To analyze the perioperative differences in a consecutive cohort of liver transplant recipients (LTRs) classified according to the indication of transplantation, and assess their impact upon early mortality 90 days after transplantation.
DesignA retrospective cohort study was carried out.
ScopeA single university hospital.
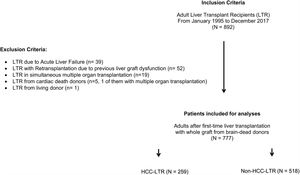
PatientsA total of 892 consecutive adult LTRs were included from January 1995 to December 2017. Recipients with acute liver failure, retransplantation or with grafts from non-brain death donors were excluded. Two cohorts were analyzed according to transplant indication: hepatocellular carcinoma (HCC-LTR) versus non-carcinoma (non-HCC-LTR).
Main variables of interestRecipient early mortality was the primary endpoint. The pretransplant recipient and donor characteristics, surgical time data and postoperative complications were analyzed as independent predictors.
ResultsThe crude early postoperative mortality rate related to transplant indication was 13.3% in non-HCC-LTR and 6.6% in HCC-LTR (non-adjusted HR=2.12, 95%CI=1.25–3.60; p=0.005). Comparison of the perioperative features between the cohorts revealed multiple differences. Multivariate analysis showed postoperative shock (HR=2.02, 95%CI=1.26–3.24; p=0.003), early graft vascular complications (HR=4.01, 95%CI=2.45–6.56; p<0.001) and multiorgan dysfunction syndrome (HR=18.09, 95%CI=10.70–30.58; p<0.001) to be independent predictors of mortality. There were no differences in early mortality related to transplant indication (adjusted HR=1.60, 95%CI=0.93–2.76; p=0.086).
ConclusionsThe crude early postoperative mortality rate in non-HCC-LTR was higher than in HCC-LTR, due to a greater incidence of postoperative complications with an impact upon mortality (shock at admission to intensive care and the development of multiorgan dysfunction syndrome).
Analizar las diferencias perioperatorias de una cohorte de trasplantados hepáticos (LTR) clasificados por la indicación de trasplante, y evaluar su impacto sobre la mortalidad precoz (90 días postrasplante).
DiseñoEstudio de cohorte retrospectivo.
ÁmbitoInstitución universitaria.
PacientesDesde 1995 hasta 2017 fueron incluidos 892 LTR. Se excluyeron los receptores con fallo hepático agudo, retrasplante o de donantes sin muerte cerebral. Se analizaron 2 cohortes según el motivo del trasplante: carcinoma hepatocelular (HCC-LTR) vs. causas diferente al carcinoma (non-HCC-LTR).
Principales variables de interésLa variable principal fue la mortalidad precoz. Las características pretrasplante de receptores, donantes, tiempo quirúrgico y complicaciones postoperatorias se estudiaron como predictores independientes.
ResultadosLa mortalidad postoperatoria temprana bruta relacionada con la indicación de trasplante fue del 13,3% en non-HCC-LTR y del 6,6% en HCC-LTR (HR no ajustada: 2,12; IC 95%: 1,25-3,60; p=0,005). La comparación de características perioperatorias entre las cohortes mostró múltiples diferencias. El shock postoperatorio (HR: 2,02; IC 95%: 1,26-3,24), complicaciones vasculares tempranas del injerto (HR: 4,01; IC 95%: 2,45-6,56) y síndrome de disfunción multiorgánica (HR: 18,09; IC 95%: 10,70-30,58) fueron predictores independientes de mortalidad. La indicación de trasplante no mostró significación en el análisis multivariante (HR ajustada: 1,60; IC 95%: 0,93-2,76; p=0,086).
ConclusionesLa mortalidad postoperatoria temprana bruta en non-HCC-LTR fue mayor que en HCC-LTR debido a la mayor incidencia de complicaciones postoperatorias con impacto en la mortalidad (shock al ingreso en la UCI y aparición del síndrome de disfunción multiorgánica).
Liver transplantation is the only potentially curative therapy for patients with end-stage liver disease and early-stages hepatocellular carcinoma (HCC) but priority and equity to access to transplantation are a controversial issue.1,2
Among liver transplant recipients (LTR), the highest incidence of mortality occurs within the early postransplant period and it is close to 10% in elective recipients at 90 days.3 Although some baseline clinical features of donors or recipients may justify some cases of mortality,4–5 in most patients the postoperative complications directly derived from transplantation surgery are responsible for early death and usually unpredictable.6–9
Before the upcoming of Milan criteria10 there was a worldwide moratorium on access to transplant of patients with HCC. Currently, liver transplantation due to HCC accounts for 15–30% of all transplants performed in most of the institutions.11,12 The indication for liver transplantation (HCC vs non-HCC) seems to have an impact on early postoperative mortality but literature around this issue is heterogeneous.13,14 Targeted studies examining the difference between mortality rates after whole-graft elective liver transplantation from brain-dead donors without inclusion of recipients with acute liver failure or retransplantation are lacking.
Liver transplant recipients due to HCC (HCC-LTR) could show lower perioperative mortality rates, mainly due to preserved pretransplant liver function, less portal hypertension15 and reduced comorbidities.16 In addition, while prioritization of LTR due to end-stage liver disease (non-HCC-LTR) depends on the MELD score in most centres in Europe and USA, prioritization of HCC-LTR is more arbitrary (ie. MELD exceptions) and may vary among different countries or even among institutions within the same country.17
Therefore, the aims of this study were to analyse the perioperative differences in a consecutive cohort of LTR classified according to indication to transplant and assess their impact on early mortality during 90 day after transplantation.
Patients and methodsThe present study is a retrospective analysis of a prospectively collected database from a single centre. A consecutive cohort of adult (>16 aged) LTR with brain-dead donors from January 1995 to December 2017 was analysed.
All LTR after liver transplantation were admitted at Intensive Care Unit (ICU) and included in the study. Exclusion criteria were as follows: patients with acute liver failure enlisted for urgent transplantation, multiple organ transplantation, retransplantation, split or reduced donation, living donation and donation after cardiac death. Subsequently, the cohort was ordered according to transplant indication (HCC-LTR vs non-HCC-LTR).
The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations18 were followed for the study design and the manuscript preparation.
The study fulfils all ethical requirements of the WHO code (Declaration of Helsinki) and was approved by the Regional Clinical Research and Ethics Committee (Code 0823-N-18, Act No. 278, ref 3948) without requirement of signed informed consent.
According to our regional prioritization system,19 patients with HCC (HCC-LTR cohort) empirically received 15 MELD points at inclusion in the waiting list. Extra-MELD points were granted only to a subgroup of patients with an increased risk of tumour progression (ie. a single tumour≥3cm, multinodular or with alpha fetoprotein>200ng/mL). In such patients one extra-MELD point was added every month within the waiting list up to 20 MELD points. The non-HCC-LTR cohort included LTR with end-stage liver disease and poor liver function irrespective of cirrhosis aetiology. They were prioritized according their actual MELD score which was updated at each patient visit. Patients enlisted for special indications such as refractory ascites or recurrent encephalopathy were also included in the non-HCC-LTR and prioritized as follows. In patients with refractory ascites, MELD-Na was considered after 3 months in the waiting list. In patients with encephalopathy, MELD 15 at inclusion, one extra MELD point every three months up to 18 points, and 1 extra MELD point every other month thereafter. Both indications were limited to MELD 20.
Throughout the study period, three different surgical techniques were performed in our institution. Until 1992, the Standard technique was the standard of care whenever the patient could tolerate the vascular clamping test. Subsequently, Preservation of inferior cava vein technique became the most commonly performed. In 2007, Preservation of inferior cava vein with a temporary portacaval shunt was adopted and since then it has been considered the technique of choice.
All patients included in the study had been admitted to ICU immediately after surgery. Initial postoperative treatment throughout the study period was fairly homogeneous, with no significant clinical changes in terms of supportive treatment, antimicrobial prophylaxis or length of stay in ICU. Doppler ultrasound was routinely performed to rule out vascular complications within the first 24h after transplant, at day 7th and whenever clinically indicated. Immunosuppressive regimens, however, have been changed according to the state of art of each period. Since 1997, tacrolimus was available in its oral formulation and after 2008 it is administered in a triple therapy regimen using initial low doses. Currently, induction therapy with anti-CD25 antibodies is restricted to patients with severe graft dysfunction or pretransplant renal impairment in order to avoid calcineurin inhibitors within the first 5–7 postoperative days.
VariablesThe main dependent variable of the study was early postoperative mortality (ie. within the first 90 days after transplant). Other outcomes were urgent retransplantation in the first 7 days due to graft non-function (primary or vascular cause) and length of stay in ICU.
The following variables were explored as potential predictors of such outcomes. As for the recipients, demographic variables (age, gender, weight, height and body mass index), indication or reason for inclusion in waiting list for transplantation (HCC vs non-HCC), aetiology of liver disease, renal function tests (blood urea and creatinine levels) and pretransplant scores related to severity and prognosis of liver disease (Child–Pugh and MELD-Na) were recorded. The MELD-Na score was calculated with laboratory data obtained just before the transplant. Moreover, pretransplant comorbidities that could affect early postoperative mortality, such as diabetes mellitus, implantation of transjugular portosystemic shunt (TIPS) or supramesocolic surgery were studied. Criteria of hepatorenal syndrome, treatment with terlipressin, need for renal support (dialysis in any of its modalities) and hospital admissions in the course of the month prior to transplantation were also obtained from clinical records.
Explanatory variables of donors such as gender, age, weight, length of stay in ICU, graft cold ischemia time and graft or donor meaningful characteristics were recorded. Suboptimal graft or donor were considered when they met two or more of the following criteria: age over 80 years, obesity, prolonged or severe hypotension episodes requiring vasoactive drugs longer than 6h, need for high doses of vasoactive agents (e.g., noradrenaline>0.4μg/kg/min), length of stay in ICU>7 days, graft injury during the harvesting process or steatosis greater than 30% found in the baseline graft biopsy.
Characteristics of surgical technique of transplantation, type of vascular clamping and need for biliary derivation as technique of biliary anastomosis, time of surgery, need for transfusions and severe intraoperative complications such as cardiac arrest or severe cardiac dysfunction were registered.
Several clinical features and analytical parameters of patients were obtained at admission and throughout their stay in ICU. Postoperative oxygenation status immediate upon ICU admission, serum lactate peak value within the first 48h, aminotransferases (AST, ALT) peak value in the first 3 days, ocurrence of shock at ICU admission and transfusion requirements in the first 5 days were recorded. Massive transfusion was defined as 6 or more units of red blood cells (RBC) transfusion in the first 5 days postransplant. Data of initial graft function, such as INR and total bilirubin levels on the 7th postransplant day and need for urgent retransplantation due to graft non-function (primary or vascular cause) were analysed. Data of early postoperative period, complications and organ dysfunctions including serum urea and creatinine peak values in the first 5 days and requirement of renal support, postransplant mechanical ventilation time, need for orotracheal reintubation or tracheostomy due to prolonged mechanical ventilation were also obtained from clinical records. Multiple Organ Dysfunction Syndrome (MODS) was considered when two or more organ functions were affected and Sequential Organ Failure Assessment (SOFA) scored greater than 6 points longer than 48h. Finally, we analysed data of infectious complications throughout ICU stay and first month postransplant in hospital ward.
Statistical analysisQuantitative variables are described as median and 25–75th percentile according to their distribution as assessed by the Kolmogorov–Smirnov test. Qualitative variables are described as frequencies.
Comparisons between cohorts were performed by univariate logistic regression, Mann–Whitney U test for asymmetric distributions and Moses tests when indicated. All covariates with statistical significance were included for multivariate analyse, except those with missing values greater than 20%. Also, other variables with proven clinical relevance in previous reports on early mortality (e.g. the age of recipients, body mass index, graft vascular complications and severe neurological complications) were also included.
Multivariate analysisWe analysed the time to main outcome (mortality or survival) of whole cohort of LTR. Patients with need of urgent retransplantation were censored. The variable selection for the final Cox proportional hazard model was based on the independent univariate logistic regression models for classification of groups (HCC and non-HCC). Those variables which had a significant differences between the studied groups are evaluates in multivariate Cox proportional hazard model. The final model includes the selected predictors by step-wise model selection using Akaike Information Criterion (AIC). A variance inflation factor test was performed to avoid collinearity in the multivariate proportional hazard Cox regression. Kaplan–Meier curves were compared using the Log-rank test. The level of statistical significance was established at p-value<0.05. All statistical analyses were performed using the statistical software R (3.5.0).
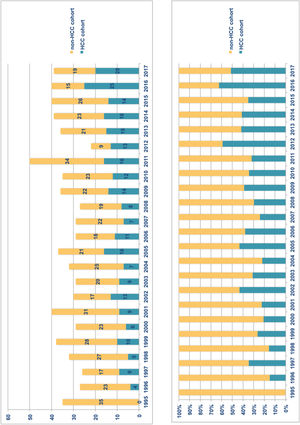
ResultsFrom January 1995 to December 2017, 892 adult patients were transplanted and all of them were considered for inclusion. Exclusion criteria were met in 115 patients (Fig. 1) and therefore the overall cohort consisted of 777 adult LTR of which 259 (33.3%) formed the HCC-LTR cohort and 518 (66.7%) the non-HCC-LTR cohort. Annual numbers and proportions of both cohorts is shown in Fig. 2.
Characteristics of the cohortsBaseline pretransplant characteristics of recipients are shown in Table 1. Significant differences between cohorts were found in all demographic data. In non-HCC-LTR there were a higher proportion of female (12.7% vs 29.3%, p<0.001), patients with lower age and body mass index (p<0.001, in both), higher severity of liver disease (Child C stage 14.3% in HCC-LTR vs 49.2% in non-HCC-LTR, p<0.001; MELD-Na score 12 [IQR 9-16] points in HCC-LTR vs 19 [IQR 16-23] points16–23 in non-HCC-LTR, p<0.001), as in parameters related to pretransplant renal function and need of previous hospital admission (p<0.001).
Baseline characteristics of liver transplant recipients. Comparison of risk between cohorts (non-HCC-LTR vs HCC-LTR) by binary logistic regression.
| N | LTR-cohortaN=777 | HCC – LTRn=259 | Non-HCC – LTRn=518 | OR | CI 95% | p | |
|---|---|---|---|---|---|---|---|
| Demographic data | |||||||
| Gender, Female (%) | 185 (23.8) | 33 (12.7) | 152 (29.3) | 2.844 | 1.885–4.291 | <0.001 | |
| Age, years | 54 (48–59) | 56 (51–61) | 53 (45–59) | 0.933 | 0.914–0.952 | <0.001 | |
| Weight, kg | 761b | 75 (65–85) | 79 (70–89) | 72 (63–81) | 0.972 | 0.962–0.982 | <0.001 |
| Height, cm | 761 | 168 (162–173) | 169 (164–173) | 167 (161–173) | 0.978 | 0.961–0.996 | 0.018 |
| BMI, kg/m2 | 761 | 26.4 (23.8–29.6) | 27.7 (24.6–30.8) | 25.9 (23.4–28.7) | 0.922 | 0.891–0.953 | <0.001 |
| Reason to transplant | |||||||
| HCC-LTR (%) | 259 (100) | ||||||
| Non-HCC-LTR (%) | 518 (100) | ||||||
| Hepatocellular insufficiency (%) | 409 (79.0) | ||||||
| Special indications and others (%) | 62 (12.0) | ||||||
| Biliary cirrhosis (%) | 47 (9.1) | ||||||
| Liver disease stage | |||||||
| Child C stage (%) | 292 (37.6) | 37 (14.3) | 255 (49.2) | 5.783 | 3.920–8.531 | <0.001 | |
| MELD-Na, points | 772 | 17 (12–22) | 12 (9–16) | 19 (16–23) | 1.229 | 1.186–1.273 | <0.001 |
| Previous comorbidities | |||||||
| Diabetes mellitus (%) | 225 (29.0) | 83 (32.0) | 142 (27.4) | 0.801 | 0.579–1.108 | 0.181 | |
| TIPS previous (%) | 776 | 41 (5.3) | 10 (3.9) | 31 (6.0) | 1.588 | 0.766–3.292 | 0.237 |
| Supramesocolic surgery (%) | 776 | 105 (13.5) | 42 (16.2) | 63 (12.2) | 0.717 | 0.470–1.094 | 0.148 |
| Blood creatinine, mg/dL | 773 | 0.9 (0.7–1.1) | 0.8 (0.7–1.0) | 0.9 (0.7–1.2) | 1.744 | 1.178–2.544 | 0.006 |
| Blood urea, mg/dL | 772 | 33 (25–46) | 30 (24–40) | 35 (25–52) | 1.013 | 1.006–1.019 | <0.001 |
| Hepatorenal syndrome (%)c | 775 | 81 (10.5) | 10 (3.9) | 71 (13.8) | 3.081 | 1.421–6.681 | 0.004 |
| Dialysis (%) | 776 | 6 (0.8) | 2 (0.8) | 4 (0.8) | 1.002 | 0.182–5.506 | 1.000 |
| Previous hospital admission (%) | 85 (10.9) | 6 (2.3) | 79 (15.3) | 7.047 | 2.757–18.012 | <0.001 | |
Donors characteristics and surgical features during transplantation time are shown in Table 2. Significant differences between cohorts were found in age of donors and cold ischemia time of grafts. The proportion of suboptimal donors was scarce in both cohorts. Non-HCC-LTR required more RBC and plasma transfusions (p<0.001, in both).
Donors characteristics and transplantation surgical time features. Comparison of risk between cohorts (non-HCC-LTR vs HCC-LTR) by binary logistic regression.
| N | LTR-cohortaN=777 | HCC – LTRn=259 | Non-HCC – LTRn=518 | OR | CI 95% | p | |
|---|---|---|---|---|---|---|---|
| Donor characteristics | |||||||
| Gender, Female (%) | 294 (37.8) | 95 (36.7) | 199 (38.4) | 1.077 | 0.791–1.466 | 0.638 | |
| Age, years | 776b | 51 (34–64) | 54 (34–66) | 49 (34–62) | 0.992 | 0.984–1.000 | 0.059 |
| Weight, kg | 769 | 75 (65–83) | 75 (67–85) | 75 (65–80) | 0.991 | 0.979–1.003 | 0.138 |
| ICU length of stay, days | 765 | 2 (1–4) | 2 (1–4) | 2 (1–5) | 1.005 | 0.966–1.045 | 0.819 |
| Cold ischemia time, min | 753 | 360 (300–540) | 360 (300–480) | 380 (300–558) | 1.001 | 1.000–1.002 | 0.011 |
| Suboptimal donors (%) | 766 | 16 (2.1) | 6 (2.3) | 10 (1.9) | 0.830 | 0.298–2.309 | 0.721 |
| Surgical features | |||||||
| Vascular clamping methods | 776 | ||||||
| Standard technique (%) | 86 (11.1) | 31 (12.0) | 55 (10.6) | ||||
| Caval flow preservation with and without porto-caval shunt (%) | 690 (88.9) | 227 (88.0) | 463 (89.4) | 1.150 | 0.720–1.836 | 0.559 | |
| Hepatic-jejunostomy as biliary reconstruction (%) | 775 | 16 (2.1) | 0 | 16 (3.1) | |||
| Intraoperative red blood cells transfusions, units | 634 | 5 (2–9) | 3 (1–6) | 6 (3–10) | 1.109 | 1.070–1.149 | <0.001 |
| Intraoperative plasma transfusions, units | 629 | 4 (0–9) | 2 (0–5) | 5 (2–10) | 1.124 | 1.083–1.167 | <0.001 |
| Transplantation surgical time, min | 764 | 270 (240–300) | 270 (240–300) | 270 (240–300) | 1.001 | 0.999–1.003 | 0.394 |
| Severe intraoperative cardiac complications (%)c | 775 | 56 (7.2) | 12 (4.7) | 44 (8.5) | 1.911 | 0.991–3.684 | 0.056 |
Explanatory variables related to early postoperative period are shown in Table 3. Immediately after ICU admission, no differences were found between cohorts regarding to pulmonary oxygenation or serum lactate peak values. However, non-HCC-LTR showed an increase rate of shock at ICU admission, which was associated with lower hemoglobin levels, a higher requirement of massive transfusions of RBC (>6 units) within the first 5 postoperative days, and an increased risk of early surgical reoperations (usually due to postoperative hemorrhage). Collinearity between shock upon ICU admission and intraoperative transfusion of RBC was demonstrated. Therefore, this latter was excluded from the final analysis.
Postoperative features in liver transplant recipients cohort.
| N | LTR cohortaN=777 | HCC – LTRn=259 | Non-HCC – LTRn=518 | OR | CI 95% | p | |
|---|---|---|---|---|---|---|---|
| At ICU admission | |||||||
| paO2/FiO2 | 770 b | 352 (262–453) | 394 (291–477) | 331 (252–429) | 0.999 | 0.998–1.001 | 0.325 |
| Lactate peak, mmol/Lc | 599 | 3.8 (2.3–6.3) | 3.4 (2.0–6.0) | 3.3 (2.0–5.5) | 0.990 | 0.949–1.034 | 0.652 |
| Shock (%) | 98 (12.6) | 18 (6.9) | 80 (15.4) | 2.445 | 1.432–4.175 | 0.001 | |
| Perioperative complications | |||||||
| Lowest hemoglobin level in the first 5 days, g/L | 761 | 84 (78–100) | 90 (80–108) | 80 (75–90) | 0.971 | 0.962–0.980 | <0.001 |
| Massive RBC transfusion (%)d | 776 | 83 (10.7) | 14 (5.4) | 69 (13.3) | 2.695 | 1.486–4.888 | 0.001 |
| Early surgical reoperations in the first 4 days (%) | 98 (12.6) | 19 (7.3) | 79 (15.3) | 2.273 | 1.345–3.842 | 0.002 | |
| Vascular thrombosis in the first 7 days (%) | 776 | 51 (6.6) | 16 (6.2) | 35 (6.8) | 1.103 | 0.598–2.032 | 0.754 |
| Late or multiple surgical reoperations (%) | 776 | 48 (6.2) | 17 (6.6) | 31 (6.0) | 0.908 | 0.493–1.673 | 0.757 |
| Graft function test | |||||||
| AST peak value in the first 3 days, U/L | 774 | 724 (379–1400) | 740 (412–1534) | 712 (369–1344) | 1.000 | 1.000–1.000 | 0.058 |
| ALT peak value in the first 3 days, U/L | 774 | 526 (271–1040) | 609 (316–1110) | 499 (245–994) | 1.000 | 1.000–1.000 | 0.011 |
| INR on day 7 | 738 | 1.1 (1.0–1.2) | 1.1 (1.0–1.1) | 1.1 (1.0–1.2) | 1.663 | 0.665–4.161 | 0.277 |
| Total bilirubin on day 7, mg/dL | 732 | 2.4 (1.1–5.7) | 1.6 (0.9–3.5) | 3.2 (1.4–6.7) | 1.106 | 1.062–1.152 | <0.001 |
| Renal function complications | |||||||
| Serum-creatinine peak value in the first 5 days, mg/dL | 764 | 1.2 (0.9–1.8) | 1.0 (0.8–1.6) | 1.3 (0.9–1.9) | 1.508 | 1.206–1.885 | <0.001 |
| Serum-urea peak value in the first 5 days, mg/dL | 762 | 99 (68–144) | 79 (59–109) | 107 (73–155) | 1.011 | 1.007–1.014 | <0.001 |
| Extra-renal depuration methods (%) | 776 | 46 (5.9) | 13 (5.0) | 33 (6.4) | 1.282 | 0.663–2.481 | 0.460 |
| Respiratory function complications | |||||||
| Post-transplant mechanical ventilation time, h | 754 | 11 (7–19) | 9 (6–15) | 12 (7–21) | 1.001 | 0.998–1.004 | 0.717 |
| Mechanical ventilation time<24h (%) | 655 (84.3) | 231 (91.3) | 424 (84.6) | 0.524 | 0.318–0.865 | 0.011 | |
| Re-intubation and mechanical ventilation (%) | 75 (9.7) | 17 (6.6) | 58 (11.2) | 1.795 | 1.023–3.150 | 0.042 | |
| Tracheostomy (%) | 19 (2.4) | 3 (1.2) | 16 (3.1) | 2.720 | 0.785–9.420 | 0.101 | |
| Infectious complications | |||||||
| Early infection (%) | 776 | 144 (18.6)) | 33 (12.7) | 111 (21.5) | 1.872 | 1.229–2.853 | 0.004 |
| Intra-abdominal infection (%) | 776 | 67 (8.6) | 17 (6.0) | 50 (9.7) | 1.555 | 0.879–2.750 | 0.127 |
| Multiorgan dysfunction | |||||||
| MODS criteria (%)e | 777 | 94 (12.1) | 20 (7.7) | 74 (14.3) | 1.992 | 1.186–3.345 | 0.009 |
| Other complications | |||||||
| Severe neurological complications (%)f | 775 | 58 (7.5) | 17 (6.6) | 41 (7.9) | 1.224 | 0.681–2.199 | 0.564 |
| Outcome variables | |||||||
| ICU length of stay, days | 775 | 6 (4–8) | 5 (4–6) | 6 (5–8) | 1.058 | 1.022–1.095 | 0.002 |
| Retrasplantation<7th days (%) | 777 | 13 (1.7) | 4 (1.5) | 9 (1.7) | 1.127 | 0.344–3.695 | 0.843 |
| Mortality (%)g | 777 | 86 (11.1) | 17 (6.6) | 69 (13.3) | 2.117 | 1.245–3.599 | 0.006 |
LTR-cohort=Overall Cohort of Liver Transplant Recipients. HCC-LTR=Patients transplanted due Hepatocellular Carcinoma. Non-HCC-LTR=Patients transplanted due to other causes than HCC.
Number of patients evaluated and followed when there were data missing or errors in the records or reports.
A greater grade of ischemia/reperfusion injury, which was estimated by an increase in postransplant ALT peak value, was found in HCC-LTR. This cohort had shorter cold ischemia time but older donors. No meaningful differences were found in liver synthesis function tests assessed by INR at postoperative day 7 (p=0.277) nor in urgent retransplantation rate (1.5% in HCC-LTR vs 1.7% in non-HCC-LTR, p=0.843). However, cholestasis (serum bilirubin at 7th postoperative day) was more frequent in non-HCC-LTR (p<0.001). No other meaningful differences were found in grafts function despite of different evolving characteristics between cohorts.
In relation to other organs function, on the one hand, the non-HCC-LTR reached higher levels of serum creatinine and urea within the first 5 postoperative days (p<0.001 in both), although renal replacement therapy rate was similar (5.0% in HCC-LTR vs 6.4% in non-HCC-LTR, p=0.459). Besides, the non-HCC-LTR had a lesser percentage of patients with mechanical ventilation shorter than 24hours and had a greater rate of reintubations than the HCC-LTR (p<0.001). However, there were no differences in the tracheostomy rate (p=0.101) due to prolonged ventilation time.
On the other hand, MODS incidence was also higher in non-HCC-LTR (OR=1.99, 95% CI=1.19–3.35). An ad hoc multivariate analysis of patients with MODS showed a higher incidence of postoperative shock (OR=5.18, 95% CI=2.54–10.57), higher surgical reoperations rate (OR=4.63, 95% CI=2.36–9.10), higher incidence of renal dysfunction (OR=4.76, 95% CI=2.57–8.81), higher dialysis rate (OR=7.08, 95% CI=2.91–17.22), and infection rate (OR=7.02, 95% CI=3.66–13.48) in non-HCC-LTR.
Outcomes of cohortsLength of stay in ICU showed no meaningful differences between cohorts in the central tendency values (Mann–Whitney U test) but it did it in variability or extreme values (Moses test, p=0.012).
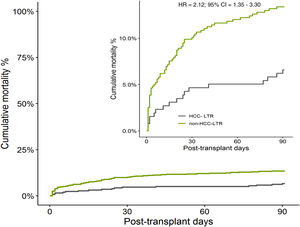
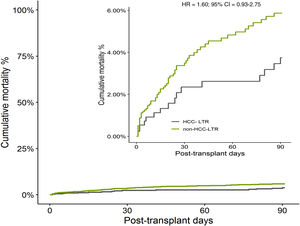
The overall cumulative mortality during 90 posttransplant days, when patients with urgent retransplant were censored, was 13.0% in non-HCC-LTR vs 5.9% in HCC-LTR (non-adjusted HR=2.12, 95% CI=1.25–3.61, p=0.005) (Fig. 3). When this outcome was adjusted by their independent predictors, the mortality related to transplant indications (non-HCC-LTR vs HCC-LTR as reference) reach no statistical significance (adjusted HR=1.60, 95% CI=0.93–2.76, p=0.086) (Fig. 4).
By a multivariate Cox proportional hazard model of differences between the transplant indication related cohorts were obtained three independent predictors of mortality in whole cohort (Table 4). These predictors were postoperative shock (HR=2.02, 95%CI=1.26–3.24, p=0.003), early vascular complications of graft (HR=4.01, 95%CI=2.45–6.56, p<0.001) and persistence of multi-organ dysfunction syndrome (HR=18.09, 95%CI=10.70–30.58, p<0.001). Two of them (postoperative shock and appareance of MODS) had a higher risk in non-HCC cohort and explain the mortality difference between indication related cohorts. Therefore the indication related to transplant (non-HCC-LTR vs HCC-LTR as reference) no showed differences for itself in the early mortality and it had no meaningful impact on mortality (Fig. 4).
Univariate and multivariate analysis of liver transplant recipients mortality endpoint using Cox's proportional hazards model.
| Univariate analysisCox regression | Multivariate analysisCox regression | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Baseline characteristics ofrecipientsa | ||||||
| Gender, female | 1.246 | 0.778–1.996 | 0.359 | |||
| Age, per each year | 1.018 | 0.993–1.043 | 0.141 | |||
| Body mass index, per each kg/m2 | 1.022 | 0.977–1.069 | 0.329 | |||
| Non-HCC-LTR cohort (HCC-LTR as reference) | 2.120 | 1.247–3.605 | 0.005 | 1.604 | 0.934–2.755 | 0.086 |
| Child C | 1.189 | 0.770–1.835 | 0.432 | |||
| MELD-Na, per each point | 1.047 | 1.014–1.081 | 0.004 | |||
| Pre-transplant creatinine level, per each mg/dL | 1.733 | 1.307–2.298 | <0.001 | |||
| Pre-transplant urea level, per each mg/dL | 1.007 | 1.003–1.011 | <0.001 | |||
| Hepatorenal syndrome | 2.087 | 1.211–3.595 | 0.008 | |||
| Previous supramesocolic surgery | 1.672 | 0.983–2.844 | 0.057 | |||
| Previuous hospital admission | 2.483 | 1.492–4.134 | <0.001 | |||
| Features of donors and transplantation time | ||||||
| Cold ischemia time, per each min | 1.001 | 0.999–1.002 | 0.067 | |||
| Intraoperative RBC transfusion, per each unit | 1.045 | 1.029–1.061 | <0.001 | |||
| Intraoperative plasma transfusion, per each unit | 1.029 | 1.008–1.051 | 0.005 | |||
| Features of postoperative period | ||||||
| Shock at ICU admission | 8.923 | 5.840–13.632 | <0.001 | 2.023 | 1.261–3.244 | 0.003 |
| Massive transfussion | 5.165 | 3.284–8.122 | <0.001 | |||
| Early surgical reoperation | 5.158 | 3.338–7.970 | <0.001 | |||
| Mechanical ventilation<24h | 0.209 | 0.134–0.326 | <0.001 | |||
| Need of tracheal reintubation and mechanical ventilation | 8.255 | 5.367–12.699 | <0.001 | |||
| ALT peak value UI/L, log scaleb | 6.272 | 3.672–10.713 | <0.001 | |||
| Graft vascular thrombosis in the first 7 days | 12.101 | 7.692–19.036 | <0.001 | 4.011 | 2.450–6.563 | <0.001 |
| S-creatinine peak value in the first 5 post-transplant days, per each mg/dL | 1.837 | 1.532–2.202 | <0.001 | |||
| S-urea peak value in the first 5 post-transplant days, per each mg/dL | 1.005 | 1.001–1.008 | 0.008 | |||
| S-total bilirrubin on day 7 per each mg/dL | 1.042 | 1.015–1.069 | 0.002 | |||
| Infection in the post-operative first month | 5.406 | 3.528–8.283 | <0.001 | |||
| Severe neurological complicationsc | 1.443 | 0.724–2.879 | 0.298 | |||
| Multi-organ dysfunction syndrome | 31.722 | 19.801–50.818 | <0.001 | 18.090 | 10.701–30.578 | <0.001 |
Mortality rates of overall and indication related cohorts are shown in Table 5 by time sequences during early postoperative period.
Mortality rates of overall and indication related cohorts by time sequences during early postoperative period.
| Postoperative day | 1 | 2 | 7 | 30 | 60 | 90 | Total |
|---|---|---|---|---|---|---|---|
| Overall cohort | |||||||
| N patients at riska | 764 | 750 | 745 | 736 | 707 | 686 | 675 |
| Mortality rate in time interval seriesb, n | 14 | 5 | 9 | 29 | 11 | 11 | 79 |
| Series aggregate mortality, n | 14 | 19 | 28 | 57 | 68 | 79 | |
| Mortality rate in time interval series, % | 1.8 | 0.7 | 1.2 | 3.9 | 1.6 | 1.6 | 10.8 |
| Series aggregate mortality, % | 1.8 | 2.5 | 3.7 | 7.6 | 9.2 | 10.8 | |
| Aggregate mortality, % overall cohort | 17.7 | 24.1 | 35.4 | 72.2 | 86.1 | 100.0 | |
| HCC-cohortc | |||||||
| N at riska | 255 | 254 | 253 | 251 | 245 | 244 | 240 |
| Mortality rate in time interval series, n | 1 | 1 | 2 | 6 | 1 | 4 | 15 |
| Mortality rate in time interval series, % | 0.4 | 0.4 | 0.8 | 2.4 | 0.4 | 1.6 | 6.0 |
| Series aggregate mortality, % | 0.4 | 0.8 | 1.6 | 4.0 | 4.4 | 6.0 | |
| Aggregate mortality, % HCC-cohort | 6.7 | 13.3 | 26.7 | 66.7 | 73.3 | 100 | |
| Non-HCCcohortd | |||||||
| N at riska | 509 | 496 | 492 | 485 | 471 | 461 | 454 |
| Mortality rate in time interval series, n | 13 | 4 | 7 | 23 | 10 | 7 | 64 |
| Mortality rate in time interval series, % | 2.6 | 0.8 | 1.4 | 4.7 | 2.1 | 1.5 | 13.2 |
| Series aggregate mortality, % | 2.6 | 3.4 | 4.8 | 9.6 | 11.7 | 13.2 | |
| Aggregate mortality, % non-HCC-cohort | 20.3 | 26.6 | 37.5 | 73.4 | 89.1 | 100 | |
In liver transplant recipients, early postoperative mortality is conditioned by complications emerged after transplant surgery and initial graft function, which could be associated with certain clinical pretransplant features and the way to access to transplant. In elective liver transplant candidates, waiting list prioritization is an eternally controversial issue since prioritization systems have to be periodically audited and further adapted to demographic changes and novel transplant indications.
The present study adds additional information to the actual body of research in this field. It does not try to demonstrate the homogeneity of the groups to obtain the validity of the results and predictors, but just the opposite, it tries to show that the cohorts HCC-LTR and non-HCC-LTR are not homogeneous and the mortality predictors have no the same risk in both cohorts.
First of all, in our population the non-HCC-LTR who received their first liver transplant from brain-dead donors showed a crude early mortality rates doubled compared to HCC-LTR (non-adjusted HR=2.12, 95% CI=1.25–3.60, p=0.005). The confirmation of a lower early perioperative mortality rate in HCC-LTR in other studies, it would be equivalent to a “possible advantage” in a short term in comparison to non-HCC-LTR. At present once the Milan criteria are universally applied, this early advantage for HCC patients could be balanced in the long term by mortality risk derived from tumour recurrence.14,20 However, taking into account the current guidelines for treatment of patients with HCC,21 these patients could have in future a lower mortality on the waiting list, a higher probability of transplantation, and a greater early postransplantation survival.22
Secondly, independent predictors of early postoperative mortality in LTR are almost exclusively related to postoperative complications: shock at ICU admission, graft vascular thrombosis and MODS occurrence. And the mortality related to transplant indication (non-HCC-LTR vs HCC-LTR as reference) reached no statistical significance when was adjusted by these independent predictors (adjusted HR=1.60, 95% CI=0.93–2.76, p=0.086). Therefore, the crude difference observed between HCC and non-HCC patients could be only justified by these predictors.
Two meaningful predictors of mortality (postoperative shock and MODS) had higher incidence in non-HCC-LTR. The first is related to a higher need for transfusions within surgery and early reinterventions.23 The MODS is the confluence of higher incidence of postoperative shock, renal dysfunction, graft dysfunction, and a higher rate of postoperative infections. All of them cause a longer mechanical ventilation time and ICU length of stay.
Some of predictors analysed in our work have been previously reported but within heterogeneous populations that included patients with acute liver failure or retransplantation.24–31 Other risk factors that are not widely accepted could also be discussed. LTR stature is not universally accepted as a risk factor.5 However, a study demonstrated that coincidence of short stature recipients and donors with larger size and weight was an independent risk factor for early dysfunction and secondary graft lost.32 Pretransplant serum creatinine is a well-known predictor of postransplant mortality33–34 but has collinearity with MELD-Na, which was expected since serum creatinine is contained in the MELD-Na. Also, blood lactic acid levels tested just after transplantation could also be considered a risk factor, although its clearance is more important than the postoperative peak.35–36
There are several aspects for practical application of our results. On the one hand, a prospective study with separated cohorts according to reason for transplant (HCC vs non-HCC) should be taken into account in future studies to analyse the early mortality in liver transplant recipients. This could prove if overall cumulative mortality is due to other predictors as a higher risk of postoperative shock and MODS. On the other hand, there is a need to assess whether this “possible advantage” of short-term mortality in HCC-LTR (to which an exceptions to the MELD are applied) is in fact compensated.37–40 On the contrary, a re-design of transplant access protocols of the “harmed cohort” should be considered.
Our study have several limitations. It is a single-centre involvement which could decrease external validity. It is a very old historic series which contain a certain grade of heterogenity of the patients. It is a retrospective study, however there are several strengths including a large sample of patients with homogeneous characteristics and the prospective database of most of the explanatory variables.
ConclusionIn conclusion, our study report a higher crude postoperative mortality in non-HCC-LTR compared to HCC-LTR due to higher incidence of shock at ICU admission and occurrence of postoperative multiple organ dysfunction syndrome. The indication to transplant (non-HCC compared to HCC as reference) did not show a meaningful statistical difference in early mortality rate during 90 days after transplantation.
AuthorshipJuan Carlos Pozo-Laderas: concept and design of study, data colleted, statistical analysis, interpretation of results and writing of the manuscript. Ipek Guler: Statistical study. Manuel Rodríguez-Perálvarez: concept and design, interpretation of results, writing and critical revision of the manuscript. Juan Carlos Robles, Ana Mula, Pedro López-Cillero and Carmen de la Fuente: critical revision of the manuscript.
Conflict of interestThe authors declare no conflict of interest.
To Carmen Pozo MD, Department of Urology, Vienna General Hospital, for her suggestions in the design, preparation and translation. To Elena Hernandez thanks for her help in translation.