“Zero-VAP” is a proposal for the implementation of a simultaneous multimodal intervention in Spanish intensive care units (ICU) consisting of a bundle of ventilator-associated pneumonia (VAP) prevention measures.
Methods/designAn initiative of the Spanish Societies of Intensive Care Medicine and of Intensive Care Nurses, the project is supported by the Spanish Ministry of Health, and participation is voluntary. In addition to guidelines for VAP prevention, the “Zero-VAP” Project incorporates an integral patient safety program and continuous online validation of the application of the bundle. For the latter, VAP episodes and participation indices are entered into the web-based Spanish ICU Infection Surveillance Program “ENVIN-HELICS” database, which provides continuous information about local, regional and national VAP incidence rates. Implementation of the guidelines aims at the reduction of VAP to less than 9 episodes per 1000 days of mechanical ventilation.
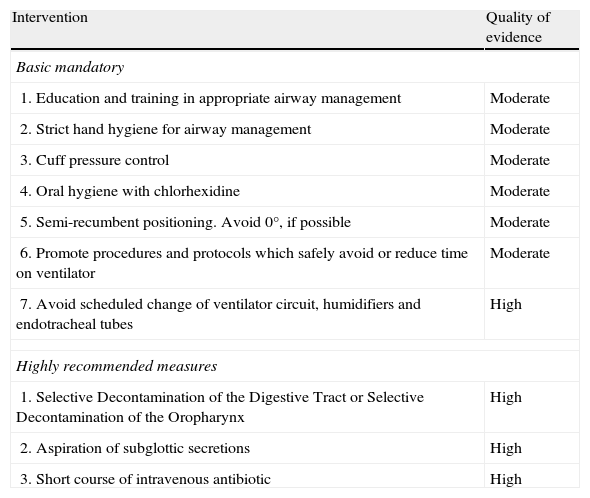
A total of 35 preventive measures were initially selected. A task force of experts used the Grading of Recommendations, Assessment, Development and Evaluation Working Group methodology to generate a list of 7 basic “mandatory” recommendations (education and training in airway management, strict hand hygiene for airway management, cuff pressure control, oral hygiene with chlorhexidine, semi-recumbent positioning, promoting measures that safely avoid or reduce time on ventilator, and discouraging scheduled changes of ventilator circuits, humidifiers and endotracheal tubes) and 3 additional “highly recommended” measures (selective decontamination of the digestive tract, aspiration of subglottic secretions, and a short course of iv antibiotic).
DiscussionWe present the Spanish VAP prevention guidelines and describe the methodology used for the selection and implementation of the recommendations and the organizational structure of the project. Compared to conventional guideline documents, the associated safety assurance program, the online data recording and compliance control systems, as well as the existence of a pre-defined objective are the distinct features of “Zero VAP”.
«Neumonía Zero» es una propuesta de aplicación de una intervención multimodal simultánea en las unidades de cuidados intensivos españolas que consiste en un paquete de medidas preventivas de la neumonía asociada a la ventilación mecánica (NAVM).
Métodos/diseñoSe trata de una iniciativa de las sociedades españolas de Medicina Intensiva y Enfermería Intensiva. El proyecto cuenta con el apoyo del Ministerio de Sanidad y la participación es voluntaria. Además de las directrices para la prevención de la NAVM, el proyecto «Neumonía Zero» incluye un programa integral de seguridad del paciente y una validación continua «online» de la aplicación de las medidas. Para ello se introducen los episodios de NAVM y los índices de participación en la base de datos en red del programa «ENVIN-HELICS» de vigilancia de las infecciones en las unidades de cuidados intensivos españolas, que ofrece información continua acerca de las tasas de incidencia de NAVM a nivel local, autonómico y nacional. La aplicación de «Neumonía Zero» pretende reducir las NAVM a menos de 9 episodios por cada 1.000 días de ventilación mecánica.
Inicialmente, se seleccionaron 35 medidas de prevención. Un grupo de expertos utilizó la metodología del Grading of Recommendations, Assessment, Development and Evaluation Working Group para crear una lista de 7 recomendaciones básicas «obligatorias» (formación y entrenamiento en el manejo de la vía aérea, higiene estricta de manos en el manejo de la vía aérea, control de la presión del neumotaponamiento, higiene bucal con clorhexidina, posición semiincorporada, fomento de medidas que de forma segura eviten o reduzcan la duración de la ventilación mecánica, y desaconsejar los cambios programados de tubuladuras, humidificadores y tubos endotraqueales) y 3 medidas adicionales «muy recomendables» (descontaminación selectiva del tubo digestivo, aspiración de las secreciones subglóticas y un breve curso de antibióticos intravenosos).
DebatePresentamos las directrices españolas para la prevención de la NAVM y describimos la metodología utilizada para seleccionar y aplicar las recomendaciones y la estructura organizativa del proyecto. En comparación con recomendaciones convencionales, el programa de seguridad asociado, el registro de datos «online» y los sistemas de control del cumplimiento, además de la existencia de un objetivo predefinido, son características distintivas de «Neumonía Zero».
Ventilator-associated pneumonia (VAP) is the most frequent ICU-acquired infection.1–3 It is associated with significant increases in the length of stay, healthcare costs and both crude and attributed mortality.4–7 Therefore, potential functional, mechanical and pharmacological prevention measures of VAP have frequently been investigated, classified and recommended in accordance with updated available evidence and feasibility.8–17 Questionnaires, however, repeatedly report that knowledge, implementation and adherence to guidelines are low among nurses and physicians working in ICUs internationally.18–20
The Spanish annual April to June ICU National Nosocomial Infection Surveillance Study (Estudio Nacional de Vigilancia de Infección Nosocomial, “ENVIN”)3,2 shows stable VAP incidence densities of approximately 15 episodes per 1000 days of mechanical ventilation for the years 2000–2009 in more than 100 ICUs. These figures compare negatively with other national surveillance programs. The United States National Healthcare Safety Network reports mean VAP incidence rates of 3.7 for 2006–2008, from as low as 2.1 in pediatric medical-surgical ICUs to 10.7 episodes per 1.000 days in burn units1.
Recently, the implementation of “bundles” of effective measures, compared to individual interventions, has been proposed to reduce the incidence of catheter-related bloodstream infections21 and VAP.22 In Spain, a highly successful bacteraemia prevention bundle, named “Zero Bacteraemia”, was started and implemented by the Spanish Society of Intensive Care Medicine (SEMICYUC) in 200823 under the auspices and financial support of the World Health Organization and the Quality Assurance Agency of the Spanish Ministry of Health (QAA). The “Zero-VAP” Project uses the organizational structure and methodology created for “Zero-Bacteraemia”. In this article we describe the methods applied to identify the recommendations to be included in the “Zero-VAP” bundle and to accomplish implementation in Spanish ICUs. We also refer to the systems used to monitor compliance and to register the data generated during the project and describe the associated quality assurance program.
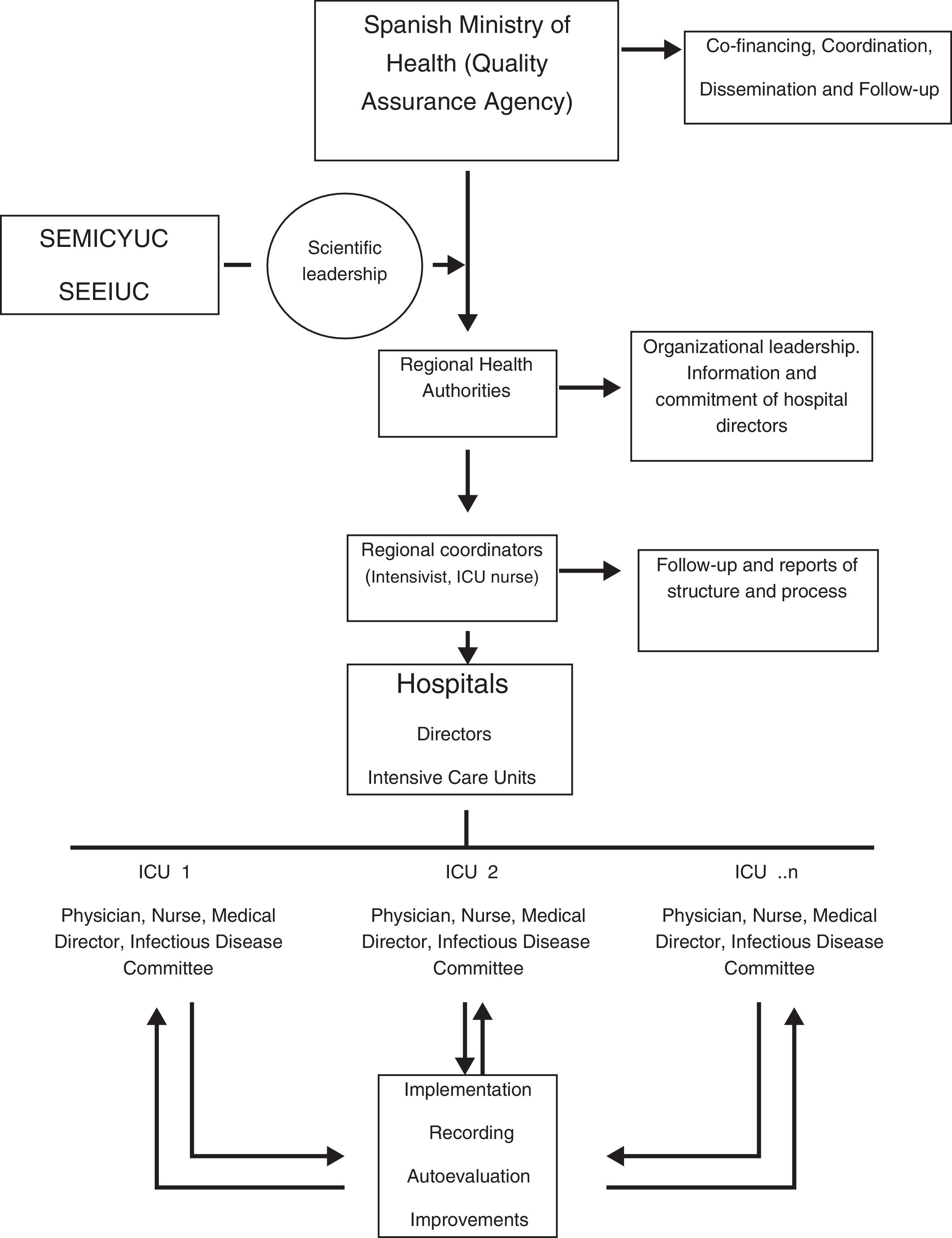
Methods and designStructure and organizationSEMICYUC and the Spanish Society of Intensive Care Nurses (SEEIUC) lead and coordinate the technical aspects of “Zero-VAP” by means of a working contract. The QAA hierarchically involves the 17 regional healthcare authorities and the hospital directors of the participating ICUs and promotes the Project through co-financing with the regional healthcare authorities, nation-wide coordination, dissemination and follow-up (Fig. 1). For every Spanish autonomous region, as well as in every ICU, an intensivist and an intensive care nurse coordinate “Zero-VAP” at their respective level. The definitions of the European CDC24–26 for VAP and tracheobronchitis have been adopted and are available in the user's manual of ENVIN-HELICS.3 In brief, the presence of these features suggest VAP: two or more serial chest X-rays or CT scans with a suggestive image for patients with underlying cardiac or pulmonary disease, or one definitive chest X-ray or CT scan in patients without underlying cardiac or pulmonary disease, with fever >38°C and/or leucocytosis ≥12,000WBC/mm3 or leucopenia ≤4000WBC/mm3, and at least one of the following: (1) new onset purulent sputum or change of character of sputum; (2) cough, dyspnea or tachycardia; (3) suggestive auscultation or (4) worsening gas exchange. VAP episodes were additionally stratified according to the microbiological sampling diagnostic method employed. The target of reduction of VAP to less than 9 episodes per 1000 days of mechanical ventilation was chosen because compared to the period 2000–2009 and the most recent results it represents reductions of 40% and 25%, respectively.3
A national task force with members of SEMICYUC and SEEIUC selected the prevention measures and will be in charge of the management of the Project.
The VAP prevention bundle, the objectives and tools for implementation and control of “Zero-VAP” were presented at a national meeting and subsequently at regional and local meetings. The “Zero-VAP” guidelines are an extension of ENVIN-HELICS, which collects epidemiological data from all patients admitted to participating ICUs from April 1st to June 30th yearly since 1994 and does not require ethics committee approval. Viewed as a quality improvement initiative, neither the Spanish Ministry of Health nor the regional healthcare authorities requested evaluation of the project by local or regional ethics committees.
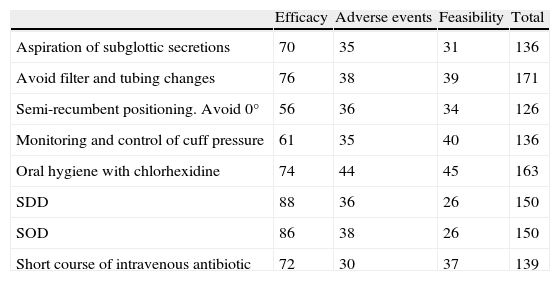
Selection of ventilator-associated pneumonia prevention measuresThirty-five interventions were derived from published clinical trials, guidelines, systematic reviews and meta-analyses. They were classified as “functional”, “mechanical” or “pharmacological” (Table 1) and evaluated independently by teams of at least two members of the task force using the Grading of Recommendations Assessment, Development and Evaluation Working Group methodology27,28 (http://www.gradeworkinggroup.org/) (Table 2). Inclusion of 8 debated interventions was resolved by quantitative assessment by the 11 members of the panel, considering (1) the quality of the evidence (10 points), (2) its safety (5 points), and its feasibility in Spanish ICUs (5 points) (Table 3). Finally, feasibility and cost criteria were applied, as recommended,29 based on which groups of 7 “basic mandatory” and 3 “highly recommended” measures (Table 4) were generated.
Classification of VAP prevention methods.
| Functional |
| 1. Semi-recumbent position |
| 2. Strict hand hygiene with alcohol-based gels or solutions before airway management |
| 3. Education and training in aspiration of bronchial secretions |
| 4. Daily sedation vacation and assessment of weaning and extubation |
| 5. Availability of weaning protocols |
| 6. Early tracheostomy |
| 7. Non-invasive mechanical ventilation |
| 8. Microbiological surveillance of cross-contamination and infection |
| 9. Instillation of normal saline prior to endotracheal suctioning |
| 10. Ventilator tubing change |
| 11. Route of endotracheal intubation. Orotracheal vs. nasotracheal |
| 12. Type of airway humidification. Preference of heat moisture exchanger or heated humidifier |
| 13. Physiotherapy |
| 14. Positive end-expiratory airway pressure (PEEP) of 5–8cmH2O vs. Zero end-expiratory pressure (ZEEP) in patients without lung injury |
| 15. Enteral feeding: route of administration and gastric residual volumes. Use of prokinetics |
| Mechanical |
| 1. Endotracheal tube cuff pressure monitoring |
| 2. Subglottic secretion drainage |
| 3. Polyurethane-cuffed endotracheal tubes |
| 4. Polyurethane-cuffed endotracheal tubes with subglottic secretion drainage |
| 5. Silver-coated endotracheal tubes |
| 6. High-volume, low-pressure endotracheal tube cuff |
| 7. Small caliber feeding tubes |
| 8. Aspiration of tracheobronchial secretions with closed vs. open systems |
| 9. Endotracheal tube biofilm removal device (Mucus Shaver®) |
| 10. Kinetic bed therapy |
| 11. Airway filters |
| 12. Water-soluble gel lubrication of the endotracheal tube |
| 13. Tooth brushing |
| Pharmacological |
| 1. Selective decontamination of the digestive tract |
| 2. Selective oropharyngeal decontamination |
| 3. Short course of intravenous antibiotic |
| 4. Oral hygiene with chlorhexidine |
| 5. Nebulized antibiotics |
| 6. Antibiotic cycling |
| 7. Probiotics |
| Quality of evidence |
| High. Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| Low. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| Very low. Any estimate of effect is very uncertain |
| Degree of recommendation |
| Strong. Indicating a judgment that most well informed people would make |
| Weak. Indicating a judgment that a majority of well-informed people would make but a substantial minority would not |
Mean individual score of measures categorized as “strong” recommendation.
| Efficacy | Adverse events | Feasibility | Total | |
| Aspiration of subglottic secretions | 70 | 35 | 31 | 136 |
| Avoid filter and tubing changes | 76 | 38 | 39 | 171 |
| Semi-recumbent positioning. Avoid 0° | 56 | 36 | 34 | 126 |
| Monitoring and control of cuff pressure | 61 | 35 | 40 | 136 |
| Oral hygiene with chlorhexidine | 74 | 44 | 45 | 163 |
| SDD | 88 | 36 | 26 | 150 |
| SOD | 86 | 38 | 26 | 150 |
| Short course of intravenous antibiotic | 72 | 30 | 37 | 139 |
Quality of evidence of individual components of the VAP prevention bundle. All interventions were categorized as “highly recommended”.
| Intervention | Quality of evidence |
| Basic mandatory | |
| 1. Education and training in appropriate airway management | Moderate |
| 2. Strict hand hygiene for airway management | Moderate |
| 3. Cuff pressure control | Moderate |
| 4. Oral hygiene with chlorhexidine | Moderate |
| 5. Semi-recumbent positioning. Avoid 0°, if possible | Moderate |
| 6. Promote procedures and protocols which safely avoid or reduce time on ventilator | Moderate |
| 7. Avoid scheduled change of ventilator circuit, humidifiers and endotracheal tubes | High |
| Highly recommended measures | |
| 1. Selective Decontamination of the Digestive Tract or Selective Decontamination of the Oropharynx | High |
| 2. Aspiration of subglottic secretions | High |
| 3. Short course of intravenous antibiotic | High |
Several systems for the surveillance of adhesion to the Project and compliance of the proposed measures have been established.
- 1.
Participation in the web-based “ENVIN-HELICS” registry. ICUs are committed to enter data required for calculation of incidence density of VAP.
- 2.
Provision of education and training to healthcare workers (HCW). Two educational modules about VAP prevention and patient safety, with their corresponding examinations, are freely accessible on-line3 and continuously monitored for number and category of ICU-HCW successfully completing the tests. Coordinators in each unit have access to this registry and report to the regional coordinators.
- 3.
Evaluation of compliance. The web-based 6-monthly compliance registry consists of 3 quality indicators, which were arbitrarily selected because of the ease of monitoring: (1) cuff pressure control prior to oral hygiene, (2) the use of chlorhexidine for oral hygiene, and (3) the number of monthly meetings and activities related to the Project. The previous registry for “Zero Bacteremia”23 remains active: (1) a checklist for the insertion of vascular catheters, (2) chlorhexidine skin disinfection, (3) availability of a vascular catheter insertion cart, (4) achievement of daily objectives, (5) meeting with hospital directors and (6) “learning from errors” meetings. Information about how many indicators are accomplished is available on-line. The optional “highly recommended” measure(s) implemented at each ICU will be captured from the general database (SDD) and from a structured questionnaire (subglottic aspiration and short course of iv antibiotic) to allow for a separate comparative statistical analysis.
“Zero VAP” data are recorded through a specific adaptation of the “ENVIN-HELICS” web page (http://hws.vhebron.net/Neumonia-zero/). Participating ICUs record data of patients meeting the definition of VAP. In addition, monthly information about risk factors, including total patient-days and ventilated patient-days, is provided. If a patient develops VAP, a database entry is created with information on demographics, risk factors, severity at ICU admission, underlying conditions, comorbidity, diagnostic criteria, microbiological sampling procedures, etiology and clinical course. Patients entered in the “ENVIN-HELICS” surveillance program are recorded automatically in the “Zero VAP” database, with no additional intervention being required.
Summary descriptive statistics are available on-line for every individual unit, which may directly access its data on a daily basis. Local results are displayed together with the corresponding regional and national values.
Detailed educational slide presentations for every component of both “basic mandatory” and “highly recommend” measures can be freely accessed at http://hws.vhebron.net/Neumonia-zero/.
Basic mandatory measures- 1.
Education and training in airway management (aspiration of bronchial secretions). A systematic review including 26 studies30 suggests that educational interventions are associated with significant reductions in nosocomial infection rates, although a causal relation cannot be established due to limitations in study design. Several studies show significant reductions in VAP incidence after implementation of educational programs31,32 and simple clinical protocols, including emphasis on strict hand hygiene.33 Although the quality of evidence for educational interventions for airway management is “moderate”, the strength of this recommendation was classified as “strong” because of its significant association with the prevention of VAP, no major threats for safety, and low cost of implementation.
The specific care practices taught in the educational program to be implemented at the beginning of the project are freely available at http://hws.vhebron.net/Neumonia-zero/.
- 2.
Strict hand hygiene with alcohol solutions before airway management. Observational studies34 report reductions in nosocomial infection rates and methicillin-resistant Staphylococcus aureus (MRSA) infection after promoting hand-washing. Hand-washing before and after patient contact and the use of gloves were introduced in 2004 as proven measures for the prevention of VAP and other nosocomial infections.9 It is now firmly established as a fundamental component of standard clinical practice.19 The use of gloves does not preclude the obligation of hand-washing with alcohol solutions before and after management of the artificial airway.
- 3.
Oral hygiene with chlorhexidine. Chlorhexidine has been recommended for MRSA skin decolonization35–38 and VAP prevention.9,10,15 Four of 6 meta-analyses39–44 show significant reductions of VAP incidence rates with oropharyngeal chlorhexidine. A recent meta-analysis43 favors chlorhexidine (OR 0.56, 95%CI 0.44–0.73), although 6 of the 10 trials were negative. The variable effect of chlorhexidine seems to be related to its limited microbiological effect, which, while reducing oropharyngeal carriage with S. aureus, leaves Gram-negative colonization largely unaffected.45 Efficacy may also be related to the local concentrations of chlorhexidine. A trial administering a high concentration 2% solution showed a significant reduction of VAP, although 10% of patients in the test group developed irritation of the oral mucosa.42 Chlorhexidine apparently has no risk of inducing cross-resistance to antibiotics. However, chlorhexidine-resistant strains of MRSA may substitute susceptible strains shortly after initiating routine chlorhexidine application.46,47 Up to 63% of European strains actually express plasmid-borne qacA/B genes coding for multidrug efflux pumps,48 which confer chlorhexidine resistance in MRSA.
Oral hygiene with aqueous chlorhexidine solutions (0.12–2%) should be performed every 8h. Before its application, cuff pressure should be above 20cmH2O. Formal training of nurse's aides, responsible for this procedure in most ICUs, will be done.
- 4.
Control and maintenance of cuff pressure. Although included in the guidelines,49 this recommendation is based on the results of a small single-centre, non-comparative study50 suggesting that a cuff pressure level below 20cmH2O is associated with an increased risk of VAP in patients not receiving systemic antibiotics. A recent randomized trial, comparing continuous monitoring of cuff pressure with intermittent or non-scheduled measurements,51 did not confirm this association in spite of a significant difference in the incidence of cuff pressures below 20cmH2O. The panel considered that routine checks of cuff pressure is a simple low cost standard clinical procedure, also controlling for inappropriately high pressures and should be scheduled at 8h intervals and set at 20–30cmH2O before oral application of chlorhexidine.
- 5.
Semi-recumbent positioning. Avoidance of 0° supine positioning. The physiologic rationale behind semi-recumbent positioning is that it may favor spontaneous ventilation and reduce aspiration of contaminated gastric content. Its effect on VAP prevention has not been validated in unstable patients or patients with increased intra-abdominal pressure, and results of randomized trials vary.52,53 A meta-analysis54 using a random effects model to compensate for significant heterogeneity observed a non-significant reduction in the incidence of VAP (OR 0.59, 95%CI 0.15–2.35) in patients in a semi-recumbent 45° position. A recent well-conducted randomized trial55 enrolling 232 patients with tetanus was negative, with 20.8% (39.2 episodes per 1000 days of ventilation) patients developing VAP in supine position and 25% (38.1 episodes) in semi-recumbence (OR 0.79, 95%CI 0.39–1.57, p=0.46). Differing study results prevent establishing a firm recommendation to elevate backrests to 45°. However, it is recommended to avoid 0° supine positioning in patients receiving enteral feeding and with no contraindication.
- 6.
Promoting procedures and protocols that safely avoid or reduce duration of mechanical ventilation. Effective interventions aimed at avoiding or shortening duration of endotracheal intubation are associated with reductions of VAP. Therefore, protocols for non-invasive mechanical ventilation (NIMV) in acute exacerbations of chronic obstructive pulmonary disease (COPD), for weaning or for sedation promoting lower infusion doses or its daily interruption should be available.
NIMV appears to be associated with reduced mortality and VAP (relative risk 0.29, 95%CI 0.19–0.45) when used as a weaning strategy in patients with COPD after extubation.56
A recent systematic review57 suggests that the use of weaning protocols is associated with a reduced duration of mechanical ventilation. However, significant heterogeneity between studies and the absence of data on VAP preclude grading of evidence of this potentially preventive measure.
To our knowledge, no study evaluating the effect of daily sedation vacation on VAP incidence as end-point has been performed. Two landmark trials evaluating daily interruption of sedation did not mention comparative VAP rates, although both found significant reductions in the duration of mechanical ventilation.58,59 Therefore, the quality of the evidence of VAP prevention by reducing sedation could not be appropriately graded. A detailed sedation protocol was deemed to be beyond the scope of the Project.
Based on the available data, the panel decided to issue a generic recommendation for the availability of updated weaning and sedation protocols and for the use of NIMV in selected patient populations, to reduce the duration of mechanical ventilation.
- 7.
Avoidance of elective changes of ventilator circuits, humidifiers and endotracheal tubes. Planned ventilator circuit changes may increase cost and the risk of VAP and should not be performed, as has already been recommended.8,11 A systematic review60 confirms that 48h circuit changes compared to 7 days almost double the risk of VAP (OR 1.93, 95%CI 1.08–3.44). It is concluded that the practice of planned ventilator circuit changes should be abandoned.
Heat-moisture exchangers (HME) have been suggested to be associated with lower incidence of VAP than heated humidifiers (HH).61 A recent meta-analysis does not confirm this effect62 and, therefore, HH should be reserved for individual cases at increased risk of airway obstruction. The adequate frequency of change of HME has not been established. The results of prospective before-after studies and randomized trials indicate that prolonging the duration of HMEs from 24 to 48h,63,64 to 5 days,65 and even to 7 days,66 reduces costs and does not increase VAP.
- 1.
Selective Decontamination of the Digestive Tract (SDD) or Selective Oropharyngeal Decontamination (SOD). This intervention aims at the reduction of endogenous infections by preventing or eradicating the aero-digestive carrier state with potentially pathogenic flora. The SDD protocol includes administration of a short 2- to 5-day course of a third generation cephalosporin and topical antibiotics administered as a paste to the oral mucosa and as a solution via nasogastric tube. The topical antimicrobials are non-absorbable to maintain high luminal gut concentrations and prevent the development of resistance. This strategy has no effect on exogenous infections, which are caused by direct inoculation, although it may reduce cross-transmission. SDD is associated with reductions of VAP of approximately 70% in 60 randomized trials and 15 meta-analyses. A recent individual patient meta-analysis67 calculated an odds ratio of 0.28 (95%CI 0.20–0.38) for the development of VAP. SDD is also associated with significant reductions in bacteraemia (OR 0.73, 95%CI 0.59–0.9)68 and mortality (OR 0.75, 95%CI 0.65–0.87).67 Although concerns about the development of resistance during SDD have been voiced, the most recent and biggest randomized multicentre trials demonstrate significant reductions of incidence rates of multi-drug resistant bacteria (MDR).69–71 SOD, studied in 9 controlled trials,72 drastically reduces VAP risk (OR 0.17, 95%CI 0.17–0.43), although not mortality, or gastro-intestinal carriage with MDR.70,71
The implementation of SDD and SOD requires the collaboration of several hospital departments, such as critical care, microbiology and pharmacy and neither the antimicrobial paste nor the oral solution is commercially available. Therefore, although the panel considered the quality of the evidence favoring SDD to be “high” and strongly recommends its use, it was not categorized as a basic mandatory measure.
In order to facilitate its implementation, the instructions for manufacture, administration and surveillance are provided.
- 2.
Continuous aspiration of subglottic secretions (CASS). A meta-analysis73 found that CASS significantly reduces early-onset VAP (EO-VAP) in patients exceeding 3 days of intubation (risk ratio for all episodes 0.51, 95%CI 0.37–0.71, for EO-VAP 0.38, 95%CI 0.16–0.88), although it does not prevent colonization or infection of the respiratory tract with Enterobacteriaceae or Pseudomonas aeruginosa. The duration of mechanical ventilation and that of ICU stay were reduced by 2 days (95%CI 1.7–2.3) and 3 days (95%CI 2.1–3.9), respectively, with no effect on mortality. A recent randomized trial in patients undergoing a major heart surgery74 was negative. A post hoc sub-group analysis of patients ventilated more than 48h found significant reductions of cumulative incidence of VAP (26.7% vs. 47.5%, relative risk 0.40, 95%CI 0.16–0.99; p=0.04), ICU length-of-stay (7 vs. 16.5 days, p=0.01) and antibiotic use, with no effect on mortality. No adverse events attributed to CASS have been reported in humans, although evidence of widespread injury to tracheal mucosa and submucosa was documented after 72h of CASS in sheep.75
As for SDD, CASS is not widely available and expensive. Instructions for its use are provided.
- 3.
Short course (2–3 days) of systemic antibiotic therapy. A short course of intravenous cephalosporin was added to topical antibiotics in the SDD protocol in order to prevent primary endogenous, mainly respiratory tract, infection in severe trauma patients.76,77 This measure has been evaluated separately for the prevention of VAP, without concomitant administration of topical antimicrobials, and was therefore included in the bundle for those ICUs not implementing the complete SDD protocol.
Patients with decreased level of consciousness are particularly at a high risk of primary endogenous VAP, typically including severe trauma, severe head trauma, stroke, cardiac arrest and metabolic or drug-related central nervous system depression. A small randomized controlled trial78 showed a significant reduction of the incidence of VAP from 36% to 18% associated with the administration of only 2 doses of cefuroxime 1.5g/12h. In a double-blind randomized multicentre trial, 3 doses of ceftriaxone 2g/24h were associated with a significant reduction of primary endogenous VAP from 51.3% to 14.3%.79 Intravenous antibiotics have shown to be protective50,80 and are therefore recommended, similar to surgical prophylaxis, in the aforementioned patient populations, although they do not reduce late infections, morbidity or mortality.
In patients with decreased level of consciousness, a short 48–72h course of intravenous cefuroxime, ceftriaxone or amoxicillin-clavulanate should be considered.
DiscussionThe last decade has witnessed the publication of rigorous and comprehensive guidelines for the prevention of VAP, generated with ever-improving and transparent methodologies,8,10,49,9,11–15 which have provided detailed reviews of the literature and thoughtful recommendations. The Spanish “Zero VAP” Project, developed under the leadership of SEMICYUC, has several additional differential features, which in our opinion extends the value of this initiative beyond that of its 7 “basic” and 3 “highly recommended” or other published “conventional” guidelines.
Beyond the usual physician-led recommendations, this project was developed from its earliest phases in collaboration with the Spanish critical care nurses (SEEIUC), who participated actively in all theoretical and practical aspects of its design, training, implementation, adherence, compliance, quality assurance and coordination. The active role of the nurses and their identification with the initiative were considered to be an essential element for success.
“Zero-VAP” is an interventional program incorporated into a firmly established observational ICU infection surveillance program, ENVIN-HELICS,3 which the Working Group for Infectious Diseases of SEMICYUC initiated in 1994. ENVIN-HELICS yearly collects data from ICU patients staying>24h and who are admitted during the surveillance period, which spans from April 1st to June 30th. The ever increasing number of participating ICUs reached 173 in 2012. The huge amount of epidemiological data and surveillance experience accumulated over 18 years in 150,000 patients by Spanish intensivists and critical care nurses constitute a solid historical control group providing the adequate baseline reference data. “Zero-VAP” may therefore be viewed as a huge, multicentre, “before-after” study, where the efficacy of a VAP prevention bundle is prospectively evaluated.
The establishment of an organizational structure and contractual commitment involving the Spanish Ministry of Health, regional authorities, hospital directors, and regional and site medical and nurse coordinators is another differential characteristic of “Zero-VAP”, with the objective to guarantee continued adherence and quality. In addition, continuous monitoring of compliance will also allow validating the prevention package and some of its individual measures.
Compared to conventional guidelines, “Zero-VAP” is a bundle of interventions with a pre-defined objective. The reduction of the national VAP incidence rate by 25% and to less than 9 episodes per 1000 days of mechanical ventilation is a simple and clear quantitative target.
Finally, the validation for efficacy and safety of expert recommendations in “real life” situations, even if these are produced by the best of methodologies, seems to be becoming more and more important. It may even be argued that future guidelines should be accompanied by evaluation for adequacy and safety, i.e. internal validity, before widespread implementation of recommendations. Performance of randomized trials before implementation of guidelines has recently been proposed.81 In addition, the results of implementing recommendations, i.e. external validity, should ideally be assessable on a continuous basis, similar to phase IV or post-marketing studies of new antimicrobial compounds, thus allowing for early detection of adverse outcomes. “Zero-VAP”, through its continuous on-line recording of results and incorporated patient safety and quality improvement program, has efficient incorporated tools to detect and correct errors and to implement modifications or improvements, thereby also guaranteeing future adherence to recommendations.
The weaknesses of “Zero-VAP” are related to the “study” design and to the absence of site monitoring. Compared to an ideal multicentre, randomized, control trial, the current project has only a historic control group, and confounding variables cannot be definitively excluded. Also, although pre-defined diagnostic criteria exist, underreporting of VAP episodes may start to occur if during the project, candidate VAP episodes are evaluated with stricter criteria than in previous practice. Although a desirable “fringe benefit” of the project, this effect may falsely improve the results of implementation of the VAP prevention bundle. Unfortunately, the financial support of the project does not cover site monitoring to detect VAP episodes that are not reported but treated as such with antimicrobials. Monitoring of more than 200 ICUs would require allocation of important economic resources.
In summary, we present “Zero-VAP”, the Spanish national VAP prevention bundle, with its organizational structure involving several levels of the healthcare administration, and provide a detailed description of the methodology used for selecting the components of the bundle. Online tools put in place facilitate implementation and adherence and measure compliance and the effect of the bundle on the incidence of VAP in Spanish ICUs. “Zero-VAP” also promotes the development of a culture of safety assurance in ICUs.
FundingThe Spanish Ministry of Health provided financial support for the meetings of the task force.
Conflicts of interestThe authors declare that they have no competing interests.
This manuscript is dedicated to the memory of Dr. María Jesús López Pueyo.
We thank Yolanda Agra (QAA) and the regional healthcare authorities for their commitment, allocation of resources and organizational support of “Zero VAP”. The regional and local medical and nurse coordinators deserve mentioning for their outstanding efforts and commitment. Our thanks go also to the intensivists and critical care nurses of the participating units, whose dedication to the implementation of the proposed measures was decisive in actually making “Zero VAP” possible.