To evaluate the impact of a history of harmful use of alcohol (HUA) on sedoanalgesia practices and outcomes in patients on mechanical ventilation (MV).
MethodsA prospective, observational multicentre study was made of all adults consecutively admitted during 30 days to 8 Spanish ICUs. Patients on MV >24h were followed-up on until discharge from the ICU or death. Data on HUA, smoking, the use of illegal (IP) and medically prescribed psychotropics (MPP), sedoanalgesia practices and their related complications (sedative failure [SF] and sedative withdrawal [SW]), as well as outcome, were prospectively recorded.
ResultsA total of 23.4% (119/509) of the admitted patients received MV >24h; 68.9% were males; age 57.0 (17.9) years; APACHE II score 18.8 (7.2); with a medical cause of admission in 53.9%. Half of them consumed at least one psychotropic agent (smoking 27.7%, HUA 25.2%; MPP 9.2%; and IP 7.6%). HUA patients more frequently required PS (86.7% vs. 64%; p<0.02) and the use of >2 sedatives (56.7% vs. 28.1%; p<0.02). HUA was associated to an eightfold (p<0.001) and fourfold (p<0.02) increase in SF and SW, respectively. In turn, the duration of MV and the stay in the ICU was increased by 151h (p<0.02) and 4.4 days (p<0.02), respectively, when compared with the non-HUA group. No differences were found in terms of mortality.
ConclusionsHUA may be associated to a higher risk of SF and WS, and can prolong MV and the duration of stay in the ICU in critical patients. Early identification could allow the implementation of specific sedation strategies aimed at preventing these complications.
Evaluar el impacto del consumo enólico de riesgo (HUA) en las prácticas de sedoanalgesia y la evolución de pacientes en ventilación mecánica (MV).
MétodosEstudio prospectivo observacional multicéntrico de todos los adultos ingresados consecutivamente durante 30 días en 8 UCIs españolas. Los pacientes en MV >24h fueron evaluados hasta el alta de UCI o exitus. Se registró el HUA, consumo de tabaco, psicótropos ilegales (IP) o bajo prescripción médica (MPP) las prácticas de sedoanalgesia y sus complicaciones asociadas (Fracaso de Sedación/SF y Síndrome de Privación/SW) así como datos sobre la evolución clínica.
ResultadosEl 23.4% (119/509) de los ingresados, requirieron VM ≥24h: Varones 68.9%; Edad 57.0 (17.9) años; APACHEII 18.8 (7.2); Ingreso por causa medica 53.9%. La mitad consumían al menos un psicotrópico (tabaco: 27.7%; HUA: 25.2%; PPM: 9.2%; PI: 7.6%). Los pacientes con HUA requirieron más frecuentemente PS (86.7% vs. 64%; p<0.02) y doble sedación (56.7% vs. 28.1%; p<0.02). El HUA se asoció a incidencias 8 (p<0.001) y 4 (p<0.02) veces superiores de SF y SW y prolongó en 151 (p<0.02) horas y 4.4 (p<0.02) días, el tiempo de VM y estancia media en UCI respectivamente respecto al grupo no-HUA. No se encontraron diferencias en la mortalidad.
ConclusionesEl HUA podría asociarse a un mayor riesgo de SF y WS y prolongar los tiempos de MV y LOS en los pacientes críticos. Su identificación precoz permitiría implementar estrategias específicas de sedación orientadas a prevenir estas complicaciones.
Analgesic and sedative agents are universally used in intensive care unit (ICU) patients1 as a cornerstone of the strategies applied to provide comfort and safety during their ICU stay by controlling pain and agitation. This is particularly true for patients on mechanical ventilation (MV), where the reduction of the physiological response to stress exerted by sedation and analgesia allows healthcare practitioners to provide adequate patient care while promoting ventilator synchrony. However, sedatives and analgesics are not exempt from complications thus implementation of strategies to maximize their effectiveness and safety has become a priority for scientific societies and experts’ committees of sedation and analgesia in ICU patients.2–4
Sedative dose requirements are highly variable among ICU patients and particularly influenced by inter and intra-individual differences.5 Pathophysiology in the critically ill patients and individual specific characteristics such as age, metabolic or genetics, impact the drugs’ metabolism and elimination6,7 and can render unpredictable responses to sedatives even when used at known safe doses.8
The difficulty to achieve an adequate level of sedation despite the application of higher doses of sedatives/analgesics along with agitation upon sedative discontinuation defined as difficult sedation (DS),5 is a common clinical scenario and represents a challenge for the ICU specialists who are frequently forced to increase sedative dose or to add new agents, which may increase the risks of toxicity and related complications. DS in ICU patients is also associated with specific clinical, haemodynamic, endocrine and metabolic responses, which negatively impact on patients’ outcome.8,9
Certain conditions have been related to sedation complications. Among these, harmful use of alcohol (HUA), reported in about 20–39% of ICU patients,10,11 and chronic use of psychotropics have been both associated with increased risks of withdrawal syndrome, particularly for patients under prolonged sedation (PS).12
Considering this scenario, we aimed to prospectively study the impact of HUA history on sedation and analgesia practices applied for ICU patients on MV, as well as to assess its influence on patients’ outcome and mortality.
MethodsProspective, observational multicentre study of all adult patients consecutively admitted in 8 ICUs of public and university hospitals of Spain between November and December of 2007. Patients were followed since admission until ICU discharge or death. Patients derived to other ICU or participating in other clinical trials were excluded. All study materials were approved by the Ethics Committee of the coordinating hospital (Ref: CEIC49/2007).
Data were recorded from the patient or their relatives or clinical records (in case patients were unable to report themselves) including age, gender, cause for admission (medical, surgical or trauma), Acute Physiology and Chronic Health Evaluation (APACHE II) and consumption habits such as tabaquism (>10cigarettes/day), regular/occasional use of illegal psychotropics (IP) during the previous year (cocaine, opioids derivatives, cannabis, psychostimulants, psychedelics and other drugs), regular use of prescribed psychotropics medications (PPM) during the last 3 months (hypno-sedatives, antidepressants, neuroleptics, antiepileptics) and a quantitative evaluation of alcohol use according to Standard Drink Units (SDU) adapted for Spain13 considering as HUA weekly consumptions above 280g (28 SDU) in men and 168g (17 SDU) in women, or 50g (5 SDU) during the weekend or once a month as recommended by the World Health Organization (WHO).14
Analgesia and sedation practices were performed in accordance to the protocol of each participating ICU, all following the sedation and analgesia guidelines published by the Sedation and Analgesia Work Group of the SEMICYUC in which preparation were actively involved (Table 1). Sedation level was evaluated using the Ramsay Scale15 (4 centres) and the Richmond Agitation–Sedation Scale (RASS)16 (4 centres), while analgesia was monitored with a Pain Visual Analogic Scale (VAS) in patients able to communicate and/or the Campbell Scale17 in the rest of cases.
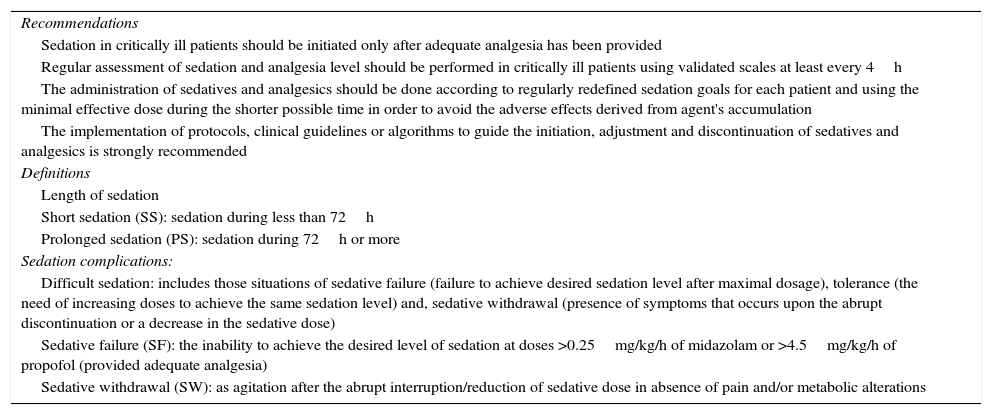
Main recommendations and definitions of the sedation and analgesia of the Sedation and Analgesia Working Group of the Sociedad Española de Medicina Intensiva, Critica y Unidades Coronarias (SEMICYUC).
| Recommendations |
| Sedation in critically ill patients should be initiated only after adequate analgesia has been provided |
| Regular assessment of sedation and analgesia level should be performed in critically ill patients using validated scales at least every 4h |
| The administration of sedatives and analgesics should be done according to regularly redefined sedation goals for each patient and using the minimal effective dose during the shorter possible time in order to avoid the adverse effects derived from agent's accumulation |
| The implementation of protocols, clinical guidelines or algorithms to guide the initiation, adjustment and discontinuation of sedatives and analgesics is strongly recommended |
| Definitions |
| Length of sedation |
| Short sedation (SS): sedation during less than 72h |
| Prolonged sedation (PS): sedation during 72h or more |
| Sedation complications: |
| Difficult sedation: includes those situations of sedative failure (failure to achieve desired sedation level after maximal dosage), tolerance (the need of increasing doses to achieve the same sedation level) and, sedative withdrawal (presence of symptoms that occurs upon the abrupt discontinuation or a decrease in the sedative dose) |
| Sedative failure (SF): the inability to achieve the desired level of sedation at doses >0.25mg/kg/h of midazolam or >4.5mg/kg/h of propofol (provided adequate analgesia) |
| Sedative withdrawal (SW): as agitation after the abrupt interruption/reduction of sedative dose in absence of pain and/or metabolic alterations |
Sedation and analgesia data included the type of sedative and analgesic prescribed, sedation duration (short sedation (SS)<72h; prolonged sedation (PS)≥72h),18 and use of sequential sedation (substitution of a medium-long half-life for a shorter half-life sedative).19 Sedation/analgesia complications were prospectively registered including difficult sedation (DS), sedative failure (SF) and sedative withdrawal (SW) defined by the Sedation and Analgesia Work Group of the SEMICYUC5 (Table 1).
MV total time, data on weaning and tracheostomy, ICU length of stay (LOS) and patients’ outcome (Exitus/Alive) was also registered. Information on other adverse effects associated with sedation and analgesia such as vasopressor rate, arrhythmias, infections, etc., were not collected.
Statistical analysisImpact of HUA on complications of sedation and clinical outcome was analyzed only for patients who required MV beyond 24h. Descriptive results were calculated based on the total number of valid cases and are presented for continuous variables as mean (SD) and median and interquartile range (IR P25–P75) in case of non-normal distribution and as frequencies for categorical variables. Categorical variables underwent univariate analysis using Pearson's Chi-squared test and Fisher's test in case of lower frequencies than expected. Continuous variables were compared with the Student t test (normal distribution) and U-Mann–Whitney test, considering a p<0.05 value as statistically significant. For the multivariate analysis, a multinomial (categorical dependent factors) and linear regression models (continuous factors) were used to determine the effect of each factor on the sedation complications and risk probabilities. Variables identified as significantly different between HUA and no HUA groups were included in the multivariate analysis to determine their impact on the prevalence of SF and WS. DS was not included in the analysis since SF and WS are part of this clinical entity. Results are presented as odds ratios (OR) along with 95% confidence intervals (95% CI) thus considering as possible risk factor those with an OR>1 and a protective factor those with an OR<1. All statistical analysis was performed with SPSS 13.0® for Windows.
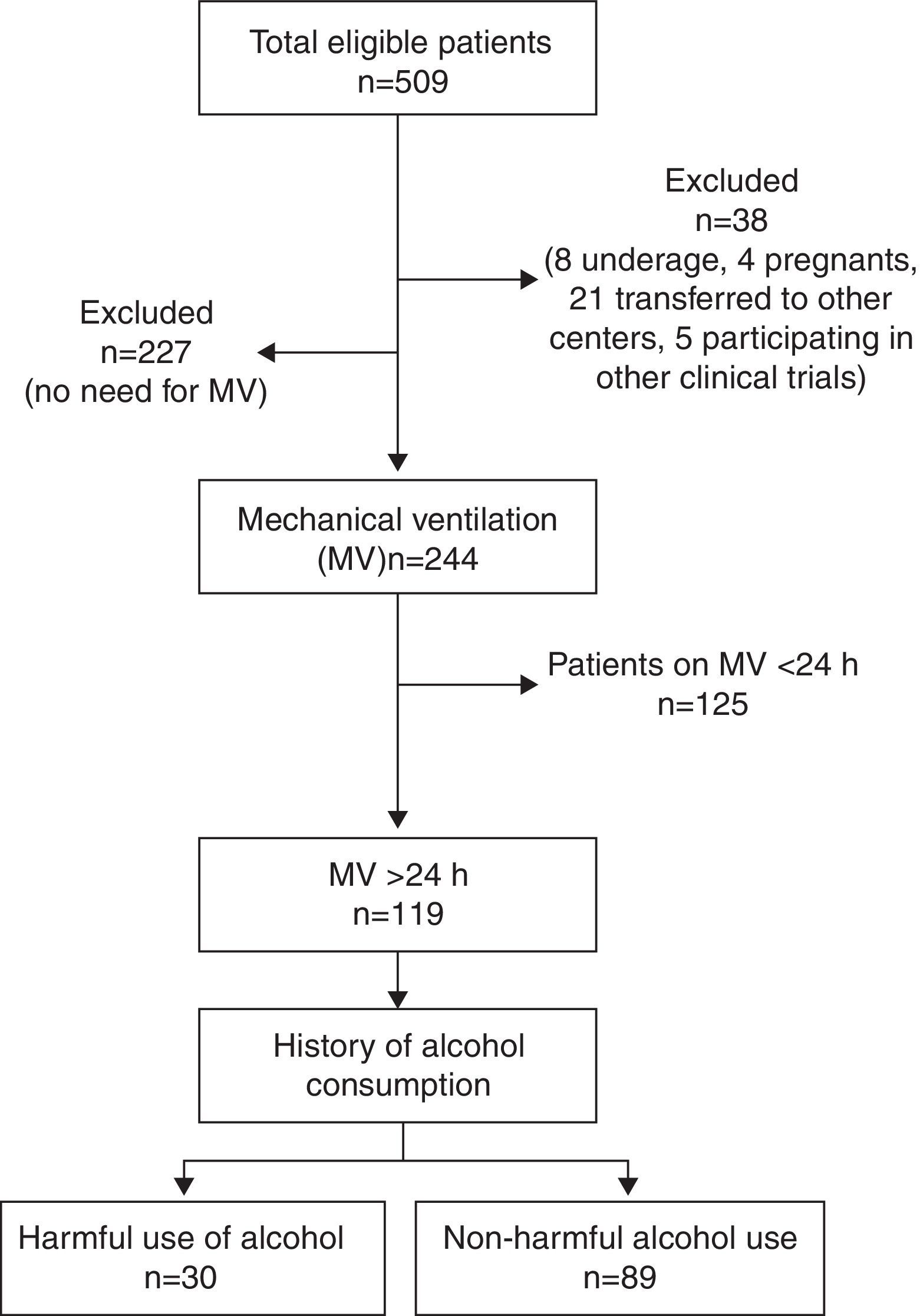
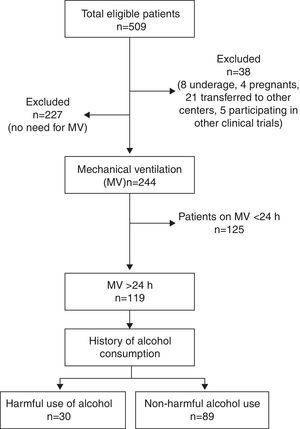
ResultsTotal cohortA total of 509 patients were admitted to the 8 participating ICUs during study period and 38 of them were excluded (Fig. 1). Total study sample (n=471) was 58.9 (17.2) years old, mostly male (66.9%), with a medical cause for admission (52.2%) and a mean APACHE II of 13.2 (8.3).
A 16.7% of cases were considered as HUA and 20% of all patients were smokers. PPM and IP were reported in 19.3% and 4.4% of patients, respectively. A total of 244 (51.8%) patients received MV and 119 (23.4%) did so for ≥24h constituting the study group.
Study groupDemographic characteristics and consumption habits of the study group are presented in Table 2. Most patients were male (68.9%) and had a medical cause of admission (53.9); mean age was 57.0 (17.9) years old and APACHEII was 18.8 (7.2); half (50.5%) of the patients referred consumption of at least one psychotropic, being tobacco (27.7%) and HUA (25.2%) the most frequently reported. PPM were reported in 15.1% of patients and hypno-sedatives (9.2%) where the most commonly prescribed, being benzodiazepines (7.5%) the more frequent type. Of the IP users, 75% were also, with cocaine (4.2%) and cannabis (3.3%) as the preferred drugs. A 75% of IP users were also tobacco or alcohol consumers. Use of PPM was reported in 15.1% of patients being hypno-sedatives (benzodiazepines 81.5%) and antidepressants (SSRI 50%) the most commonly prescribed.
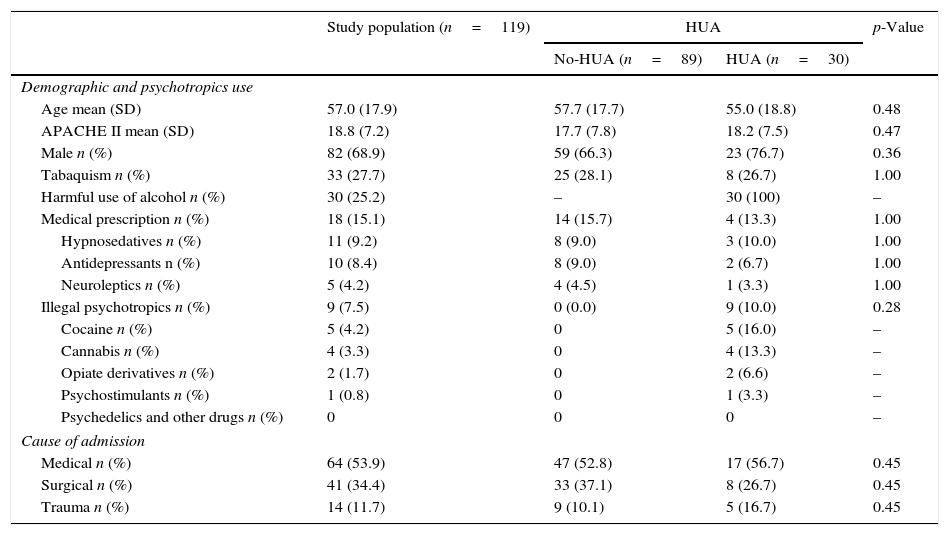
Comparison of demographic and psychotropic use variables between groups.
| Study population (n=119) | HUA | p-Value | ||
|---|---|---|---|---|
| No-HUA (n=89) | HUA (n=30) | |||
| Demographic and psychotropics use | ||||
| Age mean (SD) | 57.0 (17.9) | 57.7 (17.7) | 55.0 (18.8) | 0.48 |
| APACHE II mean (SD) | 18.8 (7.2) | 17.7 (7.8) | 18.2 (7.5) | 0.47 |
| Male n (%) | 82 (68.9) | 59 (66.3) | 23 (76.7) | 0.36 |
| Tabaquism n (%) | 33 (27.7) | 25 (28.1) | 8 (26.7) | 1.00 |
| Harmful use of alcohol n (%) | 30 (25.2) | – | 30 (100) | – |
| Medical prescription n (%) | 18 (15.1) | 14 (15.7) | 4 (13.3) | 1.00 |
| Hypnosedatives n (%) | 11 (9.2) | 8 (9.0) | 3 (10.0) | 1.00 |
| Antidepressants n (%) | 10 (8.4) | 8 (9.0) | 2 (6.7) | 1.00 |
| Neuroleptics n (%) | 5 (4.2) | 4 (4.5) | 1 (3.3) | 1.00 |
| Illegal psychotropics n (%) | 9 (7.5) | 0 (0.0) | 9 (10.0) | 0.28 |
| Cocaine n (%) | 5 (4.2) | 0 | 5 (16.0) | – |
| Cannabis n (%) | 4 (3.3) | 0 | 4 (13.3) | – |
| Opiate derivatives n (%) | 2 (1.7) | 0 | 2 (6.6) | – |
| Psychostimulants n (%) | 1 (0.8) | 0 | 1 (3.3) | – |
| Psychedelics and other drugs n (%) | 0 | 0 | 0 | – |
| Cause of admission | ||||
| Medical n (%) | 64 (53.9) | 47 (52.8) | 17 (56.7) | 0.45 |
| Surgical n (%) | 41 (34.4) | 33 (37.1) | 8 (26.7) | 0.45 |
| Trauma n (%) | 14 (11.7) | 9 (10.1) | 5 (16.7) | 0.45 |
HUA, harmful use of alcohol; n, number; %, percentage; SD, standard deviation.
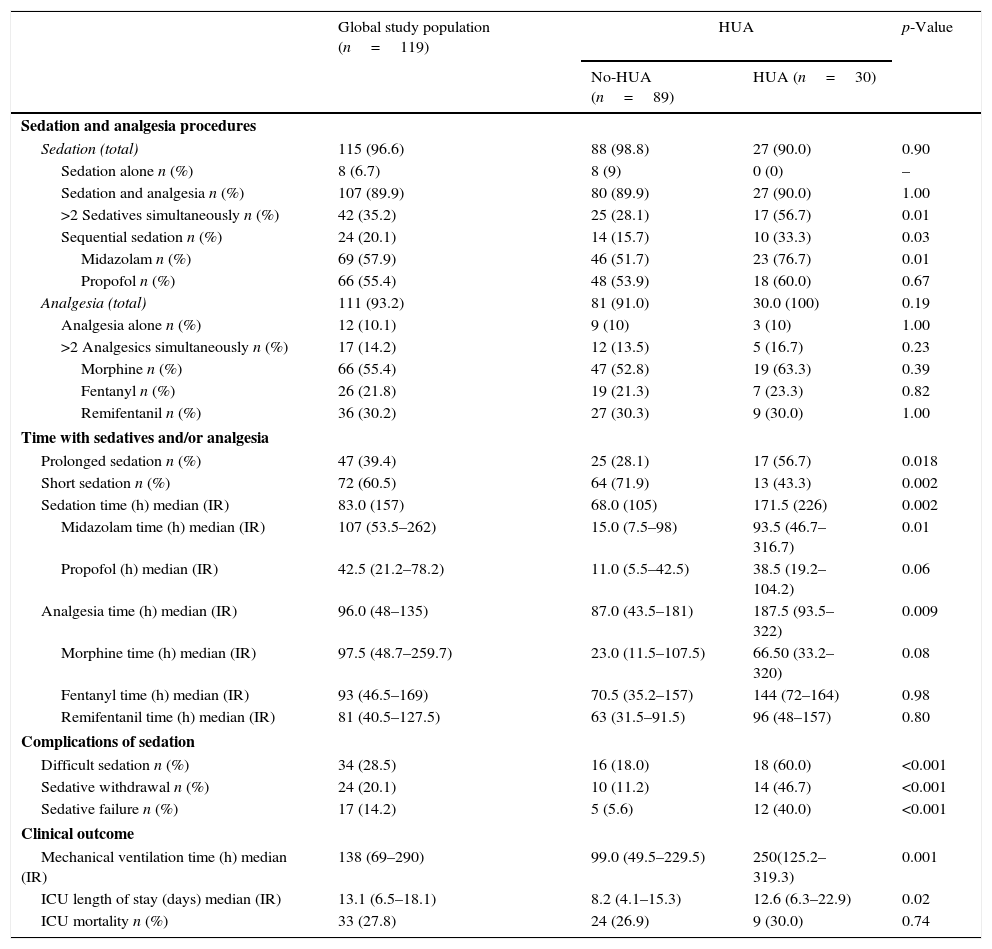
Combination of sedatives and analgesics was the most frequent sedation pattern observed (89.9%), with a 10.1% and 6.7% of patients receiving analgesia or sedation alone, while simultaneous use of two or more sedatives was observed in 35.2% of cases (Table 3). Shorter sedation times (SS<72h) was registered in 60.5% of patients and a 39.4% required PS. The strategy of sequential sedation was applied for 20.1% of patients and SW and SF were observed in 24 (20.1%) and 17 (14.2%) of them, respectively.
Comparison sedation/analgesia procedures and clinical outcome variables between groups.
| Global study population (n=119) | HUA | p-Value | ||
|---|---|---|---|---|
| No-HUA (n=89) | HUA (n=30) | |||
| Sedation and analgesia procedures | ||||
| Sedation (total) | 115 (96.6) | 88 (98.8) | 27 (90.0) | 0.90 |
| Sedation alone n (%) | 8 (6.7) | 8 (9) | 0 (0) | – |
| Sedation and analgesia n (%) | 107 (89.9) | 80 (89.9) | 27 (90.0) | 1.00 |
| >2 Sedatives simultaneously n (%) | 42 (35.2) | 25 (28.1) | 17 (56.7) | 0.01 |
| Sequential sedation n (%) | 24 (20.1) | 14 (15.7) | 10 (33.3) | 0.03 |
| Midazolam n (%) | 69 (57.9) | 46 (51.7) | 23 (76.7) | 0.01 |
| Propofol n (%) | 66 (55.4) | 48 (53.9) | 18 (60.0) | 0.67 |
| Analgesia (total) | 111 (93.2) | 81 (91.0) | 30.0 (100) | 0.19 |
| Analgesia alone n (%) | 12 (10.1) | 9 (10) | 3 (10) | 1.00 |
| >2 Analgesics simultaneously n (%) | 17 (14.2) | 12 (13.5) | 5 (16.7) | 0.23 |
| Morphine n (%) | 66 (55.4) | 47 (52.8) | 19 (63.3) | 0.39 |
| Fentanyl n (%) | 26 (21.8) | 19 (21.3) | 7 (23.3) | 0.82 |
| Remifentanil n (%) | 36 (30.2) | 27 (30.3) | 9 (30.0) | 1.00 |
| Time with sedatives and/or analgesia | ||||
| Prolonged sedation n (%) | 47 (39.4) | 25 (28.1) | 17 (56.7) | 0.018 |
| Short sedation n (%) | 72 (60.5) | 64 (71.9) | 13 (43.3) | 0.002 |
| Sedation time (h) median (IR) | 83.0 (157) | 68.0 (105) | 171.5 (226) | 0.002 |
| Midazolam time (h) median (IR) | 107 (53.5–262) | 15.0 (7.5–98) | 93.5 (46.7–316.7) | 0.01 |
| Propofol (h) median (IR) | 42.5 (21.2–78.2) | 11.0 (5.5–42.5) | 38.5 (19.2–104.2) | 0.06 |
| Analgesia time (h) median (IR) | 96.0 (48–135) | 87.0 (43.5–181) | 187.5 (93.5–322) | 0.009 |
| Morphine time (h) median (IR) | 97.5 (48.7–259.7) | 23.0 (11.5–107.5) | 66.50 (33.2–320) | 0.08 |
| Fentanyl time (h) median (IR) | 93 (46.5–169) | 70.5 (35.2–157) | 144 (72–164) | 0.98 |
| Remifentanil time (h) median (IR) | 81 (40.5–127.5) | 63 (31.5–91.5) | 96 (48–157) | 0.80 |
| Complications of sedation | ||||
| Difficult sedation n (%) | 34 (28.5) | 16 (18.0) | 18 (60.0) | <0.001 |
| Sedative withdrawal n (%) | 24 (20.1) | 10 (11.2) | 14 (46.7) | <0.001 |
| Sedative failure n (%) | 17 (14.2) | 5 (5.6) | 12 (40.0) | <0.001 |
| Clinical outcome | ||||
| Mechanical ventilation time (h) median (IR) | 138 (69–290) | 99.0 (49.5–229.5) | 250(125.2–319.3) | 0.001 |
| ICU length of stay (days) median (IR) | 13.1 (6.5–18.1) | 8.2 (4.1–15.3) | 12.6 (6.3–22.9) | 0.02 |
| ICU mortality n (%) | 33 (27.8) | 24 (26.9) | 9 (30.0) | 0.74 |
HUA, harmful use of alcohol; n, number; %, percentage; SD, standard deviation; IR (interquartile range P25–P75); ICU, intensive care unit.
Most patients were intubated outside the ICU (83%). Median length of MV was of 138 (69–290)h and 70.5% of patients required of MV ≥72h. Re-intubation was necessary in 6% of cases and a tracheostomy was performed in 15% of patients at an average of 10.2±6.1 days after admission. Mean ICU's LOS was 13.1±11.6 days and mortality rate was of 27.7%.
HUA vs. No-HUAAccording to the SDUs assessment, a total of 30 patients (25.2%) were categorized as HUA (Tables 2 and 3). No differences in demographics, severity, and cause of admission or history of psychotropic consumption were observed compared with No-HUA patients (Table 2).
Overall, HUA patients required longer sedation and analgesia times, and a higher proportion of them were under PS (86.7% vs. 64%; p<0.02).
Midazolam was the preferred sedative (p<0.02) in HUA group while no differences among use of other agents when compared with the No-HUA group were observed. The simultaneous use of ≥2 sedatives and/or the sequential application of sedatives were significantly more frequent in the HUA group (p<0.05) (Table 3).
Similarly, the number of patients in the HUA group presenting DS tripled that of the No-HUA group (60% vs. 18%), and the incidence of sedation complications such as SF (40% vs. 5.6%) and SW (46.7% vs. 11.2%) was 8 and 4 times higher in the HUA group.
HUA was also associated with longer periods on MV (250; (125.2–319.3) vs. 99; (49.5–229) hours; p<0.001) and LOS (8.2; (4.12–15.3) vs. 12.6 (6.3–22.9); p<0.02) days when compared with No-HUA group. However, no differences were found regarding mortality (Table 3).
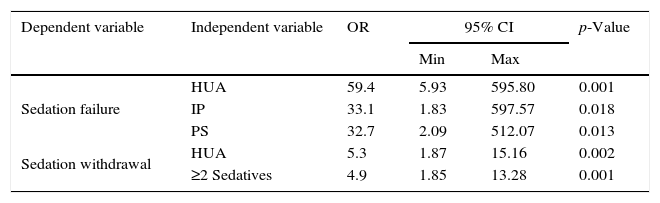
Multivariate logistic regression analysis (Table 4) identified history of HUA (OR: 59.4; 5.9–595.8), use of IP (OR: 33.1; 1.8–597.5) and PS (OR: 32.7; 2.09–512.0) as significant risk factors for SF. Incidence of SW was also influenced by HUA (OR: 5.3; 1.87–15.1) as well as the simultaneous requirement of ≥2 sedatives during ICU's stay (OR: 4.9; 1.8–13.2).
Independent factors identified as risk factors for complications of sedation.
| Dependent variable | Independent variable | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Min | Max | ||||
| Sedation failure | HUA | 59.4 | 5.93 | 595.80 | 0.001 |
| IP | 33.1 | 1.83 | 597.57 | 0.018 | |
| PS | 32.7 | 2.09 | 512.07 | 0.013 | |
| Sedation withdrawal | HUA | 5.3 | 1.87 | 15.16 | 0.002 |
| ≥2 Sedatives | 4.9 | 1.85 | 13.28 | 0.001 | |
Data is presented as odds ratio (OR) along with confidence intervals at 95% (95% CI). Min, lower CI; Max, higher CI; HUA, harmful use of alcohol; IPP, illegal psychotropic; PS, prolonged sedation (sedation ≥72h).
The present work is, to our knowledge, the first multicentre study of prospective nature that demonstrates that history of HUA may negatively impact on the sedation and analgesia's related complications in MV critically ill patients.
One out of 4 patients was categorized as HUA, similar to data reported in United States20 and Spanish national surveys, but higher than data from ICU settings.10,11 The retrospective nature and the different diagnostic criteria used in most of the evidence available could account for such differences. The definition of HUA according to the WHO recommendations (SDU adapted for Spanish population) may present some advantages over other instruments such as the Alcohol Use Disorders Identification Test21 (AUDIT) or the CAGE questionnaire22 in the critical care setting. The SDU is an objective, easy and quick way to quantify the alcohol intake that may ease the investigation of a patient's alcohol consumption habits by the critical care specialist who frequently lacks the time and training to carry out complex questionnaires.
Similar to previous reports,11 there were no differences in the type of admission diagnosis among HUA and no HUA patients. However and contrarily to published series10,11 HUA patients of this study were not younger nor predominantly male; different geo-demographical characteristics of the studies setting may account for the difference.
Sedation and analgesiaOne-third of the study population required of the simultaneous administration of ≥2 sedatives in order to achieve adequate sedation. Similarly, SF was reported in 14.2% of the cases. The incidence of SF shows important variations across the evidence, (i.e. midazolam SF 25–33%23 or propofol 3–34%24), due to the lack of a clear consensus on the definition of the maximal dose for some of the most used sedatives in ICU. Doses ranging between 4 and 5mg/kg/h have been recommended by several scientific societies as the maximal safe dose for propofol since higher infusions have been associated with direct toxicity defined as propofol infusion syndrome.25 This demarcation is less clear for midazolam thus the maximum dose reported in the available evidence ranges from 0.2 to 0.5mg/kg/h. In absence of a clear definition of a toxicity syndrome directly associated with midazolam,26 the ranges considered to define sedative failure in this study were those recommended by the SEMICYUC.23
Sedative withdrawal rate observed in our study (20.1%) was lower than the reported by other authors (20–80% in critically ill patients undergoing sedation and analgesia beyond a week,27 but again, differences in SW definition and case-mix28 may account for conflicting results. Diagnosis of SW was established by study investigators whenever the presence of specific and easily recognizable clinical signs and symptoms such as insomnia, anxiety, agitation, nausea, delirium and seizures were observed at the time of sedatives’ reduction or discontinuation (in the absence of pain or other organic causes). The use of standard definitions such as DSM-IV29 or several questionnaires/interviews designed to identify SW might be limited in the ICU setting since their implementation requires an adequate level of patients’ consciousness and/or preserved verbal, motor, hearing and visual abilities.30
Sequential sedation, a strategy aimed to avoid the adverse effects of accumulation of sedatives, was implemented in 1 of every 4 patients. Authors did not find relevant evidence in literature to compare with.
HUA vs. No-HUAHUA patients were sedated for longer periods, required more frequently the simultaneous use of ≥2 sedatives and presented higher rates of sedation complications than No-HUA patients. Moreover, HUA was identified as an independent risk factor for the development of SF and SW. Similar results were reported in the study of De Wit et al.11 where critical patients with psychotropic consumption history needed 2.5 times more sedatives and 5 times more analgesics to obtain similar sedation level as well longer infusion periods than patients without history of consumption. The need for higher doses of sedatives and analgesics observed in HUA patients might be explained by a possible cross-tolerance mechanism between sedatives and alcohol, as well as to an increased metabolism of these substances through the action of p450 enzyme.31 The same underlying mechanisms could also explain the higher incidence of SW.32 Although alcohol has no specific receptors as benzodiazepines or barbiturates do, all three substances exert their action through the stimulation of GABA A receptors (reduce brain excitability33 while inhibiting glutamate's N-methyl-d-aspartate (NMDA) receptors34 (excitatory). Chronic exposure to any of these agents may induce to a series of compensatory mechanisms at the cellular receptor level producing a gradual decrease in GABA receptors (down-regulation) and increasing NMDA ones (up-regulation) in such a way that balance is only restored in the presence of such psychotropic agents.35 Prolonged use of benzodiazepines enhances the GABA/NMDA unbalance, making necessary to increase sedative agents’ dose to reach/maintain the adequate levels of sedation. Similarly, reduction or discontinuation of these agents may disrupt the balance, increasing brain excitability which together with the interruption of the inhibitory effect, is manifested as the known symptoms and signs of withdrawal.32
In the present study, HUA patients required longer periods of MV. This finding was not found by De Wit et al.11 who described that despite the increased risk for need of MV, alcoholic patients did no required prolonged times of MV, except for those presenting with withdrawal syndrome.36 Similarly to data reported by Suchyta et al.,10 in our series, HUA patients presented longer ICU LOS. These findings have not been replied in other retrospective investigations with different case mixes.11 PS and the need for higher number and/or doses of sedatives consistently found in patients with chronic use of psychotropics may play a role in the prolongation of MV and ICU stay times. Oversedation has been associated with prolonged time on MV and complications such as pneumonia, barotrauma, upper digestive haemorrhage, bacteraemia, or venous thrombosis among others.37 On the other hand, and similarly to other reports,10,11,36 HUA was not associated with higher rates of mortality in ICU patients.
One of the main limitations of the present study is that, in order to attain an easy and rapid detection in the ICU setting, criteria used for classification of “psychotropic consumption” were less strict than those used in other studies, which may contribute to a possible overestimation of psychotropic use rates. However, several studies in the hospital and out-of-hospital setting have shown that detection of drug dependence is frequently under-diagnosed with rates ranging from 10 to 86%.38 This may be particularly true in the context of critically ill patients, where the implementation of standard criteria for disease classifications39 and screening tools may be hampered by factors such as the lack of critical care physicians specific training in drug dependencies, the need for urgent care, and the frequently imprecise sources of information available in this setting (subjective information from relatives or incomplete medical records).40
The study was conducted in 2007. Sedation and analgesia practices may have changed since then hampering the validity of data presented in this manuscript with today's practice.
The implementation of sedation and analgesia protocols were responsibility of each of the participating centres as well as for the diagnosis of SW or SF that was performed according to the clinical criteria of investigators. The knowledge of history of HUA could have influenced beforehand the sedation strategy implemented and diagnosis of WS by the attending physician affecting the internal validity of the study. However it is important to recognize that all the study investigators are active members of the Spanish National Sedation and Analgesia Work Group of SEMICYUC which may influence the highly consonance of sedoanalgesia practices described in this study with the recommended by scientific societies.
We are also aware that the definition of SW used in the present study may lead to a false overrepresentation of this clinical profile due to the inclusion of misdiagnosed ICU delirium cases in mechanically ventilated patients, a syndrome that although sharing some of the clinical symptoms has different pathophysiologic mechanisms and outcomes.
Despite the multicentre design, the low number of patients and events per variable finally included advise to take cautiously results found in multivariate analysis. Similarly, although statistically significant, the impact of IPP on sedation practices and complications should be re-evaluated due to the low number of cases. The different case-mix (age, social background, etc.) of the included patients in the different participating centres may also hamper external validity of the results making them not applicable to other hospitals with different characteristics.
In conclusion, HUA patients may be at higher risk of requiring prolonged sedation and the use of higher doses of sedatives and analgesics, increasing the risk of sedation failure and withdrawal that could ultimately impact negatively on their outcome. Early identification of HUA patients might be advisable in patients admitted to ICUs since these patients are in higher risk of sedation failure and withdrawal and could benefit from strategies oriented to the prevention of these complications.
Conflict of interestNone.
Authors thank Pilar Hernandez and Cindy L. Larios for their contribution in the statistical analysis and editorial assistance for the present work.