Editado por: Rosario Amaya Villar - Unidad de Cuidados Intensivos, Hospital Universitario Virgen del Rocio, Sevilla, España
Última actualización: Febrero 2024
Más datosProne positioning (PP) improves survival in moderate-severe acute respiratory distress syndrome induced by coronavirus-19 (C-ARDS). The attenuation of ventilator-induced lung injury (VILI) represents the most plausible mechanism resulting in reduced mortality.1–3
The mechanical power (MP) has been recently proposed by Gattinoni et al. as a unifying concept aiming to reflect the risk of VILI by integrating information about the ventilatory settings and respiratory mechanics.4
The first aim of our study was to describe the effects of the first session of PP on MP in moderate-severe C-ARDS.
We carried out a prospective cohort study in the intensive care unit (ICU) of the Sanatorio Anchorena San Martín, Buenos Aires. Consecutive sedated and paralyzed adults with moderate-severe C-ARDS who required invasive ventilation and PP were included. We excluded patients without neuromuscular blockers, not drained pneumothorax, bronchopleural fistula and obstructive pulmonary disease. The study was approved by the local review board (code#11/2020) and was registered in clinicaltrials.gov (NCT04369105). The informed consent was obtained in all patients and they were ventilated in volume-controlled mode set-point (VC-CMVs), with tidal volume (VT) of 4−8 mL/Kg of predicted body weight, plateau pressure <30cmH2O and driving pressure (DP) <15cmH2O. PEEP level was selected by a 2-cmH2O stepwise decremental titration without prior recruitment and the lowest PEEP associated with higher compliance (Crs) was set. The respiratory rate was adjusted to pH between 7.25–7.35 and the inspired oxygen to peripheral oxygen saturation between 90–95 %. The patients were deeply sedated (target RASS -5) with propofol and fentanyl and received neuromuscular blockade with atracurium.5,6
We calculated MP at three time-points: 1) supine (SUP), within 15 min before PP; 2) within the 15 min after PP (PP_1) keeping the same ventilatory settings that SUP; 3) after 2−4 h of PP (PP_2). In PP_2, changes in ventilator setting were permitted at physician’s discretion. Secretions were properly suctioned prior to data collection besides, we collected arterial blood gasses at SUP and PP_2.
Our main end-point was the MP calculated through the original4:
In addition, considering the uncertain impact of the resistive pressure gradient on VILI pathophysiology, but the clear link between VILI and DP, we analyzed the pure behavior of elastic loads in MP calculations according to Dianti et al.7:
To isolate the influence of dynamic strain, we also computed the dynamic and driving MP excluding the static strain component (i.e., considering PEEP = 0cmH2O)7:
Continuous data are expressed with mean (SD) or median [quartile 25–75] according to normality checked by Shapiro-Wilk test. Categorical data are expressed as number (%). Comparisons between MP forms, ventilator settings and monitoring variables were tested by one-way ANOVA or Friedman test and p-values were adjusted by post-hoc Tukey’s or Dunn’s correction. Gas exchange response was compared with t-test or Wilcoxon-Mann-Whitney test, as appropriate. In a post-hoc analysis, we investigated potential predictors of response in cases where prone induced significant effects. A linear model was fitted with the following continuous predictors, all collected a priori in supine: PaO2/FiO2, Crs and PEEP. A two-tailed p-value <0.05 was considered statistically significant. The analysis was performed with GraphPad Prism 8.4®.
We studied 70 C-ARDS from July 2020 to September 2021. The patients were 60 (13) years-old and were predominantly male (65%). The APACHE II at admission was 13 [10–17] points, 86% had moderate-ARDS and 14% had severe-ARDS. The total MV duration was 13 [6–22] days, the ICU stay was 15 [8–25] days and the ICU mortality was 57%.
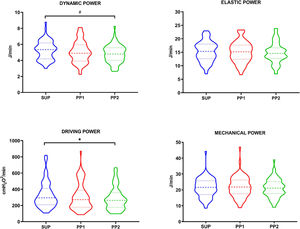
At the time of first PP session, the patients had 3 [1–6] days of intubation and a PaO2/FiO2 of 134 (37.3) mmHg (Table 1). Fig. 1 depicts the response of MP to prone positioning. Although MPglobal did not change immediately (mean-change (95% CI): 0.13 (−0.18 to 0.40); p = 0.840) or after 2−4 h (−0.02 (−0.38 to 0.35); p > 0.990), PP reduced Dynamic-Power (−0.60 (−0.86 to 0.30) J/min; p = 0.0006) and Driving-Power (−44.4 (−72 to −16.8) cmH2O2/min; p = 0.001) in PP_2. The only variable that predicted Driving-Power response was the Crs prior to prone (β = 3.36 (standard-error: 1.05–6.26); p = 0.007) (Table SM1). The response of Dynamic-Power was not predicted by any of the variables evaluated (Table SM2).
Parameters collected during the evaluation time-points
| Variables (n=70) | Baseline | PP1 | PP2 | p Value |
|---|---|---|---|---|
| Primary outcome | ||||
| Mechanical power (J/min) | 21.6 [17.1−25.7] | 21.8 [17.5−26.3] | 21.1 [17.7−25.2] | 0.5704 |
| Elastic power (J/min) | 15.28 [3.7] | 15.06 [3.8] | 14.79 [3.8] | 0.0838 |
| Dynamic power (J/min) | 5.26 [1.2] | 5.06 [1.34] | 4.81 [1.1] | 0.0006ⱡ |
| Driving power (cmH2O2/min) | 290 [210−410] | 270 [180−390] | 260 [180−360] | 0.001ⱡ |
| Setting and monitoring variables | ||||
| Tidal volume (L) | 0.39 [0.07] | 0.39 [0.07] | 0.38 [0.07] | 0.097 |
| PEEP (cmH2O) | 10 [8−14] | 10 [8−14] | 10 [8−14] | 0.3247 |
| PEEP Total (cmH2O) | 10 [8−14] | 10.9 [8−14] | 10.63 [8.1−14] | <0.0001ⱡ |
| Respiratory rate (breaths/min) | 25 [22−27] | 25 [22−27] | 24 [22−27] | 0.0381ⱡ |
| Driving pressure (cmH2O) | 11 [10−12.2] | 10.57 [9−11.8] | 10 [9−11.8] | 0.0008ⱡ |
| Respiratory system Compliance (mL/cmH2O) | 36.9 [11.28] | 38.9 [12.45] | 39.5 [12.37] | 0.0011ⱡ |
| Inspiratory Resistance (cmH2O/L/s) | 11.8 [10.1−13.6] | 12.5 [11.2−15.3] | 12.1 [10.5−14.7] | 0.0731 |
| Gas exchange | ||||
| PaO2/FiO2 ratio | 134 [27.3] | 177 [44.3] | <0.0001ⱡ | |
| Ventilatory ratio | 2.06 [1.7−2.3] | 1.94 [1.7−2.3] | 0.4218 | |
| PaCO2 (mmHg) | 51.4 [45.3−56.9] | 51.0 [43.7−57.0] | 0.7159 |
This study showed that prone ventilation did not produce significant effects on MPglobal but it may reduce the elastic energy applied to the respiratory system in C-ARDS patients.
The null impact of prone on MPglobal may have different explanations: first, PP has been shown to make ventilation more homogeneous and reduce regional pulmonary stress/strain9; MPglobal may not be able to capture such regional changes. We found improvement in oxygenation and mild increase in Crs, suggesting that recruitment occurred through redistribution of ventilation to dorsal zones. Second, the time until PP2 may not have been sufficient to find full benefits on gas exchange and/or respiratory mechanics. Accordingly, improvements in CO2 clearance, compliance, dead space, and oxygenation have been shown to be greater as the time of the prone is prolonged.10 This fact could have enabled clinicians to modify ventilator settings (e.g., reduce RR, flow or PEEP) and reduce MP; third, the original MP formula includes the resistive component, which is not expected to change significantly with PP after adequate secretion suctioning.
When resistive loads were excluded from MP calculation, PP significantly reduced Dynamic-Power and Driving-Power. Both calculations integrate Ers, RR and DP, all variables that were significantly reduced at PP2. On another side, we observed that the lower Crs in supine, the higher Driving-Power reduction with prone. Although baseline oxygenation did not predict prone response, these findings support the PROSEVA trial results and suggest that PP may have larger impact on VILI risk in patients with sicker lungs.
This study presents important limitations: we did not measure transpulmonary MP, which might show more accurate effects of PP regarding the risk of VILI. The changes in ventilator settings in PP2 were not protocolized and constitutes a bias which could have influenced the results.
In summary, our study suggests that PP is not associated with a reduction in MPglobal in the short term in C-ARDS, but may reduce the elastic energy after a 2−4 h of prone. Future research should explore whether a more prolonged follow-up during a prone session could reveal greater improvement in gas exchange and/or mechanics, which may allow for more profound modifications of ventilator settings conditioning greater MP reductions.
ContributionsMA leaded the project and performed the literature search; MA, JHD, JP, GPC and DIG carried out the data collection process and planned the study design; JP performed the analysis of data; MA, JHD, JP, GPC, MNB and DIG actively participated in the manuscript preparation; all the authors reviewed and approved the final manuscript.
Sources of financial supportThis study did not receive any grant or external financial support.
Conflict of interestThe authors declare no conflict of interest.