To evaluate the effect of enteral nutrition volume, gastrointestinal function and the type of acid suppressive drug upon the incidence of lower respiratory tract infections in critically ill patients on mechanical ventilation (MV).
DesignA retrospective secondary analysis was carried out.
SettingThe Intensive Care Unit of a University Hospital.
Patients or participantsPatients≥18-years-old expected to need MV for more than four days, and receiving enteral nutrition by nasogastric tube within 24h of starting MV.
InterventionsWe correlated enteral nutrition volume administered during the first 10 days, gastrointestinal function and the type of acid suppressive therapy with the episodes of lower respiratory tract infection up until day 28. Cox proportional hazards ratios in univariate and adjusted multivariate models were used. Statistical significance was considered for p<0.05.
Main variables of interestLower respiratory tract infection episodes.
ResultsSixty-six out of 185 patients (35.7%) had infection; 27 patients had ventilator-associated pneumonia; and 39 presented ventilator-associated tracheobronchitis. Uninfected and infected groups were similar in terms of enteral nutrition volume (54±12 and 54±9mL/h; p=0.94) and caloric intake (19.4±4.9 and 19.6±5.2kcal/kg/d; p=0.81). The Cox proportional hazards model showed neurological indication of MV to be the only independent variable related to infection (p=0.001). Enteral nutrition volume, the type of acid suppressive therapy, and the use of prokinetic agents were not significantly correlated to infection.
ConclusionsEnteral nutrition volume and caloric intake, gastrointestinal dysfunction and the type of acid suppressive therapy used were not associated to lower respiratory tract infection in patients on MV.
Valorar el efecto del volumen de nutrición enteral, la función gastrointestinal y el tipo de protección gástrica en la incidencia de infección respiratoria del tracto inferior en pacientes críticos con ventilación mecánica (VM).
DiseñoAnálisis secundario retrospectivo.
ÁmbitoLa Unidad de Cuidados Intensivos de un hospital universitario.
Pacientes o participantesPacientes con edad≥18 años que se espera que precisen de VM durante>4 días y reciban nutrición enteral en las primeras 24h.
IntervencionesCorrelacionamos el volumen de nutrición enteral administrado durante los primeros 10 días, la función gastrointestinal y el tipo de protección gástrica con los episodios de infección pulmonar del tracto inferior hasta el día 28. Utilizamos el modelo de regresión de Cox. Un valor de p<0,05 fue considerado estadísticamente significativo.
Principal variable de interésEpisodios de infección del tracto respiratorio inferior.
ResultadosSesenta y seis de los 185 pacientes (35,7%) presentaron infección, 27 pacientes neumonía y 39 traqueobronquitis. Los pacientes no infectados e infectados fueron similares en el volumen de nutrición enteral (54±12 y 54±9mL/h; p=0,94) y aporte calórico (19,4±4,9 y 19,6±5,2kcal/kg/d; p=0,81). El modelo de regresión de Cox mostró que la causa neurológica de VM fue la única variable independiente asociada con infección (p=0,001). El volumen de nutrición enteral, el tipo de protección gástrica y la función gastrointestinal no se correlacionaron significativamente con la infección.
ConclusionesEl volumen y aporte calórico de nutrición enteral, la disfunción gastrointestinal y el tipo de protección gástrica no se asociaron a la infección del tracto respiratorio inferior en pacientes con VM.
Current guidelines recommend early normocaloric (80–100% of daily estimated energy expenditure) enteral nutrition as standard of nutritional care in critically ill patients receiving invasive mechanical ventilation.1,2 Although the appropriate caloric and protein intake to be provided to critically ill patients remains unclear,3 recent studies showed that a closer to recommended enteral nutrition intake (also known as full nutrition) given during the early phase of intensive care unit (ICU) stay was associated with a favorable outcome.4–6
However, in the ICU setting, full enteral nutrition requires large amounts of enteral nutrition (greater than 85mL/h)7 and can induce gastrointestinal dysfunction, expressed by feeding intolerance and/or paralysis of lower gastrointestinal tract: This can be due to different factors, such as mechanical ventilation, use of sedatives, opiates, muscle relaxants and vasopressors.8 Feeding intolerance represents a key factor in the pathogenesis of lower respiratory tract infection, which is favored by the early use of enteral nutrition and the routine use of acid suppressive drugs to prevent gastric hemorrhage by increasing gastric pH and promoting bacterial overgrowth.7–12
The exact role of normocaloric enteral nutrition in acquired infections remains controversial. Whilst a recent study found a direct relationship between the volume of enteral nutrition and the lower respiratory tract infection,13 a recently published meta-analysis demonstrated no difference in the risk of acquired infections between patients receiving normocaloric or hypocaloric enteral nutrition.14
Therefore, the aim of this study was to evaluate the effect of the volume and caloric intake of enteral nutrition given, the gastrointestinal function and the type of acid suppressive drug on the incidence of lower respiratory tract infection in critically ill patients with mechanical ventilation.
Patients and methodsPatientsThis was a retrospective secondary analysis of a previously published study that recorded the volume of enteral nutrition given and evaluated the gastrointestinal function in critically ill patients with mechanical ventilation.15 The study was conducted in a mixed adult ICU from October 2012 to September 2013. For the current study, we specifically recorded in these patients the episodes of lower respiratory tract infection that occurred more than 48h after initiating mechanical ventilation until day 28 post-ICU admission. Patients 18 years of age or older were eligible for enrolment in the study if they were expected to need mechanical ventilation for more than 4 days and if they were receiving enteral nutrition by nasogastric tube within 24h of starting mechanical ventilation. Patients were therefore excluded if they did not tolerate enteral nutrition. Patients were ineligible if they had recent bowel surgery or any abdominal disease. The Institutional Research Committee of our hospital approved the current study and waived the need to obtain specific informed consent due to the retrospective and observational nature of this secondary analysis.
Patient managementAll patients receiving mechanical ventilation were treated with a continuous intravenous infusion of morphine (0.01–0.15mg/kg/h) for analgesia and/or midazolam (0.01–0.15mg/kg/h) or propofol (1–2mg/kg/h) for sedative purposes. Muscle relaxants were used as required.
Caloric requirements and type of enteral feeding formula or a combination of enteral and parenteral nutrition was left to attending physician criteria. Enteral feedings were started within the first 24h after admission and delivered continuously at a constant rate by an infusion pump. Enteral nutrition products without fiber consisted of polymeric formulas containing 1–1.25kcal/mL of energy (approximately 20% of proteins, 30% of lipids, and 50% of carbohydrates). Full feeding rates were calculated with goals of 25–35kcal/kg per day and 1.2–1.5g/kg per day of protein.16 Blood glucose control was accomplished using institution specific insulin protocols targeting ranges from 80 to 150mg/dL. Additional 100mL of water each 6h were administered through nasogastric tube with a syringe to prevent obstruction of the nasogastric tube. All patients received acid suppressive drugs to prevent gastric hemorrhage with histamine-2 receptor antagonists or proton pump inhibitors.
The management of absent or decreased peristalsis and feeding intolerance was performed by the physician in charge aiming to maintain or restore gastrointestinal function by using prokinetics (erythromycin 250mg and/or metoclopramide 10mg every 6–8h, intravenously)17–19 or modifying the volume of enteral nutrition. In patients who did not tolerate enteral feeding, supplementary parenteral nutrition was administered temporarily.5 The protocol of management of the paralysis of lower gastrointestinal tract was published elsewhere.15
Measures taken to prevent the infection of the lower respiratory tract included: strict hand hygiene and training in the airway management, semirecumbent positioning whenever possible,20 preferred use of thin nasogastric tubes,21 control and maintenance of cuff pressure each 8h performed with a manual manometer (Smiths Medical, Portex®, Germany),7,10 oral hygiene with aqueous chlorhexidine solutions 0.2% performed every 8h, and short 48–72h course of intravenous antibiotics in patients with decreased level of consciousness.22 Infection antibiotic management was performed according to local lower respiratory tract infection based on international guidelines.23
DefinitionsLower respiratory tract infectionsLower respiratory tract infections, including ventilator-associated pneumonia (VAP) and ventilator-associated tracheobronchitis (VAT) were defined as infection occurred more than 48h after initiating mechanical ventilation until day 28.
VAP was defined by the presence of new or progressive radiographic infiltrates associated with at least two of the following criteria: (a) temperature >38.5°C or <36.5°C; (b) leukocyte count >12,000/μL or <4000/μL, and (c) purulent endotracheal aspirate and positive endotracheal aspirate (≥105CFU/mL) or bronchoalveolar lavage (≥104CFU/mL) cultures.24
VAT was defined using all the following criteria: fever (>38°C) with no other recognizable cause, purulent sputum production, positive endotracheal aspirate culture (≥105CFU/mL) or from bronchoalveolar lavage fluid (≥104CFU/mL), and no radiographic signs of new pneumonia.24,25
Gastrointestinal dysfunctionFor the purpose of the study, gastrointestinal dysfunction included feeding intolerance and paralysis of lower gastrointestinal tract. Feeding intolerance is a general term indicating intolerance of enteral feeding for whatever clinical reason (vomiting, gastroesophageal reflux or high gastric residuals volume greater than 500mL).26 Paralysis of lower gastrointestinal tract was the inability of the bowel to pass stool due to impaired peristalsis.17 Clinical signs included absence of stool for more than 3 consecutive days without mechanical obstruction.17 We considered as having paralysis of lower gastrointestinal tract all patients treated with laxatives.
Study endpointsThe primary endpoint was the association between the volume of enteral nutrition intake and the occurrence of lower respiratory tract infection (VAP and VAT) that occurred more than 48h after initiating mechanical ventilation until day 28. According to the primary objective, patients were classified in: infected group, patients who had a first lower respiratory tract infection in the above mentioned period, and the uninfected group, patients who did not have lower respiratory tract infection in this period.
Secondary endpoints analyzed the association between gastrointestinal dysfunction and the type of acid suppressive therapy with the incidence of lower tract infection.
Data collectionWe collected demographic characteristics, including age, gender and weight. Severity of illness was evaluated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score, the Simplified Acute Physiology Score (SAPS) II, and the Sepsis-related Organ Failure Assessment (SOFA) score, calculated after the first 24h of ICU stay. Comorbidities were evaluated by the Charlson index. Reason for mechanical ventilation was noted. Pharmacological treatment associated with gastrointestinal dysfunction, such as prokinetic agents, laxative, and opiates up to 10 days of start mechanical ventilation was collected, as well as the type of acid suppressive therapy (histamine-2 receptor antagonist or proton pump inhibitor). Nutrition characteristics included daily enteral and parenteral nutrition routes. Enteral intake was collected until ICU discharge or death, or up to 10 days of initiating mechanical ventilation. The average daily energy intake was calculated by dividing the cumulative energy intake during ICU stay for up to 10 days by the number of ICU days with enteral intake. The average daily protein intake was calculated in the same manner. Episodes of lower respiratory tract infection that occurred more than 48h after initiating mechanical ventilation until day 28 were collected. Length of mechanical ventilation, ICU and in-hospital length of stay, and ICU and in-hospital mortality were recorded.
Statistical analysisVariable summaries were shown as frequency, proportion, mean (SD), or median (IQR) as appropriate. Descriptive analyses were performed by using the χ2 or Fisher's exact tests for categorical variables, and the Student's t test or Mann–Whitney–Wilcoxon test for continuous variables as appropriate. To determine the occurrence of lower respiratory tract infection that occurred more than 48h after initiating mechanical ventilation until day 28 (dependent variable) in relation to gastrointestinal dysfunction (independent variable), we used Cox proportional hazards ratios in univariate and adjusted multivariable models. We selected variables for the adjusted multivariable analysis if their p value was 0.20 or lower in the univariate analysis and according to their clinical relevance. We generated survival analysis curves during 28-day follow-up using the Kaplan–Meier method and log-rank test to compare the association of different variables with lower respiratory tract infection. Patients who died during 28 days follow-up were censored. All statistical tests were 2-sided. A p value of <0.05 was considered statistically significant. Data were analyzed using the SPSS statistical package, version 19.0 (SPSS Inc., Chicago, IL).
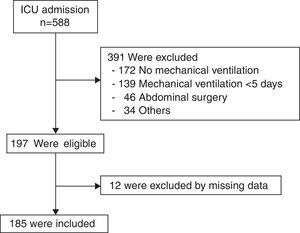
ResultsOf 588 patients admitted to the ICU during the study period, 197 patients were eligible and 12 were excluded from the analysis due to missing data, leaving 185 patients (Fig. 1). Sixty-six out of 185 patients (35.7%) had lower respiratory tract infection, 27 patients had VAP and 39 VAT. Nine out of 66 patients (13.6%) had a second episode of lower respiratory tract infection (4 patients VAP and 5 VAT).
At baseline, the uninfected and infected groups were similar with respect to demographic characteristics, the severity of illness evaluated by SAPS II, APACHE II and SOFA, and the time between ICU admission and start of mechanical ventilation (Table 1). Infected group had lower Charlson comorbidity index than the uninfected group (Table 1). The probability of a first lower respiratory tract infection more than 48h after initiating mechanical ventilation until day 28 was significantly higher in neurological patients than in the other patients [49 of 95 patients (51.6%) vs. 17 of 90 patients (18.9%), respectively, p<0.001] (Table 1). The first episode of lower respiratory tract infection was detected at a mean time of 7.7 (5.2) days and a median of 7 (4–9) days.
Demographic and clinical characteristics of patients with and without lower respiratory tract infection.
| Uninfected (n: 119) | Infected (n: 66) | p | |
|---|---|---|---|
| Female gender, n (%) | 43 (36.1) | 22 (33.3) | 0.75 |
| Age, years, mean (SD) | 56 (17) | 54 (18) | 0.92 |
| Body weight, kg, mean (SD) | 77 (18) | 77 (17) | 0.81 |
| Charlson comorbidity index, median (IQR) | 2 (3) | 1 (2) | <0.001 |
| APACHE II score, mean (SD) | 19 (7) | 20 (6) | 0.53 |
| SAPS II score, mean (SD) | 44 (14) | 45 (14) | 0.32 |
| SOFA score, mean (SD) | 7.4 (3.1) | 7.8 (2.5) | 0.32 |
| Reason for mechanical ventilation, n (%) | <0.001 | ||
| Neurologic failure | 46 (38.7) | 49 (74.2) | |
| Respiratory failure | 39 (32.8) | 7 (10.6) | |
| Severe sepsis/Septic shock | 14 (11.8) | 1 (1.5) | |
| Cardiac failure | 5 (4.2) | 1 (1.5) | |
| Others | 15 (12.6) | 8 (12.1) | |
| Time to mechanical ventilation, days, median (IQR) | 2 (3) | 1 (3) | 0.59 |
SD, standard deviation; IRQ, interquartile range; APACHE, Acute Physiology and Chronic Health Evaluation; SAPS II, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment.
Bacteria most frequently isolated from respiratory samples were gram negative in 29 (43.9%) patients and gram positive in 11 (16.7%) patients. Polymicrobial infection was found in 21 (31.8%) patients and in 5 patients the diagnosis of VAP was done although negative cultures were obtained. Taking into account the polymicrobial infection in 21 patients, the most common microorganisms isolated were: Staphylococcus aureus (18 episodes), Pseudomona aeruginosa (11 episodes), Escherichia coli (10 episodes), Enterobacter cloacae (9 episodes), Enterobacter aerogenes (7 episodes), Klebsiella pneumoniae (7 episodes), Serratia marcescens (4 episodes), Citrobacter (4 episodes), Stenotrophomonas maltophilia (3 episodes) and others (9 episodes).
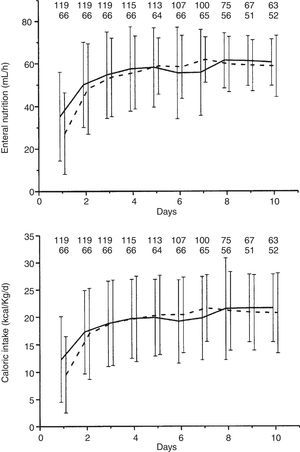
The uninfected and infected groups were similar in the volume of enteral nutrition and caloric intake (Fig. 2), frequency and days of interrupted enteral nutrition and supplemental parenteral nutrition, type of acid suppressive therapy and percentage of patients with continuous opiate perfusion therapy (Table 2). Median days of prokinetic agents, laxative polyethylene glycol, and opiates therapy were significantly higher in the infected group (Table 2).
Enteral and parenteral nutrition and feeding intolerance management from day 1 to day 10 in patients with and without lower respiratory tract infection.
| Uninfected (n: 119) | Infected (n: 66) | p | |
|---|---|---|---|
| Enteral nutrition, n (%) | 119 (100) | 66 (100) | – |
| Enteral nutrition, days, median (IQR) | 10 (3) | 10 (1) | 0.001 |
| Volume of enteral nutrition, mL/h | |||
| Mean (SD) | 54 (12) | 54 (9) | 0.94 |
| Median (IQR) | 57 (15) | 57 (13) | 0.62 |
| Enteral caloric intake, kcal/d, mean (SD) | 1455 (312) | 1442 (234) | 0.75 |
| Enteral caloric intake, kcal/kg/d, mean (SD) | 19.4 (4.9) | 19.6 (5.2) | 0.81 |
| Enteral protein intake, g/d, mean (SD) | 80 (18) | 80 (14) | 0.96 |
| Enteral protein intake, g/kg/d, mean (SD) | 1.1 (0.3) | 1.1 (0.3) | 0.83 |
| Interrupted EN, n (%) | 26 (21.8) | 17 (25.8) | 0.59 |
| Interrupted EN, days, median (IQR) | 2 (1.25) | 1 (2) | 0.49 |
| Parenteral nutrition, n (%) | 11 (9.2) | 11 (16.7) | 0.16 |
| Parenteral nutrition, days, median (IQR) | 2 (1) | 2 (2) | 0.36 |
| Acid suppressive therapy, n (%) | 0.35 | ||
| Histamine-2 receptor antagonist | 43 (36.1) | 29 (43.9) | |
| Proton pump inhibitor | 76 (63.9) | 37 (56.1) | |
| Prokinetic agents, n (%) | 39 (32.8) | 28 (42.4) | 0.20 |
| Prokinetic agents, days, median (IQR) | 3 (5) | 7 (4) | 0.01 |
| Laxative PEG, n (%) | 81 (68.1) | 47 (71.2) | 0.74 |
| Laxative PEG, days, median (IQR) | 3 (2) | 4 (2) | 0.003 |
| Opiates, n (%) | 102 (85.7) | 61 (92.4) | 0.24 |
| Opiates, days, median (IQR) | 4 (6) | 8 (7) | 0.001 |
SD, standard deviation; IRQ, interquartile range; EN, enteral nutrition; PEG, polyethylene glycol.
The Cox proportional hazards model found that the reason for mechanical ventilation (neurologic failure vs. others) was the only independent variable related to lower respiratory tract infection (p=0.001) (Table 3). Others variables entered in the Cox hazard model, including the volume of enteral nutrition, type of acid suppressive therapy, prokinetic agents, laxative polyethylene glycol, and opiates were not significantly related to lower respiratory tract infection that occurred more than 48h after initiating mechanical ventilation until day 28 (Table 3).
Univariate and multivariate Cox regression model for first lower respiratory tract infection occurred more than 48h after initiating mechanical ventilation until day 28.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Gender (women vs. men) | 0.91 (0.54–1.51) | 0.70 | ||
| Age (1-year increase) | 1.0 (0.98–1.01) | 0.63 | ||
| Reason for mechanical ventilation (neurologic vs. others) | 3.46 (1.99–6.02) | <0.001 | 5.79 (2.00–16.80) | 0.001 |
| Enteral nutrition (1mL/h increase) | 1.0 (0.98–1.02) | 0.63 | ||
| Histamine-2 receptor antagonist vs. proton pump inhibitor | 1.35 (0.83–2.20) | 0.22 | ||
| Prokinetic agents (1-day increase) | 1.22 (1.05–1.41) | 0.009 | ||
| Laxative PEG (1-day increase) | 1.13 (1.02–1.25) | 0.02 | ||
| Opiates (1-day increase) | 1.12 (1.04–1.21) | 0.002 | ||
CI, confidence interval; PEG, polyethylene glycol.
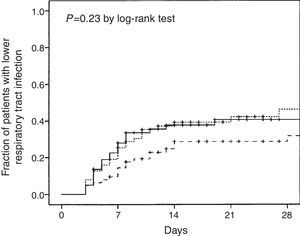
The Kaplan–Meier curves showed no differences in the time to present lower respiratory tract infection by the volume of enteral nutrition evaluated in intertertiles (p=0.23 by the log-rank test) (Fig. 3). Mean (SD) values and ranges of intertertiles of enteral nutrition volume were: first 41 (9) (6-51) mL/h, second 56 (2) (51-59) mL/h, and third 64 (5) (59-78) mL/h.
The infected group had higher duration of mechanical ventilation and ICU and in-hospital length of stay. No differences were found in ICU and in-hospital mortality (Table 4).
Outcomes of patients with and without lower respiratory tract infection.
| Uninfected (n: 119) | Infected (n: 66) | p | |
|---|---|---|---|
| Duration of MV, days, median (IQR) | 9 (9) | 18 (13) | <0.001 |
| ICU length of stay, days, median (IQR) | 11 (9) | 22 (14) | <0.001 |
| In-hospital length of stay, days, median (IQR) | 22 (26) | 38 (31) | <0.001 |
| ICU mortality, n (%) | 20 (16.8) | 10 (15.2) | 0.84 |
| In-hospital mortality, n (%) | 29 (24.4) | 13 (19.7) | 0.58 |
MV, mechanical ventilation; IQR, interquartile range; ICU, intensive care unit.
The main result of our study was we found no association between the volume of enteral nutrition given and the incidence of lower respiratory tract infection in patients with mechanical ventilation. In addition, nor the presence of gastrointestinal dysfunction neither the type of acid suppressive therapy were associated with lower respiratory tract infection.
Our results are in consonance with the meta-analysis of Marik and Hooper,14 who found no difference in the risk of acquired infections between patients receiving intentional hypocaloric as compared to normocaloric nutritional goals. The existence of gastrointestinal dysfunction and the type of acid suppressive therapy used were not associated with lower respiratory tract infection, being the neurological failure as a reason for mechanical ventilation the only independent risk factor for lower respiratory tract infection, a well-known phenomenon in the literature.27–29
Our main findings are in disagreement with the recent study by Chung et al.13 This could be due, in part, to differences in the study design (they included patients without enteral nutrition), in the pathology of patients (they included patients with severe abdominal injury), and in the number of patients (they included a higher number of patients). Another factors could potentially had an influence, such as the measures implemented to prevent regurgitation and aspiration as semirecumbent positioning,20 preferred use of thin nasogastric tubes 21 and routine care of monitoring tracheal cuff pressure every 8h.30,31
Undernutrition seems common in critically ill patients, as shown by a recent study including more than 200ICUs, reporting a mean caloric intake of 14.5kcal/kg/d.32 Our mean caloric intake was 19.5kcal/kg/d, lower than the 25–35kcal/kg/d necessary to achieve a full nutrition.5,16,33 Thus, we cannot definitively answer whether the intake of full nutrition by enteral route could increase feeding intolerance and the incidence of lower respiratory tract infections. Anyway, this highlights that other strategies would be necessary to minimize undernutrition in critically ill patients, such as using supplemental parenteral nutrition.5
Assessing gastrointestinal dysfunction in patients with mechanical ventilation is difficult, due to the lack of agreement on the definition and, in our case, because of the observational and retrospective nature of the study in which the management was performed by the physician in charge. To assess feeding intolerance we did not monitor the residual gastric volume because it has been proved ineffective in preventing the development of VAP.7,34 In addition, residual gastric volume monitoring leads to unnecessary interruptions of enteral nutrition delivery with subsequent inadequate feeding.11 Therefore, we evaluated gastrointestinal dysfunction by the days of treatments with prokinetic agents and polyethylene glycol laxative. The median days of prokinetic agents and laxative polyethylene glycol were significantly higher in the infected group than in the uninfected group, suggesting a potential association between gastrointestinal dysfunction and lower respiratory tract infection. Therefore, and even though it was not confirmed by our multivariate analysis, more studies are required to rule out this possibility.
We found no differences in the incidence of lower respiratory tract infection among patients receiving proton pump inhibitor and those treated with histamine-2 receptor antagonists. The acid suppressive drugs to prevent gastric hemorrhage could increase susceptibility to lower respiratory tract infection because these drugs increase gastric pH, thus allowing bacterial colonization.35 Controversy remains regarding the optimal regimen to use when prophylaxis against stress-related gastric hemorrhage is indicated.36,37 A recent large observational study showed that the use of proton pump inhibitor increased the risk of VAP more than histamine-2 receptor antagonist.38
We acknowledged several limitations: first, this is a retrospective secondary analysis of a previously published study recording gastrointestinal function in critically ill patients with mechanical ventilation. Second, the volume of enteral nutrition intake, the management of feeding intolerance and the decision of initiating supplementary parenteral nutrition was performed by the physician in charge the patient. However, we believe that this reflects daily clinical practice.
In conclusion, the volume of enteral nutrition, the caloric intake, the existence of gastrointestinal dysfunction and the type of acid suppressive therapy were not associated to lower respiratory tract infection in critically ill patients with mechanical ventilation. The neurologic reason for mechanical ventilation was associated to lower respiratory tract infection.
FundingNone declared.
Authors’ contributionAsunción Colomar: data collection, manuscript preparation and manuscript review. Begoña Guardiola: data collection, manuscript preparation and manuscript review. Juan A. Llompart-Pou: manuscript preparation and manuscript review. Ignacio Ayestarán: data collection, manuscript preparation and manuscript review. Javier Rodríguez-Pilar: data collection and manuscript review. Mireia Ferreruela: data collection and manuscript review. Joan M. Raurich: literature search, data collection, study design, data analysis, manuscript preparation, manuscript review.
Conflict of interestThe authors declare no conflict of interest.