The present study describes our experience with the high-flow humidified nasal cannula (HFNC) versus non-invasive ventilation (NIV) in children with severe acute asthma exacerbation (SA).
MethodsAn observational study of a retrospective cohort of 42 children with SA admitted to a Pediatric Intensive Care Unit (PICU) for non-invasive respiratory support was made. The primary outcome measure was failure of initial respiratory support (need to escalate from HFNC to NIV or from NIV to invasive ventilation). Secondary outcome measures were the duration of respiratory support and PICU length of stay (LOS).
ResultsForty-two children met the inclusion criteria. Twenty (47.6%) received HFNC and 22 (52.3%) NIV as initial respiratory support. There were no treatment failures in the NIV group. However, 8 children (40%) in the HFNC group required escalation to NIV. The PICU LOS was similar in both the NIV and HFNC groups. However, on considering the HFNC failure subgroup, the median length of respiratory support was 3-fold longer (63h) and the PICU LOS was also longer compared with the rest of subjects exhibiting treatment success.
ConclusionsDespite its obvious limitations, this observational study could suggest that HFNC in some subjects with SA may delay NIV support and potentially cause longer respiratory support, and longer PICU LOS.
El objetivo de este estudio es comparar nuestra experiencia con el uso de oxigenoterapia de alto flujo (OAF) frente a la ventilación no invasiva (NIV) en niños con estatus asmático (EA).
MétodosEstudio observacional de una cohorte retrospectiva de 42 niños con EA ingresados en nuestra Unidad de Cuidados Intensivos Pediátricos (UCIP) con soporte respiratorio no invasivo. El objetivo principal del estudio fue valorar el éxito/fracaso del soporte respiratorio inicial (necesidad o no de escalar a un soporte respiratorio superior). El objetivo secundario fue comparar la duración del soporte respiratorio y del ingreso en la UCIP.
ResultadosCuarenta y dos niños cumplieron con los criterios de inclusión. Veinte (47,6%) fueron tratados con OAF y 22 (52,3%) con VNI como soporte respiratorio inicial. No hubo fracaso terapéutico en el grupo VNI, si bien 8 niños (40%) del grupo OAF fueron cambiados a VNI. La duración de la estancia en la UCIP y en el hospital fue similar en ambos grupos NIV y HFNC. Sin embargo, en el subgrupo de fracaso de OAF, la duración del soporte respiratorio (el triple, 63h) y la estancia en la UCIP fueron mucho mayores en comparación con los sujetos que tuvieron éxito en el tratamiento.
ConclusionesEste estudio observacional, con sus evidentes limitaciones, podría sugerir que el uso de HFNC en algunos sujetos con EA puede retrasar el inicio de la VNI y potencialmente causar un soporte respiratorio más prolongado y una mayor estancia en la UCIP.
Severe acute asthma exacerbation (SA) can be a potentially life-threatening condition1 and is a frequent cause of admission to a pediatric intensive care unit (PICU).2 Endotracheal intubation and invasive mechanical ventilation (invasive MV) are associated with a substantial risk of complications.3–6 Therefore, the use of other forms of respiratory support such as non-invasive ventilation (NIV)7 and high flow nasal cannula (HFNC) have been considered in an attempt to avoid mechanical ventilation in SA subjects.
Despite the fact that NIV is widely used in asthma subjects admitted in ICU7–13 only few randomized controlled studies (RCT) have been published on pediatric population.10,13 Nevertheless, there is a strong physiological basis behind the use of NIV in asthma.7
On the other hand, HFNC has become popular as it is easy to use and very well tolerated.14–17 Since last decade, HFNC has been introduced in pediatrics as respiratory support, mainly during seasonal bronchiolitis.18 Currently, HFNC has spread to hospital wards, emergency rooms19–21 and transport services.22
There is an ongoing discussion on the indications of high flow nasal cannula therapy or non-invasive ventilation (NIV) in subjects with acute respiratory failure. The clinical advantages of HFNC have not been established yet and there is a lack of published evidence comparing NIV and HFNC during SA.
The aim of this study is to describe our experience with HFNC and NIV in children with SA admitted to the PICU.
Patients and methodsStudy designThis is a retrospective observational study in children with asthma exacerbation admitted to PICU for respiratory support.
SettingA multidisciplinary Pediatric Intensive Care Unit (PICU) from a tertiary university hospital with 12 beds and 600 admissions per year. Our hospital covers a population of 200,000 children within 0–14 years. In 2014, there were 52,335 visits to the Emergency Department (ED) and 4024 hospitalizations per year. With a prevalence of asthma of 10% in the pediatric population, asthma exacerbations count for approximately 5% of the total number of visits to the ED. Of these children, only 82 (3.3%) needed hospital admission and 21 (0.8%) PICU for management.
PatientsConsecutive sampling of all children from 1.5 to 14 years old admitted to the PICU with the diagnosis of SA, from January 2012 to December 2014. The only exclusion criteria was age below 18 months aiming to exclude bronchiolitis.
Acute exacerbation of asthma was considered as an acute episode of increased work of breathing with wheeze and prolonged expiratory phase in a child with similar previous episodes unresponsive to nebulized bronchodilators, steroids and magnesium sulphate in the ED. Treatment of acute exacerbation of asthma is performed according to our local guideline at Cruces University Hospital (Supplementary material). Our subjects are admitted to PICU if there is no response to appropriate therapy in the ED, if the frequency of required aerosol treatments was greater than what could be administered on the ward (usually hourly), or if the patent was deteriorating significantly despite appropriate therapy.
The initial support with HFNC or NIV was decided by the attending PICU physician. The decision relayed solely on the physician's self-confidence with the technique. In subjects receiving NIV, full face masks (Respironics PerforMax, Philips, Netherlands) or oronasal masks (Respironics PerformaTrak, Philips, Netherlands) were used as interfaces, and BiPAP vision or V60 (Respironics Philips, Philips, Netherlands) were used as mechanical ventilators.
Initially, Inspiratory Positive Airway Pressure (IPAP) of 8cmH2O and End-positive Airway Pressure (EPAP) of 4cmH2O were set up to achieve a tidal volume of 6–9ml/kg. Inspiratory and expiratory pressure was titrated in increments of 2cmH2O based on tidal volume, continuous pulse oximetry, work of breathing, respiratory rate and subject-ventilator synchrony. The EPAP was limited to 5cmH2O unless an improvement in PaO2 or pulse oximetry was proven with higher levels of EPAP. Fraction of inspired oxygen (FIO2) was also titrated to maintain a SpO2>92%.
In subjects receiving HFNC, a cannula of suitable size, an appropriate circuit, humidifier and air/oxygen blender were selected. Cannula size was selected based on subject weight (Fisher&Paykel OPT316 infant or OPT318 pediatric for infants and children up to 12.5kg with maximum flow 20–25L/min, and Fisher&Paykel size S OPT542, size M OPT544, size L OPT546 adult cannula in children >12.5kg). The circuit was also selected based on subject weight (Fisher&Paykel RT 329 small volume circuit tubing for children <12.5kg and Fisher&Paykel RT203 adult circuit tubing for children ≥12.5kg). Each HFNC system has a humidifier (MR850, Fisher&Paykel, Auckland, New Zealand). Flow rates were also adjusted to body weight: 2L/kg/min for the first 10kg18+0.5L/kg/min for each kg above that (maximum flow 50L/min).23
Failure of initial support was considered if subject respiratory condition did not improve or even worsened according to the clinical judgment of the attending physician. In this situation, change to a higher level of respiratory support was carried out. Subjects receiving HFNC would be switched to NIV and those receiving NIV would be changed to invasive MV.
Study-related assessment of subject status and data collectionThe following information was extracted from subject medical records: age, gender, weight, treatment prior to PICU, score Wood–Downes, heart rate (HR), respiratory rate (RR), fraction of inspired oxygen (FIO2), pH, and PCO2. Therapy-related complications were also recorded. Blood gas analysis was not routinely performed.
Subjects were classified into two groups, taking into account the respiratory support initially applied by the attending physician, i.e. intention to treat analysis:
- (a)
NIV cohort: subjects receiving NIV.
- (b)
HFNC cohort: subjects receiving HFNC.
In a secondary subanalysis, subjects were classified into three categories based on the mode of support finally received, i.e. per protocol analysis:
- a.
NIV group: subjects receiving NIV. For the analysis, this was considered the reference category.
- b.
HFNC-success group: subjects receiving HFNC successfully.
- c.
HFNC-failure group: subjects who initially received HFNC, but they failed and were changed to NIV.
In this study, primary outcome was failure of the initial respiratory support, defined as need to change to a higher level of support (HFNC to NIV, NIV to invasive MV). Secondary outcomes assessed were the duration of respiratory support, and PICU length of stay. The follow-up period was extended until the respiratory support was fully weaned.
Statistical methodsAll data were managed with a relational database (MS Access for Windows). Categorical variables were described as percentages. Continuous variables if normal (Shapiro–Wilks, p>0.05) were expressed as mean±standard deviation, if not as median with 25th–75th percentile. Statistical significance was considered with p-value <0.05. Bivariate analysis for categorical variables was made with χ2 test or Fisher's exact test. For continuous data, Student t test or Mann–Whitney U test were used as appropriate. Survival times are described by medians and the Kaplan Meier graphic method, meanwhile bivariate analysis was done with log-rank test.
For the multivariate analysis, a logistic regression model is adjusted in binary outcome variables, whereas a Cox proportional hazards model is adjusted in survival times. Adjustment was made according to the Akaike information criterion in both cases. Presence of interactions was initially assessed and proportionality of hazards has been tested graphically, being the result expressed as odds ratios or hazard ratio and their 95% confidence intervals. The discriminatory power of logistic regression model was measured by the area under the ROC curve (trapezoidal method). Its statistical significance was evaluated with De Long test. Its diagnostic accuracy was established with the Sensitivity, Specificity, and Likelihood Ratios computed for the optimal cut-off settled closest to the left-upper corner of the ROC curve.
Ethical issuesThe study was approved by the hospital institutional review board (IRB). The ethics committee waived the need for consent in this retrospective review of medical records.
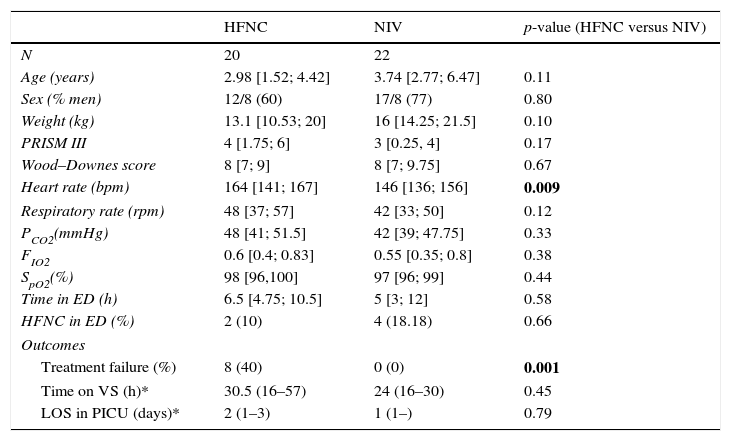
ResultsForty-two children met the inclusion criteria and were included in the analysis. Twenty were in the HFNC cohort and 22 were in the NIV cohort. The baseline characteristics of the total sample, as well as the two cohorts are shown in Table 1. Age, gender, weight, PRISM III score, Wood–Downes score, respiratory rate, pH, PCO2, and oxygen saturation at admission, were similar between the two groups. The only significant difference was heart rate with a median (p25–p75) of 164 (141–167) in the HFNC cohort versus 146 (136–156) in the NIV cohort (p=0.009). No children had comorbidities. Apart from an episode of subcutaneous emphysema, no major complications were reported. None of the subjects required intubation. All patients survive.
Baseline characteristics prior to respiratory support and outcomes.
| HFNC | NIV | p-value (HFNC versus NIV) | |
|---|---|---|---|
| N | 20 | 22 | |
| Age (years) | 2.98 [1.52; 4.42] | 3.74 [2.77; 6.47] | 0.11 |
| Sex (% men) | 12/8 (60) | 17/8 (77) | 0.80 |
| Weight (kg) | 13.1 [10.53; 20] | 16 [14.25; 21.5] | 0.10 |
| PRISM III | 4 [1.75; 6] | 3 [0.25, 4] | 0.17 |
| Wood–Downes score | 8 [7; 9] | 8 [7; 9.75] | 0.67 |
| Heart rate (bpm) | 164 [141; 167] | 146 [136; 156] | 0.009 |
| Respiratory rate (rpm) | 48 [37; 57] | 42 [33; 50] | 0.12 |
| PCO2(mmHg) | 48 [41; 51.5] | 42 [39; 47.75] | 0.33 |
| FIO2 | 0.6 [0.4; 0.83] | 0.55 [0.35; 0.8] | 0.38 |
| SpO2(%) | 98 [96,100] | 97 [96; 99] | 0.44 |
| Time in ED (h) | 6.5 [4.75; 10.5] | 5 [3; 12] | 0.58 |
| HFNC in ED (%) | 2 (10) | 4 (18.18) | 0.66 |
| Outcomes | |||
| Treatment failure (%) | 8 (40) | 0 (0) | 0.001 |
| Time on VS (h)* | 30.5 (16–57) | 24 (16–30) | 0.45 |
| LOS in PICU (days)* | 2 (1–3) | 1 (1–) | 0.79 |
HFNC=high-flow nasal cannula; NIV=non-invasive ventilation; SpO2=saturation measured via pulse oximetry; PCO2=carbon dioxide tension; FIO2=fraction of inspired oxygen; ED=Emergency Department. Continuous variables are expressed in median [Perc 25, Perc 75], except (*) in median (95% CI).
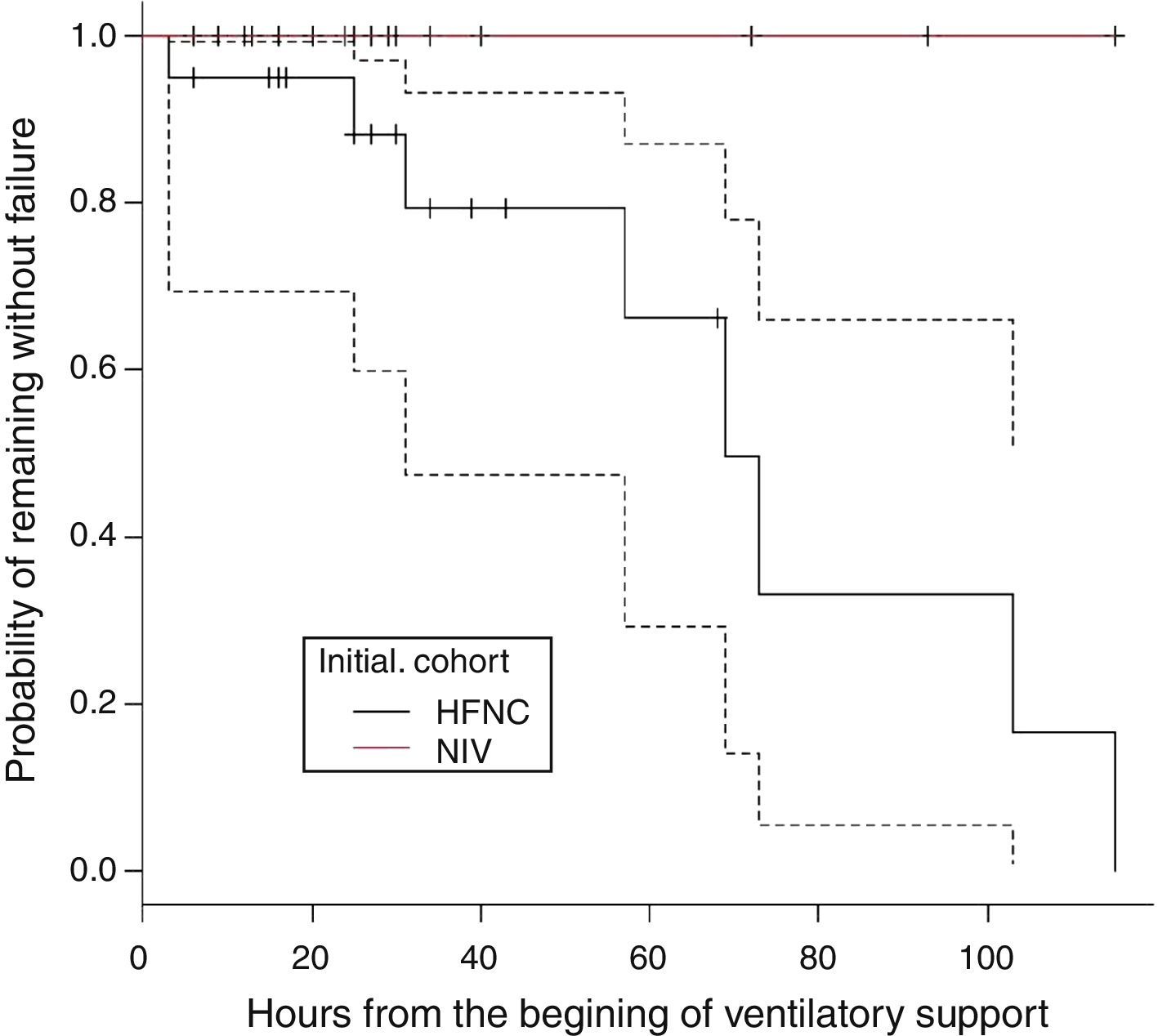
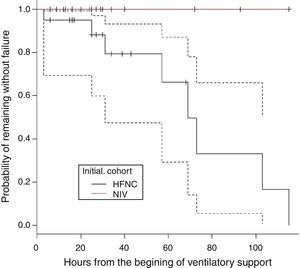
There were 22 subjects in the initial mode of NIV. The respiratory support mode in this group was bi-level positive pressure in 93.5% and in one subject CPAP. The mean EPAP was 5 (4–7) and the mean IPAP was 12 (8–17). There was no treatment failure in this group. In the HFNC group, treatment failure occurred in 8 subjects. These subjects were changed to NIV with clinical improvement observed in all cases. Therefore, the need to change to a higher respiratory support in HFNC group was 40% (8/20), whereas no subject in the group NIV required higher respiratory support (p=0.001). Fig. 1 shows the probability of remaining free from treatment failure.
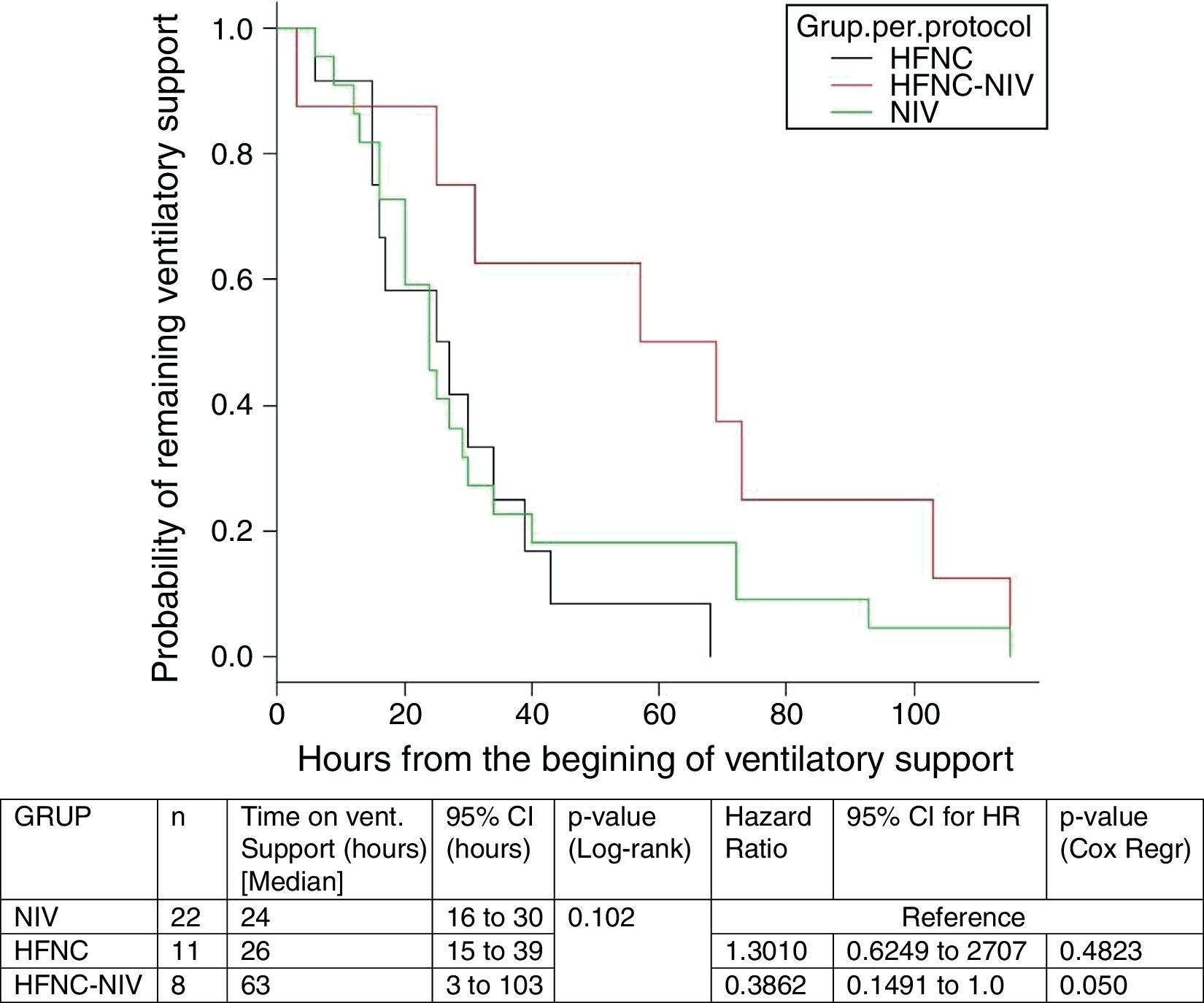
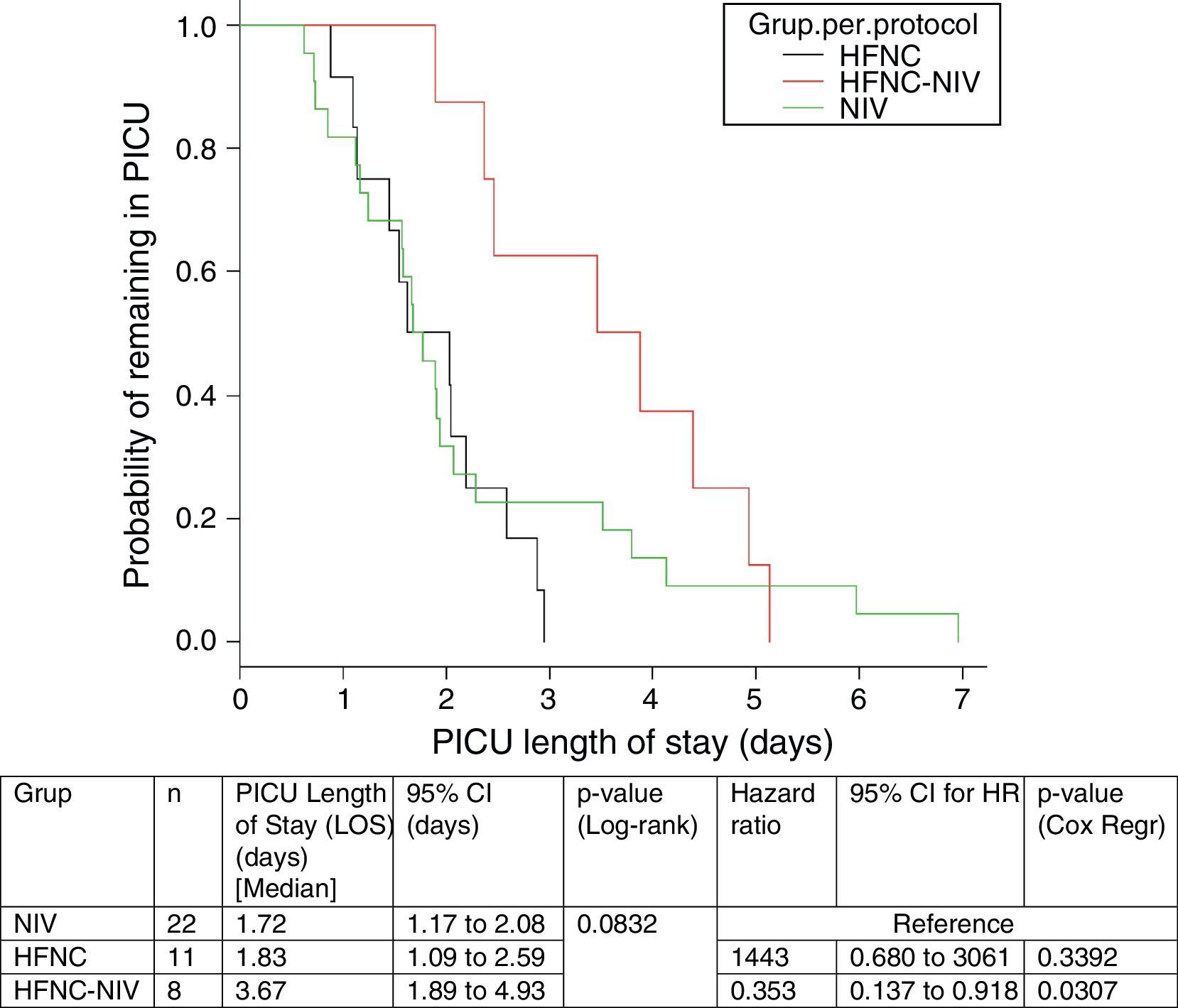
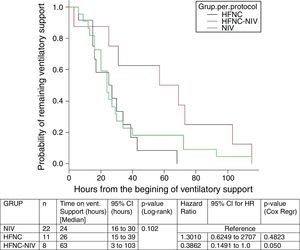
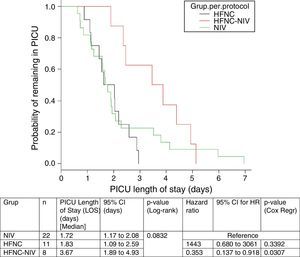
In the subgroup analysis, the mean length of ventilator support and PICU LOS were similar in the groups with no failure. However, the Cox model shows that overall length of ventilatory support was three fold higher in HFNC-failure group as compared to NIV group. Figs. 2 and 3 show the length of ventilatory support (hours) and PICU LOS (days) for the three final (per protocol) groups.
A multivariate logistic regression analysis was performed to identify factors associated with treatment failure in children receiving respiratory support. Heart rate was introduced into the model, in order to avoid confusion because at first both groups were different in relation to this variable. Independent contribution of other variables was tested, and only the heart rate and respiratory rate measured prior to ventilatory support remained in the final model, as shown in Table 2.
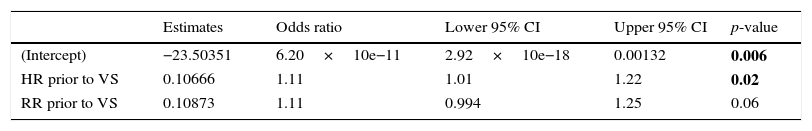
Logistic regression model. Outcome:treatment failure.
| Estimates | Odds ratio | Lower 95% CI | Upper 95% CI | p-value | |
|---|---|---|---|---|---|
| (Intercept) | −23.50351 | 6.20×10e−11 | 2.92×10e−18 | 0.00132 | 0.006 |
| HR prior to VS | 0.10666 | 1.11 | 1.01 | 1.22 | 0.02 |
| RR prior to VS | 0.10873 | 1.11 | 0.994 | 1.25 | 0.06 |
Deviance=24.662; Akaike information criteria=30.662; VS=ventilatory support; HR=heart rate; RR=respiratory rate.
The model introduces a highly good discriminatory power: Area under ROC curve (AUC)=0.90 [95% CI: 0.81–0.99 (DeLong)]. And for an optimal threshold of p>0.22, its diagnostic accuracy is excellent: Sensitivity=0.87 [95% CI=0.47–0.99], Specificity=0.82 [95% CI=0.65–0.93], likelihood ratio of a positive test=4.95 [95% CI=2.29–10.73] and likelihood ratio of a negative test=0.15 [95% CI=0.02–0.95]. In our population, this logistic model shows that a HR below 146bpm and a RR below 55bpm would predict HFNC success whereas in the face of a HR above 164bpm, only a RR below 37 would predict HFNC success.
DiscussionIn this study we present our experience with HFNC and NIV for SA in PICU. Even though both therapies are broadly used in asthmatic children all over the world, the lack of literature comparing them is significant. We have not found any randomized controlled trials on this topic and, to our knowledge; this is the first retrospective study looking at both ways of respiratory support in pediatric asthma.
Our hospital covers a population of 200,000 children within 0–14 years with a prevalence of asthma of 10%. Being asthma such a common disease in children, it is mostly managed by general pediatricians in the communities with very good rates of treatment compliance and success. However, SA is still a common presentation to the ED. Only a small percentage of these children fail to respond to medical treatment and require admission to PICU for further management. Increased use of NIV has been associated with less invasive MV and shorter hospital LOS.2,8,10,12,24 Several RCTs have proven NIV efficacy in decreasing work of breathing when used as an adjuvant to medical therapy with nebulized bronchodilator and anti-inflammatory therapy.2,25,26 In Spain, despite the lack of published data, there is a long standing tradition on the use of NIV in pediatric asthmatic subjects with good clinical results and very low rates of intubation.11 In our study 30 subjects were supported by NIV and no one was intubated. In terms of HFNC in asthma, there is also significant paucity of evidence. Kelly et al. described the largest observational study to date, which included 38 children younger than 2 years admitted with status asthmaticus.20 In this study the authors identified three variables associated with increased risk for intubation following HFNC trial: triage RR greater than 90th percentile for age (OR, 2.11; 95% CI, 1.01–4.43), initial venous PCO2 greater than 50mmHg (OR, 2.51; 95% CI, 1.06–5.98), and initial venous pH less than 7.30 (OR, 2.53; 95% CI, 1.12–5.74). We identify heart rate and respiratory rate prior to respiratory support as factors related to failure of HFNC. Also Abboud et al. identified the absence of a reduction in PCO2 as a predictive factor of HFNC failure in children affected by bronchiolitis.27 On the other hand, HFNC has been shown useful in hypoxemic, not hypercapnic subjects (PaCO2<45mmHg) requiring FIO2>0.4.28
We compared two groups of children treated with HFNC or NIV for SA in the PICU. Except for the heart rate, which was higher in the group of HFNC, the two groups in our study were similar at the time of PICU admission in terms of age, weight, PRISM III, Wood–Downes score and FIO2. The reason why HR was higher in the HFNC cannot be determined retrospectively.
We found favorable outcomes in the initial cohort of NIV. The absence of children requiring intubation could be interpreted as less severity of the illness. If we consider that most children with asthma are intubated for “severe work of breathing” or “exhaustion”, our subjects had a mean Wood–Downes score of 8 which is an indicator of severity. Also, we think that the fact that NIV is initiated relatively early in the course of SA management may play a role in preventing the need of invasive mechanical ventilation.
An interesting finding in our study is that in the HFNC group, 8 subjects (40%) needed to be escalated to NIV. Subjects in the HFNC-success group showed similar results to those in the NIV group but those in the HFNC-failure group had a three-fold longer time of respiratory support (p=0.05), compared with the initial NIV group. In addition, the PICU LOS was higher compared with the same group.
Even though causality cannot be established with our study, it is not unreasonable to think that HFNC, which is a lower level of support, could only be beneficial in selected subjects and that it could delay the initiation of NIV and therefore prolong PICU and hospital LOS.
We performed a multivariate logistic regression analysis to identify factors associated with treatment failure in children receiving respiratory support. Only heart rate and respiratory rate measured prior to ventilatory support remained in the final model. According to it, we noticed that children with heart rate less than 146bpm and respiratory rate less than 55bpm, HFNC would probably not fail. When heart rate is greater than 164bpm, HFNC would only be potentially successful in those children with RR less than 37rpm. In other words, in the most severely affected pediatric asthma subjects, it is highly likely that HFNC will fail if it is the initial mode of respiratory support applied. Validation of this study, in a randomized controlled trial with a larger sample, would help to identify a more reliable cut-off value which could guide clinicians on an appropriate algorithm to choose one or the other treatment.
Failure criteria have been described in both groups (NIV and HFNC).29 The respiratory support failure is associated with increased mortality. NIV is more likely to fail in hypoxemic subject30 and HFNC in hypercapnic subject.20 It has also been pointed out that their use may delay intubation.31 Because of low sample size and absence of intubation rate, we were not able to compare the differences in mortality or need for intubation.
Several limitations to our study must be mentioned. On the one hand, the sample size is small and there are no subjects in the NIV failure group limiting the value of our findings. On the other hand, this is a retrospective study with lack of randomization. Also, both initial therapy as well as treatment failure were determined by the attending physician preference. Therefore, the potential for bias is significant in terms of physician preference of one technique over the other or when to consider treatment failure. We tried to manage these confounding factors by using multivariate analysis. However, sample size and the fact that one of the groups had different initial HR limits possible comparisons between groups.
Since this audit, our current therapeutic approach, as other researchers reported,17 is to use HFNC in the Emergency Department in children with mild to moderate SA. Once decision is made to admit the child to PICU, NIV is initiated.
ConclusionsIn this study comparing NIV with HFNC for SA in children, we observed that early initiation of NIV in association with bronchodilators and systemic steroids is a safe and feasible initial alternative for the treatment of SA. However, more severe cases HFNC could potentially delay the initiation of NIV resulting in a longer stay in PICU, and the consequent increase in morbidity and costs.
Author's contributionJavier Pilar: Study design and writing.
Vicente Modesto i Alapont: Analysis of data.
Yolanda M. López-Fernández: Manuscript preparation.
Olaia López-Macias: Data collection.
Diego Garcia Urabayen: Literature search.
Irene Amores Hernández: Review of manuscript.
Conflicts of interestThe authors have not disclosed any potential conflicts of interest.