COVID-19 acute respiratory distress syndrome (ARDS) shares the common histological hallmarks with other forms of ARDS. However, the chronology of the histological lesions has not been well established.
ObjectiveTo describe the chronological histopathological alterations in the lungs of patients with COVID-19 related ARDS.
DesignA prospective cohort study was carried out.
SettingIntensive Care Unit of a tertiary hospital.
PatientsThe first 22 consecutive COVID-19 deaths.
MeasurementsLung biopsies and histopathological analyses were performed in deceased patients with COVID-19 related ARDS. Clinical data and patient course were evaluated.
ResultsThe median patient age was 66 [63–74] years; 73% were males. The median duration of mechanical ventilation was 17 [8–24] days. COVID-19 induced pulmonary injury was characterized by an exudative phase in the first week of the disease, followed by a proliferative/organizing phase in the second and third weeks, and finally an end-stage fibrosis phase after the third week. Viral RNA and proteins were detected in pneumocytes and macrophages in a very early stage of the disease, and were no longer detected after the second week.
LimitationLimited sample size.
ConclusionsThe chronological evolution of COVID-19 lung histopathological lesions seems to be similar to that seen in other forms of ARDS. In particular, lung lesions consistent with potentially corticosteroid-sensitive lesions are seen.
El síndrome de dificultad respiratoria aguda (SDRA) asociado a la COVID-19 comparte características histológicas con otros tipos de SDRA. Sin embargo, no se ha establecido adecuadamente la cronología de las lesiones histológicas.
ObjetivoDescribir las alteraciones histopatológicas cronológicas en los pulmones de los pacientes con síndrome de dificultad respiratoria aguda asociado a COVID-19.
DiseñoEstudio prospectivo de cohortes.
ÁmbitoUnidad de cuidados intensivos de un hospital terciario.
PacientesLas primeras 22 muertes consecutivas por COVID-19.
IntervencionesSe llevaron a cabo biopsias pulmonares y análisis histopatológicos en pacientes fallecidos por SDRA asociado a COVID-19. Se evaluaron los datos clínicos y la evolución médica.
ResultadosLa mediana de edad de los pacientes fue de 66 (63-74) años y el 73% eran varones. La mediana de la duración de la ventilación mecánica fue de 17 (8-24) días. La lesión pulmonar inducida por COVID-19 se caracterizó por una fase exudativa durante la primera semana de la enfermedad, seguida de una fase proliferativa/organizativa en la segunda y tercera semana y, por último, una fase de fibrosis en fase terminal tras la tercera semana de evolución. Se detectaron proteínas y ARN vírico en neumocitos y macrófagos en una fase muy temprana de la enfermedad, pero estos ya no se volvieron a detectar a partir de la segunda semana.
LimitaciónTamaño limitado de la muestra.
ConclusiónLa evolución cronológica de las lesiones histopatológicas pulmonares asociadas a la COVID-19 parece ser similar a la de otras formas de SDRA. En particular, se observan daños pulmonares coherentes con las lesiones potencialmente sensibles a los corticosteroides.
While most patients only develop a common mild form of COVID-19, approximately 15% of the patients require assisted oxygenation or mechanical ventilation.1 In the lungs, SARS-CoV-2 enters the cells via the angiotensin-converting enzyme 2 (ACE2) expressed in lung alveolar cells (type I and II alveolar epithelial cells), bronchial epithelium and vascular endothelial cells; thus explaining why respiratory tract and lung serve as a primary point of viral entry.2–4 COVID-19 respiratory phenotypes seem to be slightly different from “typical” acute respiratory distress syndrome (ARDS) phenotypes.5–7 Thus, pulmonary radiology studies in COVID-19 showed lesions more similar to organizing pneumonia, especially in the early stages of the disease.8,9 Although several studies have now described clinical, radiographic and pathologic features of COVID-19,10,11 no studies have been conducted on the basis of early biopsies in the deceased. Some of the reasons for the lack of biopsies include suddenness of the outbreak, massive influx of patients in hospitals, shortage of medical staff, and high rate of transmission.
The present study has been designed to assess the chronology of histologic features of SARS-CoV-2-induced pulmonary disease, by analyzing immediate postmortem transthoracic biopsies performed in patients hospitalized in intensive care unit for severe forms of COVID-19. Indeed, in a recent report, Sapino et al. suggested that tissue sampling using biopsies may be an interesting and safer alternative to autopsy for research projects during outbreaks such as COVID-19.12
MethodsStudy designBetween March 25, 2020 and May 2, 2020, all patients who died from ARDS related to COVID-19 were prospectively included in four intensive care units (ICUs) in two centers of a French tertiary hospital in Strasbourg. During their ICU stay, patients were treated in accordance with standard practice and ventilated with protective ventilation to reduce the risk of ventilator induced lung injury (VILI).13 There was no exclusion criterion. Trained intensivists performed lung biopsies at bedside within the hour following death. The area to be biopsied was selected based on the lung injuries on the most recent imaging and the procedure was carried out under ultrasound guidance. All lung biopsies were analyzed at the hospital's Department of Pathology for histopathologic evaluation. Medical conditions and medications, current medical course, and antemortem diagnostic findings were recorded.
The study was approved by the local ethics committee of the University Hospital of Strasbourg (reference CE-2020-34). Approval for the post-mortem biopsies was obtained from the biomedical agency and the French ministry of research.
Histologic examinationTissue samples for histopathologic analysis were immediately fixed in 4% neutral buffered formalin for 24h, which ensures inactivation of the virus, and then processed via standard procedure to slides stained with hematoxylin–eosin (HE), Masson's trichrome, PAS and Perls staining. All the samples were blindly analyzed by an experienced pathologist, trained in pulmonary pathology.
Microscopic examination included a systematic search of the histologic criteria described at the 3 phases of diffuse alveolar damage (DAD), the histological hallmark for ARDS. Capillary congestion, intra-alveolar edema, loose fibrin deposition and hyaline membranes were considered as exudative changes. Type II pneumocyte hyperplasia, alveolar septa thickening with fibroblast proliferation and organizing fibroblastic tissue in air spaces were considered as features of the proliferative/organizing phase. Prominent interstitial collagenous fibrosis with architectural remodeling and/or honeycomb-like change defined the fibrotic phase. Extensive intra-alveolar fibrin deposition forming fibrin “balls” in alveolar ducts and alveoli, coexisting with intraluminal loose connective tissue polyps were histologic criteria of acute fibrinous and organizing pneumonia (AFOP).
Interstitial and intra-alveolar inflammatory infiltrate was analyzed using an automated chromogenic multiplexed immunohistochemistry assay on the Discovery Ultra automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA)14 with four antibodies targeting CD4, CD8, CD20 and CD68. Presence of SARS-CoV-2 virus in the lung tissue sections was evaluated by in situ hybridization (ISH) using RNAscope technology with a SARS-Cov-2 spike probe to COVID-19 coronavirus15 and by immunohistochemistry with a polyclonal Sars nucleocapsid protein antibody (200-401-A50; Rockland Immunochemicals, Inc., USA).16,17
Statistical analysisThe aim of this study is descriptive. No formal hypothesis was implemented to drive the sample size calculation and the maximum number of patients who met the inclusion criteria was included. Descriptive data were expressed as mean (SD) or median (IQR) for continuous variables and categorical variables as number (%). Tests were two-sided with significance set at α less than 0.05. Differences between groups were considered to be significant at a P value of<0.05. Students’ t-test was performed to compare two groups sampled from normal distributions with equal variances. One-way analysis of variance (one-way ANOVA) was used to compare means of more than two groups. Statistical analyses were performed with GraphPad Prism 8 (GraphPad Software, Inc., San Diego, CA).
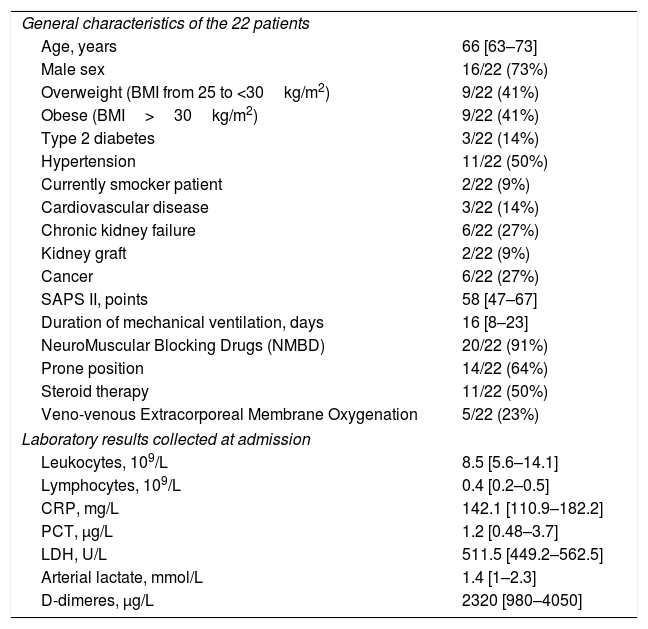
ResultsBaseline characteristics of the patientsA Total of 183 patients were admitted in ICU during this period. Twenty-two patients were included in this study. Median age was 66 [63–74] years. 16/22 (73%) patients were male. Predominant medical history of these patients was overweight and obesity. Few patients were active smokers. Table 1 provides an overview of initial laboratory results. Table 2 provides an overview of ventilatory parameters at admission and at time of death.
Baseline characteristics of the 22 patients.
| General characteristics of the 22 patients | |
| Age, years | 66 [63–73] |
| Male sex | 16/22 (73%) |
| Overweight (BMI from 25 to <30kg/m2) | 9/22 (41%) |
| Obese (BMI>30kg/m2) | 9/22 (41%) |
| Type 2 diabetes | 3/22 (14%) |
| Hypertension | 11/22 (50%) |
| Currently smocker patient | 2/22 (9%) |
| Cardiovascular disease | 3/22 (14%) |
| Chronic kidney failure | 6/22 (27%) |
| Kidney graft | 2/22 (9%) |
| Cancer | 6/22 (27%) |
| SAPS II, points | 58 [47–67] |
| Duration of mechanical ventilation, days | 16 [8–23] |
| NeuroMuscular Blocking Drugs (NMBD) | 20/22 (91%) |
| Prone position | 14/22 (64%) |
| Steroid therapy | 11/22 (50%) |
| Veno-venous Extracorporeal Membrane Oxygenation | 5/22 (23%) |
| Laboratory results collected at admission | |
| Leukocytes, 109/L | 8.5 [5.6–14.1] |
| Lymphocytes, 109/L | 0.4 [0.2–0.5] |
| CRP, mg/L | 142.1 [110.9–182.2] |
| PCT, μg/L | 1.2 [0.48–3.7] |
| LDH, U/L | 511.5 [449.2–562.5] |
| Arterial lactate, mmol/L | 1.4 [1–2.3] |
| D-dimeres, μg/L | 2320 [980–4050] |
Data are n (%) or mean (SD) unless otherwise stated. SAPS 2, simplified acute physiological score; CRP, C-reactive protein; PCT, procalcitonin; LDH, lactate dehydrogenase.
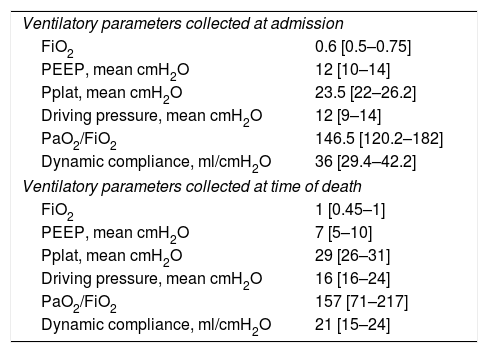
Ventilatory parameters at admission and at time of death.
| Ventilatory parameters collected at admission | |
| FiO2 | 0.6 [0.5–0.75] |
| PEEP, mean cmH2O | 12 [10–14] |
| Pplat, mean cmH2O | 23.5 [22–26.2] |
| Driving pressure, mean cmH2O | 12 [9–14] |
| PaO2/FiO2 | 146.5 [120.2–182] |
| Dynamic compliance, ml/cmH2O | 36 [29.4–42.2] |
| Ventilatory parameters collected at time of death | |
| FiO2 | 1 [0.45–1] |
| PEEP, mean cmH2O | 7 [5–10] |
| Pplat, mean cmH2O | 29 [26–31] |
| Driving pressure, mean cmH2O | 16 [16–24] |
| PaO2/FiO2 | 157 [71–217] |
| Dynamic compliance, ml/cmH2O | 21 [15–24] |
Data are n (%) or mean (SD) unless otherwise stated. PEEP, positive end-expiratory pressure; Pplat, plateau pressure.
Median SAPS II and SOFA scores on admission in ICU were respectively 58 [47–67] and 11 [8–15]. The median duration of mechanical ventilation was 17 [8–24] days. 20/22 patients (91%) received neuromuscular blocking drugs (NMBD) with a median of 8 [6–15] days. 11/22 patients (50%) received inhaled nitric oxide. 13/22 patients (59%) developed ventilator-associated pneumonia (VAP). 11/22 (50%) patients received corticosteroids for a median of 9 [7–10] days. Median dynamic respiratory system compliance at death was 21 [15–24]mL/cmH2O. 5/22 patients (23%) received veno-venous extracorporeal membrane oxygenation (VV-ECMO). 8/22 patients (36%) developed a pulmonary embolism.
Patients were stratified according to the delay since the beginning of clinical criteria for ARDS related to COVID-19: 4 (18%) patients with severe pneumonia for less than 1 week, 11 (50%) patients between 1 and 3 weeks, and 7 (32%) patients for more than 3 weeks.
Regarding the four patients who died during the first week, one died of cardiac arrest induced by tension pneumothorax and three had limit in therapeutic effort (one because of Ewing Sarcoma and multiple myeloma, one because of acute promyelocytic leukemia with stroke and one because of multiple organ failure due to COVID-19 and a staphylococcus aureus VAP).
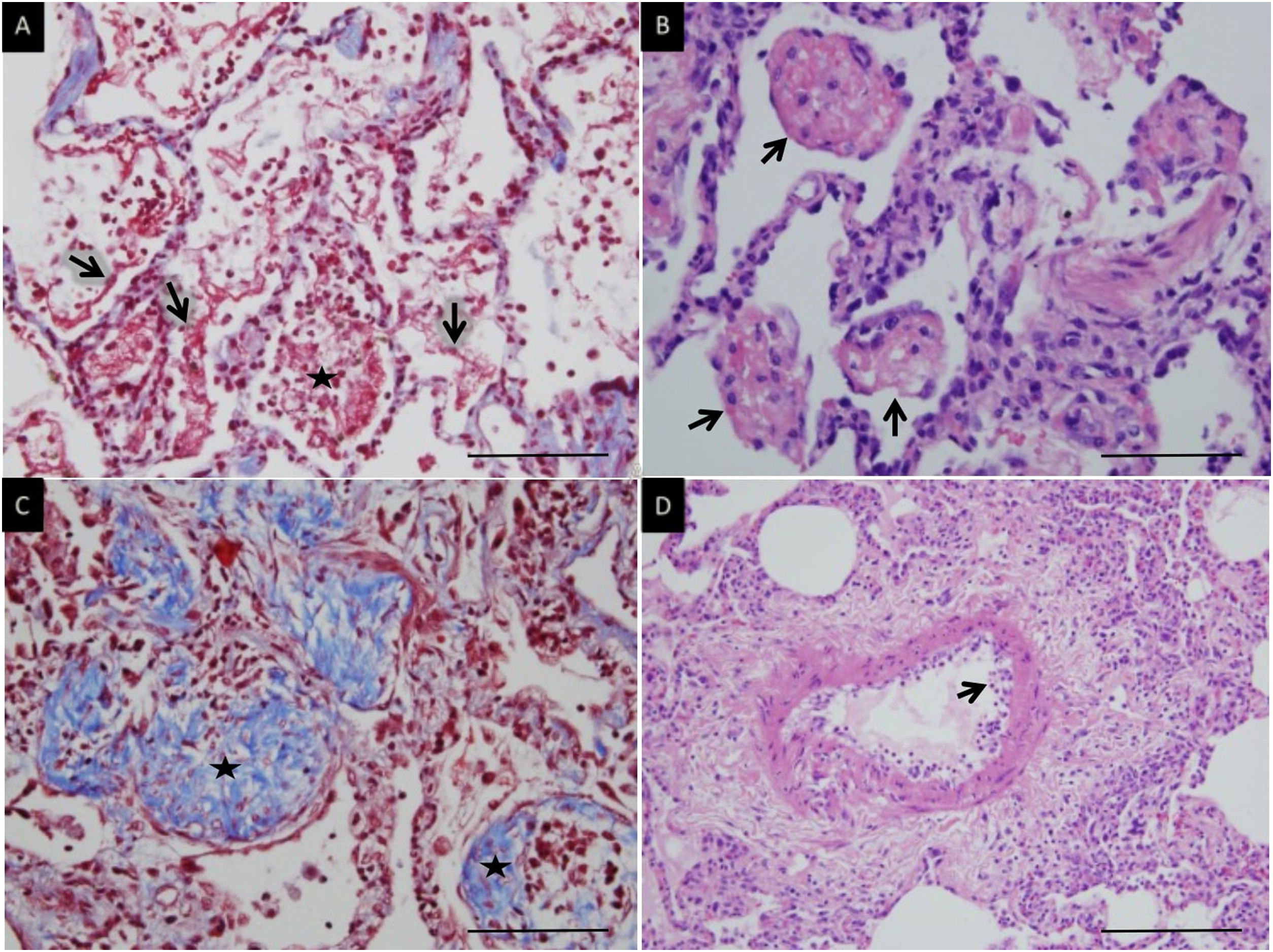
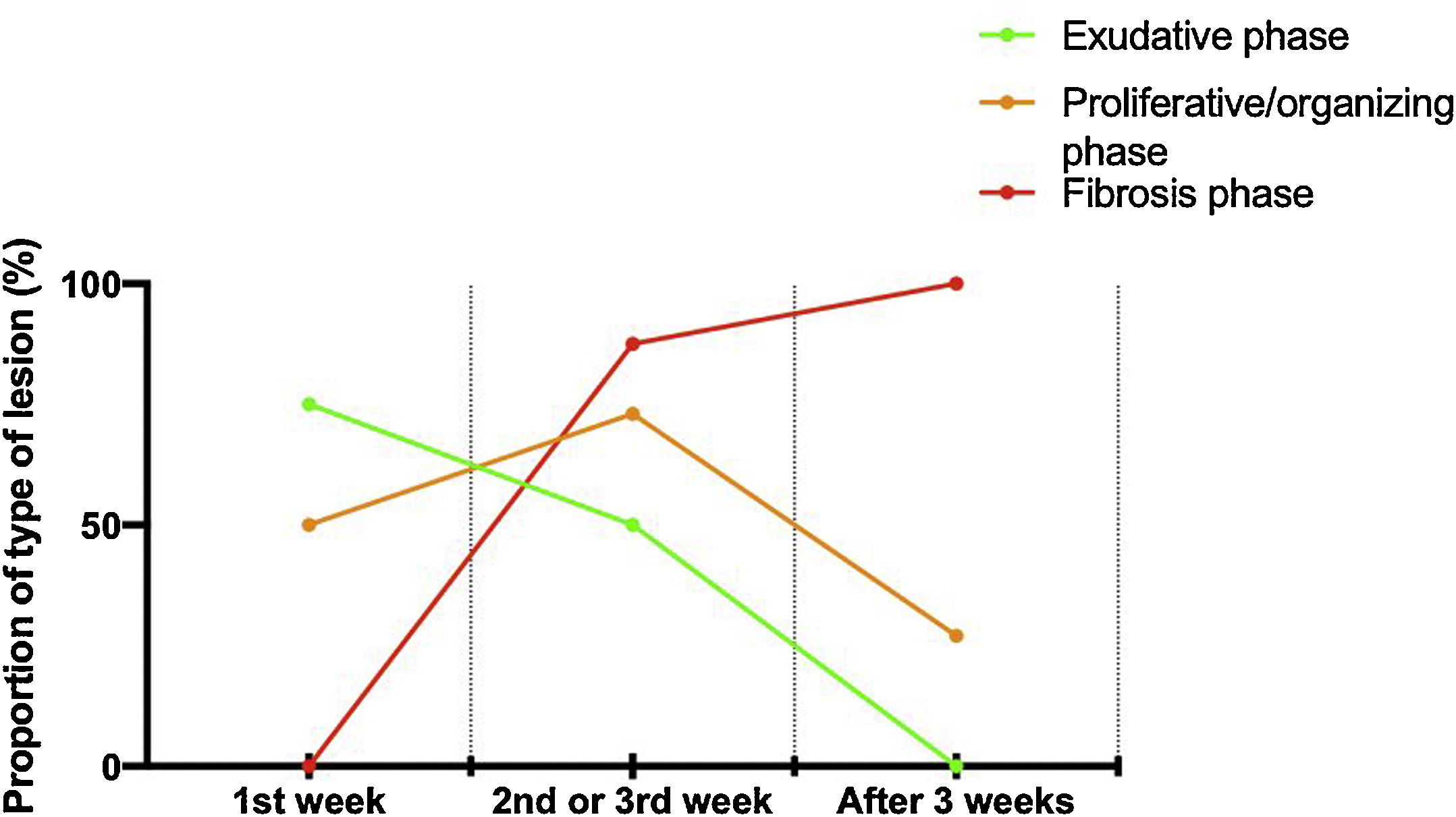
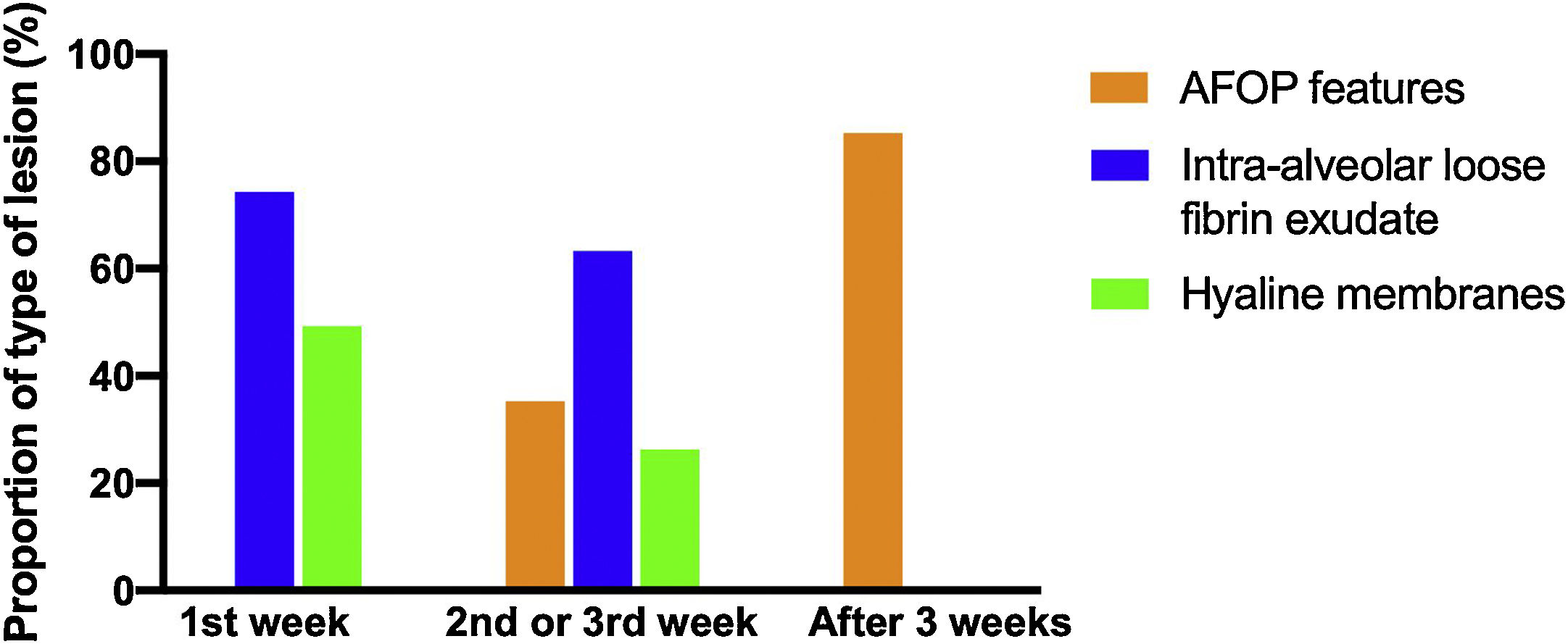
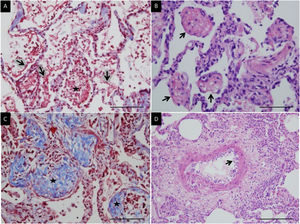
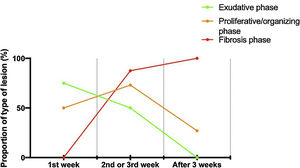
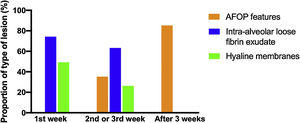
Histologic patternsThe average number of lung tissue probes obtained by needle biopsies from different lobes was 9.4. As expected, histologic features of exudative, proliferative and fibrotic phase of DAD (Fig. 1 shows different histological findings) were observed. Time to onset of histological lesions was closely related to the duration of evolution of ARDS related to COVID-19 (Fig. 2). Exudative changes were present in 3 of the 4 patients (75%) within the first week of ARDS related to COVID-19, 8 of the 11 patients (72%) within the second and third weeks, and none of the 7 patients with more than 3 weeks of evolution (p<0.01; Fig. 2). Intra-alveolar loose fibrin exudate and hyaline membranes gradually decreased over weeks (p=0.006 and p=0.01 respectively; Fig. 3).
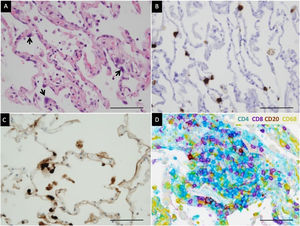
Microscopic lung findings in Covid-19. (A) Loose intra-alveolar fibrin exudates (arrow), admixed with inflammatory cells (star). Masson's trichrome. (B) Intra-alveolar “fibrin balls” (arrow). Hematoxylin-eosin staining. (C) Intra-alveolar loose fibrous plugs (star) of organizing pneumonia. Masson's trichrome. (D) Endotheliitis (arrow) in a small arterial vessel. Hematoxylin–eosin staining. The scale bar corresponds to 50μm for A, B and C, and 100μm for D.
By contrast, the incidence of proliferative changes increased over time and were reported in 2/4 patients (50%) within the first week, 8/11 patients (73%) within the 1–3 weeks, and 6/7 patients (88%) after the third week (Fig. 2). Hyperplasia of type II pneumocytes mostly with cytologic atypia was noted in 17 of the 19 patients (89%) presenting proliferative changes.
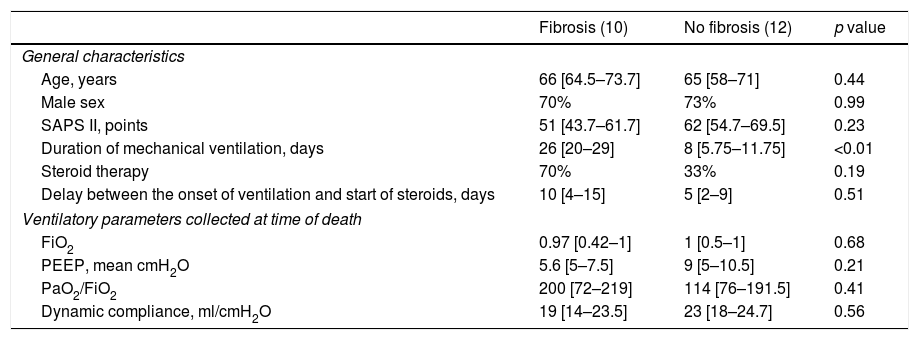
The presence of end-stage fibrosis increased over time: the pattern appeared in none of the 4 patients with disease for less than 1 week, in 3 of the 11 patients (27%) with disease for 1–3 weeks, and in all (100%) of the patients with a disease of more than 3 weeks (p<0.01; Fig. 2). Patients with end-stage fibrosis had been on mechanical ventilation for a longer period of time than patients without fibrosis (Table 3). It should be noted that histological criteria of more than one phase coexisted in 16 of 22 patients (73%).
Characteristics of 22 patients with or without fibrosis at histological examination.
| Fibrosis (10) | No fibrosis (12) | p value | |
|---|---|---|---|
| General characteristics | |||
| Age, years | 66 [64.5–73.7] | 65 [58–71] | 0.44 |
| Male sex | 70% | 73% | 0.99 |
| SAPS II, points | 51 [43.7–61.7] | 62 [54.7–69.5] | 0.23 |
| Duration of mechanical ventilation, days | 26 [20–29] | 8 [5.75–11.75] | <0.01 |
| Steroid therapy | 70% | 33% | 0.19 |
| Delay between the onset of ventilation and start of steroids, days | 10 [4–15] | 5 [2–9] | 0.51 |
| Ventilatory parameters collected at time of death | |||
| FiO2 | 0.97 [0.42–1] | 1 [0.5–1] | 0.68 |
| PEEP, mean cmH2O | 5.6 [5–7.5] | 9 [5–10.5] | 0.21 |
| PaO2/FiO2 | 200 [72–219] | 114 [76–191.5] | 0.41 |
| Dynamic compliance, ml/cmH2O | 19 [14–23.5] | 23 [18–24.7] | 0.56 |
Data are n (%) or mean (SD) unless otherwise stated. SAPS 2, simplified acute physiological score; PEEP, positive end-expiratory pressure.
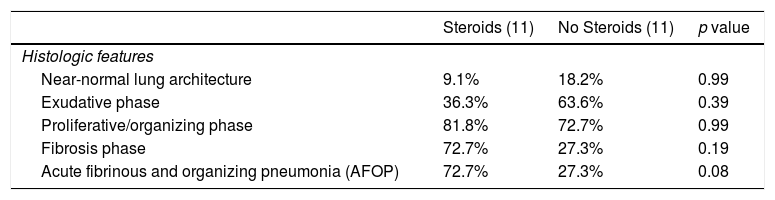
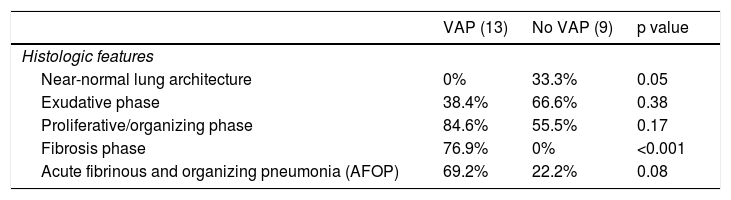
No differences in histologic findings were observed between patients who received steroids and those who did not (Table 4). Regarding patients who developed a VAP and those who did not, there was significantly more fibrosis in the patients who developed VAP (Table 5).
Histologic features of 22 patients who received steroids or not.
| Steroids (11) | No Steroids (11) | p value | |
|---|---|---|---|
| Histologic features | |||
| Near-normal lung architecture | 9.1% | 18.2% | 0.99 |
| Exudative phase | 36.3% | 63.6% | 0.39 |
| Proliferative/organizing phase | 81.8% | 72.7% | 0.99 |
| Fibrosis phase | 72.7% | 27.3% | 0.19 |
| Acute fibrinous and organizing pneumonia (AFOP) | 72.7% | 27.3% | 0.08 |
Data are expressed as the percentage (%) of patients within each subpopulation.
Histologic features of 22 patients who developed a ventilator-associated pneumonia (VAP) and those who did not.
| VAP (13) | No VAP (9) | p value | |
|---|---|---|---|
| Histologic features | |||
| Near-normal lung architecture | 0% | 33.3% | 0.05 |
| Exudative phase | 38.4% | 66.6% | 0.38 |
| Proliferative/organizing phase | 84.6% | 55.5% | 0.17 |
| Fibrosis phase | 76.9% | 0% | <0.001 |
| Acute fibrinous and organizing pneumonia (AFOP) | 69.2% | 22.2% | 0.08 |
Data are expressed as the percentage (%) of patients within each subpopulation.
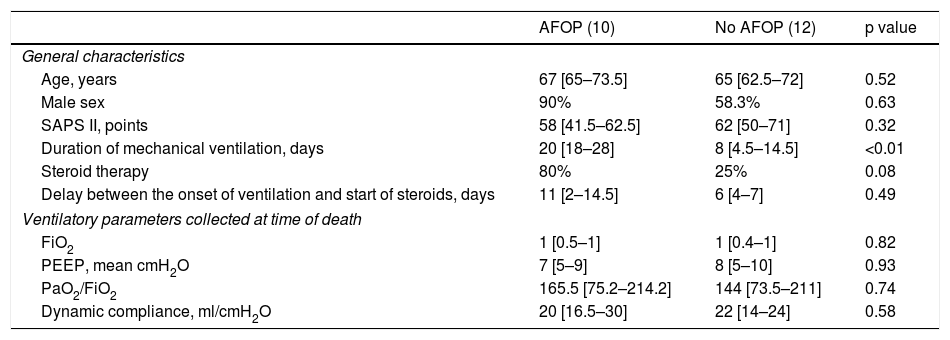
Features of AFOP were found in 10 patients (45%) (Table 6). They began to appear during the second–third week in 36% of the patients, with a large number of fibrin balls; and were found in 86% of the patients after the third week (p<0.02; Fig. 3), showing remnants of fibrin plugs associated with loose fibrous polyps of organizing pneumonia (OP).
Characteristics of 22 patients with or without acute fibrinous and organizing pneumonia (AFOP) at histological examination.
| AFOP (10) | No AFOP (12) | p value | |
|---|---|---|---|
| General characteristics | |||
| Age, years | 67 [65–73.5] | 65 [62.5–72] | 0.52 |
| Male sex | 90% | 58.3% | 0.63 |
| SAPS II, points | 58 [41.5–62.5] | 62 [50–71] | 0.32 |
| Duration of mechanical ventilation, days | 20 [18–28] | 8 [4.5–14.5] | <0.01 |
| Steroid therapy | 80% | 25% | 0.08 |
| Delay between the onset of ventilation and start of steroids, days | 11 [2–14.5] | 6 [4–7] | 0.49 |
| Ventilatory parameters collected at time of death | |||
| FiO2 | 1 [0.5–1] | 1 [0.4–1] | 0.82 |
| PEEP, mean cmH2O | 7 [5–9] | 8 [5–10] | 0.93 |
| PaO2/FiO2 | 165.5 [75.2–214.2] | 144 [73.5–211] | 0.74 |
| Dynamic compliance, ml/cmH2O | 20 [16.5–30] | 22 [14–24] | 0.58 |
Data are n (%) or mean (SD) unless otherwise stated. SAPS 2, simplified acute physiological score; PEEP, positive end-expiratory pressure.
Interstitial inflammatory infiltrate was found in all patients regardless of the duration of ventilation. It was mostly mild to moderate, and composed of lymphocytes with some monocytes. Plasma cells were present after the third week. Neutrophils were scarce. Endo-alveolar infiltrate was present in 19/22 (86%) of patients and almost exclusively composed by macrophages.
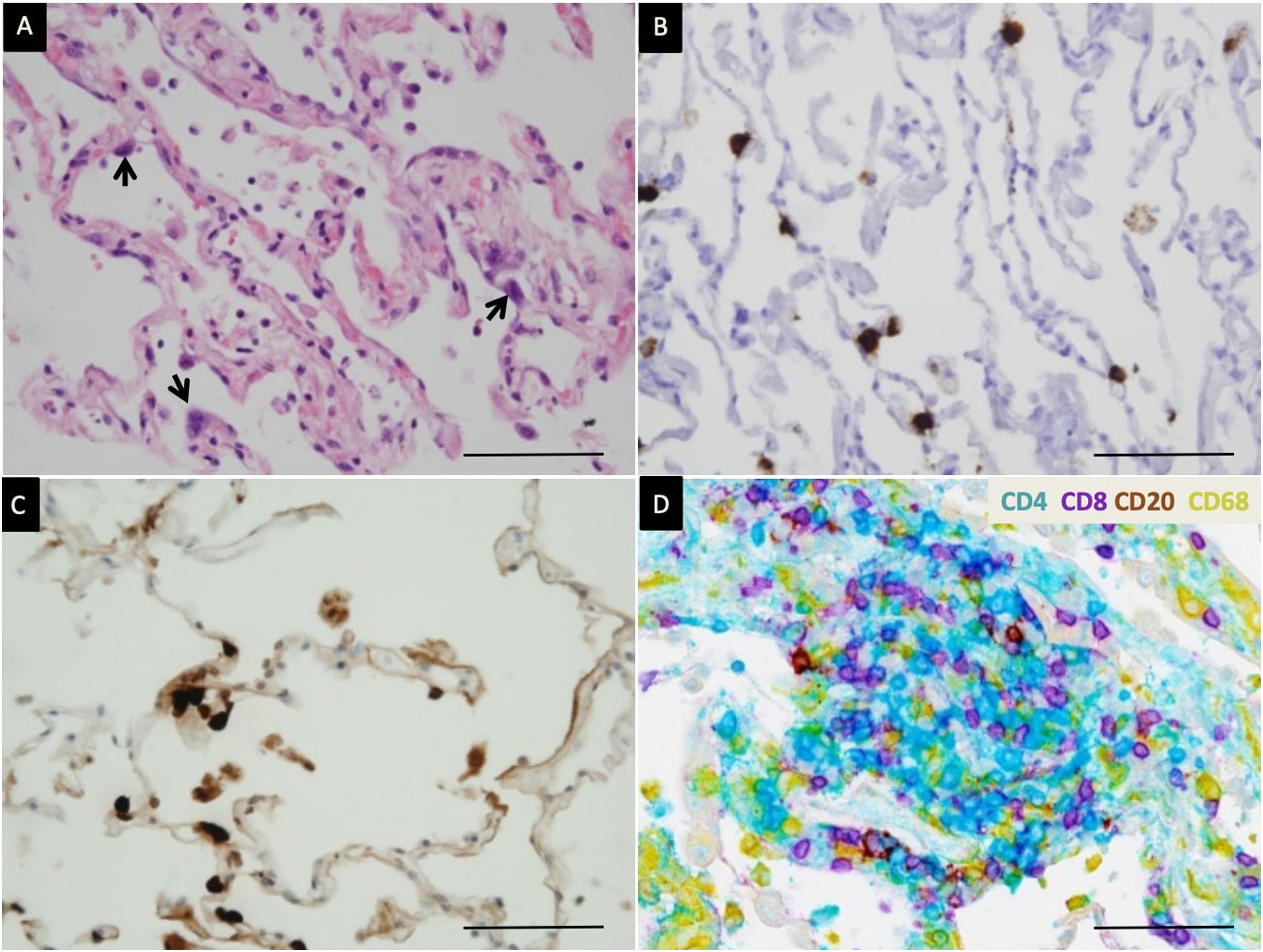
Immune cell subpopulations were analyzed in 12/22 cases (from day 1 to day 25 after ventilation) using an automated chromogenic multiplexed immunohistochemistry (IHC) assay with four antibodies (Fig. 4). In all cases only rare scattered interstitial CD20+ B-cells were observed. The infiltrate consisted of T-cells, with a mixture of CD4+ and CD8+ lymphocytes. There were slightly more CD4+ than CD8+ lymphocytes in 6/12 (50%), and slightly more CD8+ than CD4+ lymphocytes in 6/12 (50%) of patients. CD68+ macrophages were mostly located in alveolar spaces.
Detection of SARS-CoV-2 and characterization of inflammation. (A) Edema of alveolar walls and atypical pneumocytes with enlarged nuclei (arrow). Hematoxylin–eosin staining. (B) SARS-Cov-2 mRNA detection in atypical pneumocytes by in situ hybridization. (C) Viral nucleocapsid protein detection in atypical pneumocytes by immunohistochemistry. (D) Multiplex chromogenic detection by DAB staining (brown) of CD20+ lymphocytes, purple staining of CD8+ lymphocytes, teal staining (blue) of CD4+ lymphocytes and yellow staining of macrophages. CD4+ lymphocytes outnumber CD8+ lymphocytes in this case. The scale bar corresponds to 50μm.
The presence of the SARS-CoV2—tested by immunohistochemistry (IHC)—was only found in 2/22 patients (9%) (Fig. 4). Both died early, during the first week of ICU stay, after 4 and 7 days of symptomatic illness.
As IHC, RNA scope allowed detection of SARS-CoV-2 viral RNA only in the two same patients (9%) (Fig. 4).
Both viral RNA and protein were located in atypical pneumocytes and in some intra-alveolar macrophages. No staining of endothelial cells could be identified.
Although RT-PCR testing for the virus in tracheobronchial secretions was positive up until death in the majority of our patients (20/22 patients)—using the two methods (IHC and RNAscope)—no trace of the virus was found in lung tissue of patients who died after the first week.
Endothelial and microcirculation injuryEven if 8/22 patients (36%) developed a pulmonary embolism, only two of the 22 patients had recent thrombosis of small pulmonary arteries and one patient had endotheliitis in lung vessels (Fig. 1). To note, all patients received heparin therapy. Six received prophylactic anticoagulation (27.3%) and 16 therapeutic anticoagulation (72.7%). Other common findings were diffuse small vessel platelet aggregation, intra-alveolar hemorrhage or areas of hemorrhagic necrosis of the lung parenchyma.
DiscussionHerein, we report the histopathological features of SARS-CoV-2-induced pulmonary disease. Although this ARDS is typical in term of histological injuries and chronology,18 it is characterized by predominant acute fibrinous and organizing pneumonia (AFOP) and potential more pro-fibrotic evolution.
As described by Katzenstein et al. in 1976, the histological hallmark of ARDS is diffuse alveolar damage (DAD), consisting of changes such as hyaline membranes, interstitial edema, cell necrosis and proliferation, or/and fibrosis.19 In our series, such changes were present, but the predominant feature was loose fibrin deposition and/or fibrin balls within the alveolar spaces, that evolved towards organizing pneumonia,20 which is uncommon in classic DAD.21,22 Such histologic patterns of lung injury define AFOP—first described in 2002 by Beasley et al.—as a possible variant of DAD because of its similar aggressive behavior and mortality rate.23 Nowadays, AFOP is not considered as a specific entity, but rather as a histological pattern that can occur in lung tissues from patients with clinical/radiological pictures of either DAD or OP.24,25 It sometimes follows an aggressive course.26 Finding it is not completely unexpected, since this type of lung injury was previously found in SARS-CoV-1 positive cases.27 Moreover, Copin et al. described AFOP on post-mortem biopsies and concluded that lung injury in severe SARS-CoV2 infection is not a DAD.28 These lung lesions are therefore rather a fibrinous variant of DAD with AFOP features.29
ARDS is a clinical syndrome introduced for the first time in the medical literature in 1967.30 In the management of “typical” ARDS, the use of corticosteroids remains controversial.31 However, in our work, the analysis of lung biopsies in patients who died of ARDS related to COVID-19 revealed a high incidence of acute fibrinous and organizing pneumonia (AFOP) features (50% of all patients). These cortico-sensitive lesions were described from the second week of ICU, while the presence of the virus was no longer detected. Thus, whether corticosteroids are beneficial at this timing should be questioned.
The presence of AFOP features—which is uncommon in ARDS—could add some knowledge to understand why several reports suggest that ARDS related to COVID-19 is different from typical ARDS.5,6,32 Moreover, consistent with our study, lung lesions seem to evolve during the time course of the disease, suggesting that the same treatment could have a different impact depending on when it started. Indeed, contrary to the fulminant presentation of AFOP, the subacute presentation is a potentially cortico-sensitive state.33,34 Thus, understanding the chronology of lesion onset is fundamental to determine the best time to introduce a potential treatment, such as corticosteroids or maybe other immunosuppressive therapies. Hence, corticosteroids could potentially be beneficial in the early stages of ARDS by reducing inflammation, by modulating cytokine storm35 but also by acting on AFOP.36 Our results could partly explain the results of the RECOVERY trial,37 which provides evidence that treatment with dexamethasone reduces 28-day mortality in patients with COVID-19 receiving respiratory support. This study did not uncover any benefit but, on the contrary, there seemed to be an increased risk of death in patients with a mild form of COVID-19 who did not require oxygen.
Our present data show the occurrence of end-stage fibrosis in 100% of the patients after 3 weeks of ventilation appearing to be more important than in usual ARDS, where 42% of patients had fibrosis in extrapulmonary ARDS and 82% in pulmonary ARDS after 3 weeks of ventilation.21 Thus, deceased patients after severe forms of COVID-19 appear to have more pro-fibrotic activity and survivors who have been on mechanical ventilation for several days are potentially at risk of developing pulmonary fibrosis leading to potential restrictive lung disease, as reported in SARS-CoV-138 and MERS-CoV.39 Thereby, one could suggest that VAP might worsen the established histopathological injuries and facilitate the progression toward fibrosis in COVID-19 pneumonia.
A recent case-control study by Konopka concluded that there are no distinctive morphologic features to confidently differentiate COVID-19-related DAD from DAD due to other causes.40 However, this is a study based on few autopsies, with the limitations that it implies compared to early post-mortem biopsies. Furthermore, these results do not seem to be in agreement with the medical literature on ARDS where AFOP lesions are uncommon.21 Although RT-PCR testing for the virus in tracheobronchial secretions was positive up to death in the majority of our patients, no histological trace of the virus was found in patients who died after the first week. The presence of viral RT-PCR in the patients’ tracheobronchial secretions does not necessarily indicate the presence of infectious virus, as it has been demonstrated in other organs.41 These results are consistent with other histological studies in which less frequently, SARS-COV-2 were seen beyond 2 weeks.42,43 One explanation is that SARS-COV-2 replicates faster, more efficiently and to a greater extent in the epithelial cells of the nasal cavity than in the alveoli.44
As in the study by Ackermann et al.,45 we found very few neutrophils in lungs. But we did not find CD4-positive T cells being always more numerous than CD8-positive T cells. In some patients, we even observed the opposite. The CD4/CD8-positive T cells ratio did not correlate with the duration of ICU stay or the use of corticosteroids.
Some studies have shown endothelial cell infection and endotheliitis in COVID-19,46 as well as coagulation disorders with a higher than average risk of life-threatening thrombotic complications.47 However, we found no evidence of endothelial cell infection and only one patient had evidence of endotheliitis.
To our knowledge, this is the first report assessing immediate samples of lung biopsies following death, which could make our histological analysis much more accurate as stated by others.12 This is in contrast to most studies in which autopsies were performed several days after death.45,48 Hence, post-mortem autolysis could be one of the explanations why some studies find the presence of DAD, without predominant AFOP features. It is also the first study to investigate the chronology of injuries.
Some limitations can be advanced. The main one is the absence of a control group, given the urgency of the situation. The sample size was small compared to autopsy cases. This could in fact explain why we have a lower incidence of vascular thrombosis and endotheliitis in our series, especially in needle biopsies which are less prone to collect pulmonary arteries or veins. On the opposite, needle biopsies taken in different lobes well reflect the heterogeneity of the disease and help to illustrate the variety of morphological features.
ConclusionChronological evolution of COVID-19 lung histopathologic lesions seems to be close to other ARDS. COVID-19 ARDS seems to be more prone to evolve towards pulmonary fibrosis after 3 weeks than other ARDS. Moreover, there are notably pulmonary injuries consistent with potentially corticosteroid-sensitive lesions.
Authors’ contributionsAll authors whose names appear on the submission contributed substantially to the conception and design of the study, the acquisition of data or the analysis and interpretation of the data.
HM, MS, WO, RCJ, SC, JEH, RJL, JH, FM have been involved in the care of patients and performed lung biopsies.
SM, AN and MPC performed the histopathological analyses.
HM, FM and MPC collected the data and wrote the manuscript.
FM and MPC contributed equally to this work.
Data availabilityAll available data are published in the current manuscript.
Ethical approvalThe local ethics committee of the University Hospital of Strasbourg approved the study (reference CE-2020-34).
Consent for publicationNot applicable.
FundingThis work has been supported by the Association for Research Development in Intensive Care (ADRER), Strasbourg, France.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Nathalie BERVAS and Karine LEMARCHAND (Tetu Bio) for the SARS-CoV antibody. We are also grateful to Martine MUCKENSTURM, Catherine LEBERQUIER, Florence GUENARD and Tuy-Tien TONG for technical assistance.
The authors thank Ms. H. GOETZ for her English proofreading of the manuscript.
This study provides information regarding the evolution of histopathological lung lesions caused by COVID-19. Collaboration between pathologists and clinicians serving patients.