Ventilator-associated lower respiratory tract infections (VALRTIs) in mechanically ventilated patients include ventilator-associated pneumonia (VAP) and ventilator-associated tracheobronchitis (VAT). It is important to recognize that one of the major clinical issues related to the management of VALRTIs is the increasing occurrence of multi-drug resistant (MDR) or extremely-drug resistant (XDR) pathogens.1,2 The available evidence suggests that the overall prevalence of nosocomial infections attributed to MDR/XDR pathogens, as well as the global use of antibiotics in the hospital setting is on the rise despite efforts to curb these infections.3 VAP and VAT are recognized to be among the most common infections associated with MDR/XDR bacteria including Pseudomonas aeruginosa, Acinetobacter species, and KPC (Klebsiella pneumoniae carbapenemase) containing Enterobacteriaceae. Moreover, the recent recognition of colisitin resistance among MDR/XDR Gram-negative bacteria raises the real possibility of endemic spread of common enteric bacteria possessing resistance to all currently available antibacterial agents. However, it is paramount for clinicians to be aware of the pathogens within their local hospitals associated with VALRTIs in order to optimize treatment strategies and avoid unnecessary use of broad-spectrum antibiotics.
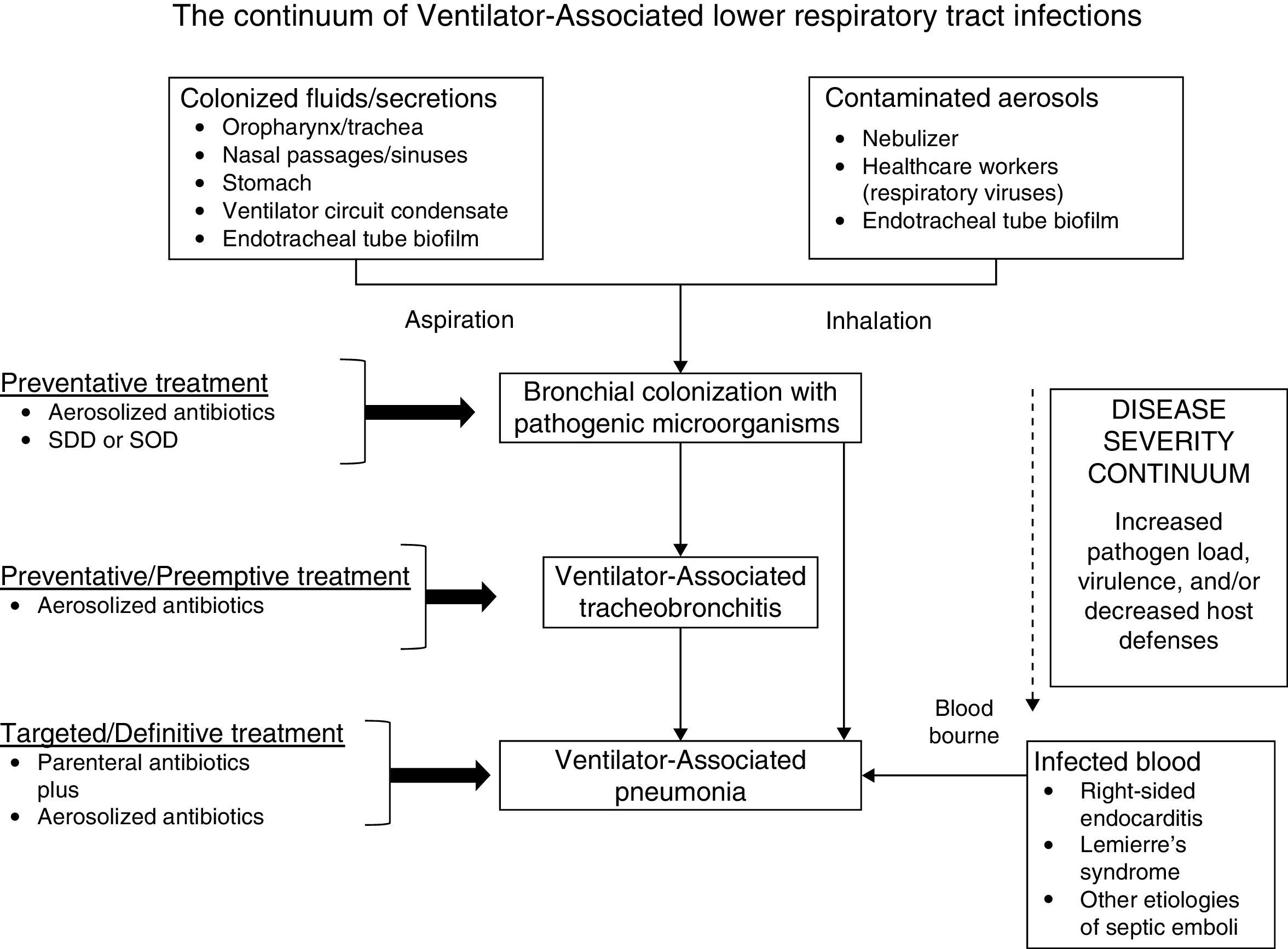
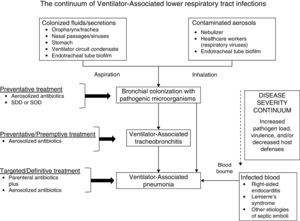
Traditionally the goal for clinicians has been to understand local patient characteristics that increase the risk for infection due to MDR/XDR pathogens above a threshold where broader empiric antibiotics would be needed. However, in the face of rising resistance the focus has shifted in many centers toward excluding patients at minimal risk for MDR/XDR bacteria. Within the hospital where I practice the prevalence of MDR/XDR bacteria associated with VAP and VAT is high enough to warrant initial coverage with broad-spectrum empiric antibiotics targeting methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa, the two most common pathogens for these infections. However, I also aggressively practice antimicrobial de-escalation in order to limit the unnecessary use of broad-spectrum agents.4Fig. 1 provides an overview of my strategy for the assessment and treatment of VALRTIs. Establishing an accurate diagnosis of VALRTIs is important in order to target antimicrobial therapy to patients who are most likely to benefit from it.
The continuum of ventilator-associated lower respiratory tract infections progressing from bronchial colonization to ventilator-associated tracheobronchitis and ventilator-associated pneumonia. Selective decontamination of the digestive tract (SDD) and selective oropharyngeal (the mouth and throat) decontamination (SOD) would only be recommended in locations with a low prevalence of colonization with multidrug-resistant bacteria.
I define VAT as the presence of all of the following in a patient receiving mechanical ventilation for >48h: body temperature >38.3°C or <36.0°C, new or increased purulent tracheal secretions, positive culture of tracheal secretions at a concentration of ≥105cfu/mL, and no new or progressive infiltrate on portable chest radiograph.5 I define VAP as the presence of a new or progressive pulmonary infiltrate and two of the following: temperature >38.3°C or <36.0°C, leukocyte count >12,000/μL or <4000/μL, or purulent tracheal secretions. I consider the diagnosis of VAP to be microbiologically confirmed if cultures, preferentially quantitative bronchoalveolar lavage cultures, demonstrate significant growth at ≥104cfu/mL. Unfortunately, portable chest radiographs often are of poor quality for assessing the presence of new or progressive infiltrates making it difficult to differentiate VAP and VAT. Nevertheless, the microbiologic confirmation of VAT and VAP is necessary in order to distinguish these infections from other noninfectious causes in mechanically ventilated patients such as congestive heart failure, atelectasis, pulmonary emboli, or neoplastic disease. It is my experience that when I obtain computed tomography in mechanically ventilated patients I frequently identify infiltrates or consolidated lung, especially at the lung bases, that was not seen on the portable films. The definition for VAT that I employ, requiring systemic evidence of infection, is similar to that of Martin-Loeches et al. describing their recent cohort of patients with VALRTIs.6
At the present time I do not routinely perform surveillance cultures from the respiratory tract in mechanically ventilated patients. I only obtain cultures when patients have systemic signs of infection as noted above. The rationale for this is to avoid the unnecessary use of antibiotics and to avoid treating simple colonization. In my published experience over a one year period, 2060 patients admitted to the medical and surgical ICUs of Barnes-Jewish Hospital required mechanical ventilation for >48h of which 111 (5.4%) were identified as having either VAT or VAP.5 There were 28 (25.2%) with VAT and 83 (74.8%) with VAP. VAT progressed to VAP in nine patients (32.1%) despite concurrent therapy with appropriate antibiotics. Martin-Loeches et al. performed a multicenter study in 114 ICUs in Spain, France, Portugal, Brazil, Argentina, Ecuador, Bolivia, and Colombia over ten months.6 They obtained data from 2960 patients receiving mechanical ventilation for >48h, of whom 689 (23%) developed VALRTIs. The incidence of VAT and VAP were 11% and 12% respectively. They also found that twelve percent of patients with VAT progressed to VAP and that patients with VAT who received appropriate antibiotic therapy were significantly less likely to progress to VAP. The data from these two studies suggest that VAT and VAP clearly overlap and that some patients with VAT may actually have early VAP. Moreover, appropriate and timely antibiotic therapy seems to be an important element in improving patient outcomes.
I traditionally have employed systemic antibiotics for the treatment of VAT and VAP avoiding the use of aerosolized antibiotics (AAs) due to the limitations of the available delivery devices. However, over the past ten years I have increasingly employed AAs for difficult to treat pathogens such as Pseudomonas aeruginosa and Acinetobacter baumannii in VAP.7 I have also progressively more considered the use of AAs for VAT due to similar pathogens. I have strictly avoided antibiotic therapy for colonization without systemic signs of infection. However, emerging data suggests that this paradigm, and my practices, may be changing in the future with the advent of improved aerosol delivery devices.
Palmer et al. recently performed a randomized study in critically ill intubated patients receiving AAs or saline using a jet nebulizer aerosol delivery system for 14 days or until extubation.8 They demonstrated that AAs were successful in eradicating existing MDR/XDR bacteria and reducing the emergence of subsequent resistance from systemically administered antibiotics.8 Advances in the design of aerosol generators has allowed for the delivery of high antibiotic concentrations into the lung. Niederman et al. studied an investigational drug-device combination (BAY41-6551) of amikacin formulated for inhalation using a primary endpoint of 25 times the MIC of 256μg/ml, representing a tracheal aspirate amikacin maximal concentration greater than or equal to 6400μg/ml.9 Response rates for this endpoint were 50% for amikacin delivered every twelve hours. Similarly, the administration of 300mg/120mg amikacin/fosfomycin combination to mechanically ventilated adults using an investigational vibrating mesh nebulizer achieved tracheal aspirate amikacin concentrations of 12,390μg/g and fosfomycin concentrations of 6174μg/g.10
VAP and VAT are important infections requiring appropriate treatment in order to maximize outcomes. However, clinicians must also me mindful of the excessive use of antibiotics, to include AAs, for fear of generating greater resistance. From my perspective, VALRTIs should be managed according to the algorithm in Fig. 1. However, the findings of ongoing trials could change the way AAs are employed for the treatment and possibly prevention of VALRTIs.
Conflict of interestDr. Kollef's efforts are supported by the Barnes-Jewish Hospital Foundation.
This work was supported by the Barnes-Jewish Hospital Foundation.