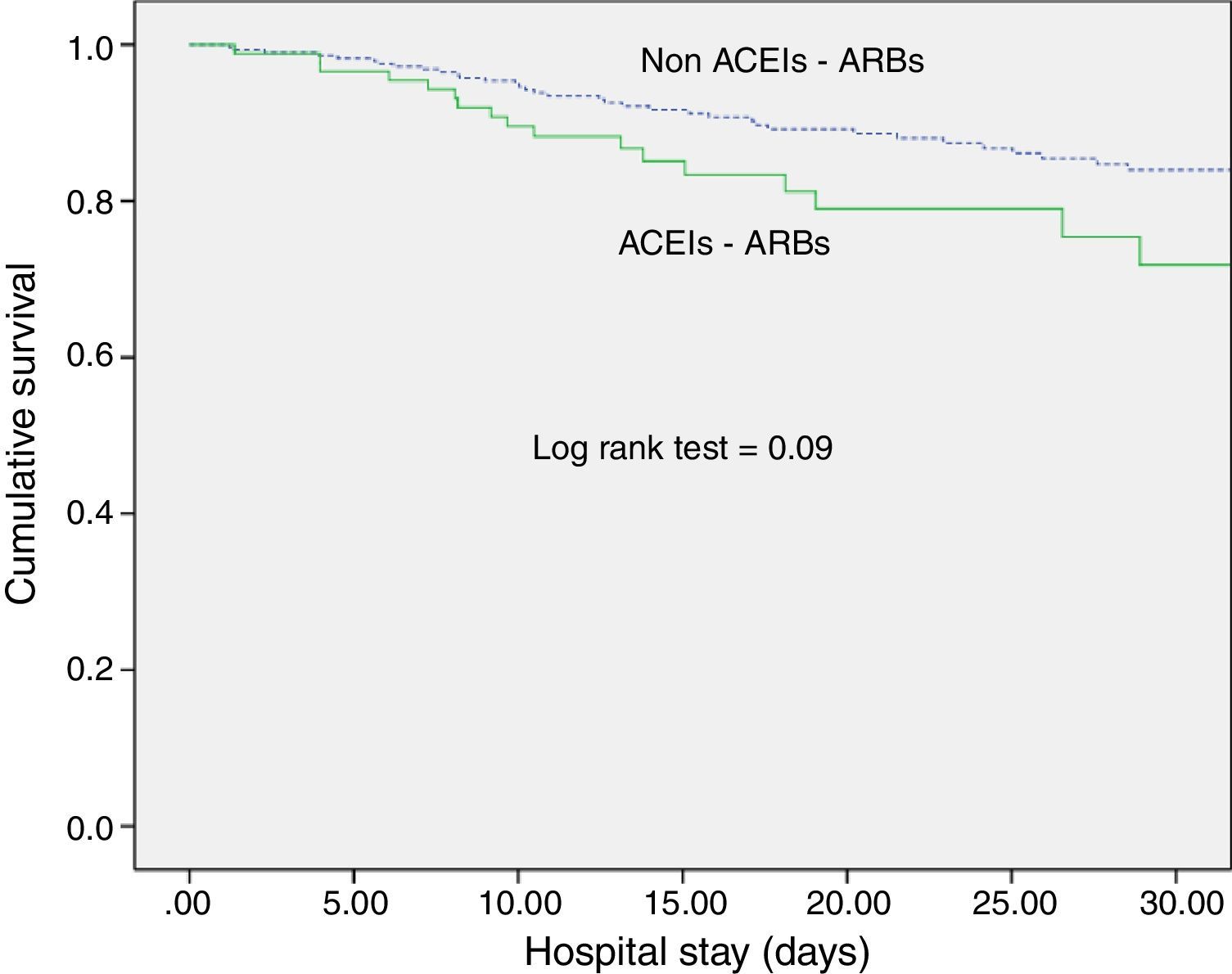
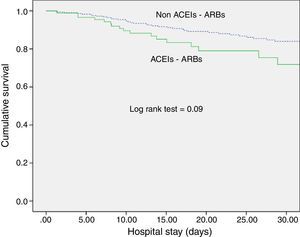
ACEI/ARB use and long-term outcomesWith respect to kidney function recovery, the group of patients on ACEIs/ARBs had significantly higher levels of creatinine at ICU discharge and needed haemodialysis more frequently thereafter. However, creatinine levels 1 month and 3 months later were similar for the groups with and without ACEI/ARB exposure. Likewise, use of ACEIs/ARBs before septic shock development neither affected recovery of previous creatinine levels (80.5% vs. 74.2%; p=0.29) nor significantly delayed recovery (Table 2). In addition, ACEI/ARB use had no effect on mortality risk for both the ICU and the hospital (Table 2 and Fig. 1).
DiscussionExposure to ACEIs/ARBs prior to septic shock episodes is associated with AKI development and severity but has no influence on ICU and hospital survival and on kidney function recovery after sepsis resolution.
This is, to our knowledge, the first study that evaluates the association of ACEIs/ARBs not only with AKI development but also with longer-term kidney function recovery in patients with septic shock. The use of ACEIs/ARBs affects kidney injury in septic patients because they are known to decrease intraglomerular pressure. This action mechanism is potentially hazardous in cases of severe sepsis and septic shock where renal blood flow tends to decrease, resulting in further reductions in intraglomerular pressure and GFR.20,21
Our finding that use of ACEIs/ARBs prior to ICU admission was associated with a doubled risk of AKI development in patients with sepsis corroborates the findings of previous studies.11,22 Both Plataki et al.11 and Suh et al.22 found ACEI/ARB use to be an independent risk factor for AKI development in patients with sepsis, for OR=1.88 and OR=2.06, respectively. However, we observed no significant differences in 1-month and 3-month creatinine levels in relation to ACEI/ARB exposure. Furthermore, previous use of ACEIs/ARBs did not affect kidney function recovery and did not extend the recovery period.
We observed no association of ACEIs/ARBs with clinical outcomes (ICU or hospital survival), contrary to Mortensen et al., who found angiotensin II receptor blocker exposure previous to sepsis episodes to be associated with a halving of the 30-day mortality rate.23 Our study, however, is not directly comparable because of differences in population characteristics and survival outcomes.
The role of the renin-angiotensin-aldosterone system in sepsis is unclear. Some studies have reported an association between high angiotensin II levels and organ failure and mortality24 and a more recent study has found low serum levels of angiotensin II and angiotensin-converting enzyme to be associated with worse outcomes.25
The causal link between sepsis and AKI is extensively established.26–29 Sepsis is the trigger for the development of approximately 50% of cases of AKI among critically ill patients and sepsis-related AKI is associated with sepsis mortality rates of up to 50–60%, depending on severity.30 This study reports a rate for KDIGO-defined AKI of 80.8% (312 of 386 patients with septic shock), a rate consistent with the previous data. An observational cohort study of 390 patients with septic shock in a single-centre ICU reported that some 2 out of 3 patients developed AKI.11 In a recent retrospective multicentre study of 4532 patients with septic shock from Canada, USA and Saudi Arabia, a similar percentage of patients (77.6%) developed AKI.31
In our study, septic AKI was more prevalent in older and male patients. The impact of demographic factors on septic AKI development is uncertain. Two studies showed that age was a risk factor6,22 for AKI onset, whereas Plataki et al.11 found no significant differences in age between patient groups with and without AKI. Whether there is a predisposition for men to develop AKI is also unclear. In our study, being male was a risk factor for AKI development, a finding corroborated by that of a German study that reported male predominance in the development of AKI in patients with severe sepsis and septic shock.29 In contrast, no significant differences were observed between the sexes in two other studies.6,22
The negative impact of septic AKI on survival is unquestionable.27,29 The presence of AKI in our population of septic shock patients was associated with tripled ICU and hospital mortality rates. Note that the global mortality rates observed in our study (16.8% ICU and 23.8% hospital) are noticeably lower than those reported by other studies of patients with septic shock and AKI6,29 – possibly explained by the implementation of a surviving sepsis campaign during the study period.32
The pathophysiology of septic AKI is not completely understood. Recent evidence suggests that septic AKI may represent a unique form of AKI with a distinct pathophysiology. Although AKI is often considered a haemodynamic disease resulting from renal ischaemia, inflammation and apoptosis have been implicated in its pathogenesis in septic patients.33 Our results support the role of hypoperfusion in the pathogenesis of septic AKI. Our patients with septic AKI had significantly higher lactate levels and lower mean arterial pressure rates on ICU admission. Nonetheless, a resuscitation protocol focused on maintaining adequate tissue perfusion with haemodynamic support failed to reduce AKI development. We thus observed no significant differences between the patients with and without sepsis AKI with respect to the number of EGDT goals achieved and compliance with the whole package. Our finding corroborates the results of a recently published paper.34 Moreover, three randomized controlled trials conducted in the USA, Australia/New Zealand and England showed that EGDT compared with standard care did not significantly decrease mortality in patients with septic shock.35–37
The main limitation of the study is that, as a single-centre observational study, its findings cannot be generalized. Also, we had no data on dosages and length of exposure to ACEIs/ARBs and so cannot dismiss the potential effects of these factors on our results. In addition, we evaluated together the potential effect of both, ACEIs and ARBs, and for that reason it cannot be determined to which of the two study drugs in particular corresponds the effect found. Neither did we evaluate the potential effects of other nephrotoxic drugs, such as non-steroidal anti-inflammatory drugs or iodinated contrast or clinical factors like diabetes mellitus, which could also contribute to AKI development. Finally, another limitation is the possibility of temporal trends in AKI biasing our results. During the study period a surviving sepsis campaign with accompanying guidelines was implemented in our hospital, but variability in compliance could have influenced AKI development and, in particular, mortality. We believe this source of bias to have been small because we found no differences regarding the number of EGDT goals achieved and compliance with the whole package. EGDT reflects the quality of resuscitation and is the factor that potentially could have most biased our results.
ConclusionsIn this cohort study, use versus non-use of ACEIs/ARBs before septic shock episodes was associated with AKI development and severity, but did not affect ICU and hospital survival rates or kidney function recovery after sepsis resolution. Large, multi-centre randomized trials are needed to provide information on optimal ACEI/ARB use in relation to sepsis and AKI.
Conflicts of interestThe authors declare no conflict of interest.