Acute respiratory distress syndrome (ARDS) is an inflammatory lung disorder, and its pathological hallmark is diffuse alveolar damage (DAD). Given that open lung biopsy (OLB) can sometimes result in severe side effects, it is rarely performed in patients with ARDS.
AimThe aims of this study were to describe: (a) the rate of treatment change associated with the histological result; and (b) the incidence of side effects induced by OLB.
Design and patientsA retrospective, single-center, descriptive observational study was carried out in Hospital Santa Clara (Bogotá, Colombia) from February 2007 to January 2014.
Inclusion criteria: Critically ill patients over 18 years of age, undergoing invasive mechanical ventilation, diagnosed with ARDS of unknown etiology, and with OLB performed at the bedside. ARDS was diagnosed according to the Berlin definition. DAD was defined by the presence of a hyaline membrane plus at least one of the following: intra-alveolar edema, alveolar type I cell necrosis, alveolar type II cell (cuboidal cells) proliferation progressively covering the denuded alveolar-capillary membrane, interstitial proliferation of fibroblasts and myofibroblasts, or organizing interstitial fibrosis. The rate of treatment change (RTC) was established according to whether the OLB pathology report resulted in: a) the prescription or discontinuation of an antimicrobial; b) the indication of new procedures; c) medical interconsultation; or d) limitation of therapeutic effort.
Patients were followed-up until death or hospital discharge. This study was approved by the Ethics Committee.
ResultsA total of 32 OLBs were performed during the study period; 17 were ruled out as they did not involve ARDS, and 15 were considered for further analysis. A histological diagnosis was reached in 14 of the 15 patients (12 DAD, one case of bronchiolitis obliterans organizing pneumonia and one case of Wegener's granulomatosis with alveolar hemorrhage). The RTC rate was 0.73. The most frequent intervention was discontinuation of antimicrobial or corticosteroid treatment.
No deaths but four side effects (3 airway leaks and one hemothorax) were associated with the OLB procedure. All were resolved before ICU discharge.
ConclusionThe information provided by OLB performed at the bedside in ARDS patients of unknown etiology could be relevant, as it may optimize treatment. The risk associated with OLB seems to be acceptable.
El síndrome de dificultad respiratoria aguda (ARDS, acute respiratory distress syndrome) es una enfermedad pulmonar inflamatoria y su característica distintiva patológica es el daño alveolar difuso (DAD, diffuse alveolar damage). Dado que la biopsia pulmonar abierta (OLB, open lung biopsy) a veces puede dar lugar a efectos secundarios graves, rara vez se realiza en pacientes con SDRA.
ObjetivosLos objetivos de este estudio fueron describir: a) la tasa de cambio de tratamiento asociado con el resultado histológico y b) la tasa de efectos secundarios inducidos por la OLB.
DiseñoEstudio observacional, descriptivo, unicéntrico y retrospectivo realizado en el Hospital Santa Clara, Bogotá (Colombia) desde febrero de 2007 a enero de 2014.
Criterios de inclusión: Pacientes críticamente enfermos mayores de 18 años sometidos a ventilación mecánica invasiva, diagnosticados con SDRA de etiología desconocida en quienes se realizó la OLB al lado de la cama. El SDRA fue diagnosticado según la definición de Berlín. El DAD se definió por la presencia de membrana hialina y al menos uno de los siguientes criterios: edema intraalveolar, necrosis de células alveolares tipo I, proliferación de células alveolares tipo II (células cuboidales) con denudación progresiva de la membrana alveolar-capilar, proliferación intersticial de fibroblastos y miofibroblastos o fibrosis intersticial organizada. La tasa de cambio de tratamiento asociada con el resultado de la biopsia pulmonar abierta (RTC) se definió si, basándose en el análisis patológico de la biopsia de pulmón abierto: a) se prescribió o suspendió un antimicrobiano, b) se indicó un nuevo procedimiento, o c) interconsulta médica, o d) limitado el esfuerzo terapéutico. Los pacientes fueron seguidos hasta la muerte o el alta hospitalaria. Este estudio fue aprobado por el comité de ética.
ResultadosDurante el período de estudio, se realizaron 32 OLB; 17 pacientes fueron descartados, ya que no tenían ARDS y 15 fueron considerados para análisis. Se llegó a diagnóstico histológico en 14 (12 casos con DAD, un caso con bronquiolitis obliterante con neumonía de organización y un caso con granulomatosis de Wegener asociada a hemorragia alveolar) de los 15 pacientes; RTC: 0,73. La intervención más frecuente fue la interrupción del tratamiento con antimicrobianos o esteroides.
No hubo muertes, pero 4 acontecimientos adversos (3 neumotórax y un hemotórax) se asociaron con el procedimiento de OLB. Todos fueron resueltos antes del alta de la UCI.
ConclusiónLa OLB constituye un procedimiento de diagnóstico de alto rendimiento que determina un impacto relevante en el tratamiento de pacientes con SDRA. El riesgo asociado a este procedimiento es aceptable. La información proporcionada por la OLB, realizada junto a la cama en la UCI, en pacientes con SDRA de etiología desconocida es relevante, ya que puede optimizar el tratamiento. El riesgo asociado con la OLB parece ser aceptable.
Acute respiratory distress syndrome (ARDS) constitutes a cataclysmic respiratory entity described half a century ago by Ashbaugh et al.1 The Berlin consensus is the most recent ARDS definition2 and considers the syndrome based only on clinical variables (hypoxemia, presence of risk factor and bilateral infiltrate in the X-ray). This definition also mentions that diffuse alveolar damage (DAD) is the ARDS histological hallmark, but it was ruled out from the definition given the procedure to diagnose DAD was considered unfeasible in the real world.3 Despite all efforts, many pharmacological treatments currently tried out on patients with ARDS were unable to demonstrate their effectiveness.4–6 A plausible hypothesis to explain these negative results postulates that ARDS is a syndrome that can harbor several different diseases.5,7–9 Furthermore, this fact may determine that patients who carry conditions that mimic ARDS (e.g. pulmonary embolism, lung cancer or alveolar hemorrhage)9,10 are not diagnosed and thus not specifically treated. Therefore, their outcome could be more prone to deterioration.7
Currently, despite several techniques existing to study the histology in patients with diffuse parenchymal lung diseases, open lung biopsy continues to be the gold standard.11 In patients with ARDS, open lung biopsy may be indicated in two situations: (a) early in the course of an ARDS when a curable etiology is highly suspect and less invasive diagnostic procedures are inconclusive and/or toward the end of the first week of evolution in order to diagnose the fibroproliferative phase.12,13
The two primary endpoints of this study are to describe the rate of treatment change associated with the histological result and to describe the rate of side effects associated with open lung biopsy.
MethodsAuthorization to report the present results was obtained from the ethics committee of the Hospital Santa Clara, Bogota, Colombia. Informed consent was obtained to perform the open lung biopsy.
PatientsWe included all patients over 18 years old, undergoing invasive mechanical ventilation, diagnosed with ARDS of unknown etiology with an open lung biopsy performed at bedside in our 32 bed medical-surgical ICU (Hospital Santa Clara, Bogota, Colombia) between February 2007 to January 2014. ARDS was diagnosed according to the Berlin definition.2
Open lung biopsyOpen lung biopsy was indicated for persistent acute hypoxemic respiratory failure with bilateral lung opacities after other causes of respiratory failure were ruled out (e.g. heart failure or absence of microorganism in the broncho-alveolar lavage). The decision to carry out the open lung biopsies requires the previous agreement of the following departments: intensive care, pulmonology, pathology, radiology and thoracic surgery.
According to our protocol, open lung biopsies were performed at bedside in the ICU. Anticoagulation therapy was suspended 12hours before the procedure. Each patient underwent a single procedure during which samples for microbiological (bacterial cultures, mycobacteria, parasites, and fungi) and histopathological study were obtained. A detailed explanation of the surgical procedure can be found in the supplementary material. All specimens were 1–2cm at the largest diameter and were inflated by injecting formalin with a syringe, fixed in 10% buffered formalin for 24h at room temperature and then embedded in a paraffin block. Criteria for the diagnosis of DAD included the presence of hyaline membranes plus at least one of the following: intra-alveolar edema, alveolar type I cell necrosis, alveolar type II cell (cuboidal cells) proliferation progressively covering the denuded alveolar-capillary membrane, interstitial proliferation of fibroblasts and myofibroblasts, or organizing interstitial fibrosis.14,15 The presence of hyaline membranes was qualitatively assessed. Likewise histological pneumonia was defined by the presence of intense neutrophilic infiltration in the interstitium, in the intra-alveolar spaces and around terminal bronchioles.14 Interstitial lung diseases were defined according to the American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias.16,17
Data collection and analysisThe following data were recorded at the time of ICU admission: age, gender, diagnosis, comorbidities and Simplified Acute Physiology Score (SAPS) II.18 Ventilator settings (tidal volume [VT], FiO2, PEEP, breathing frequency) were recorded at time of ARDS diagnosis and open lung biopsy.
Patients were followed until death, ICU or hospital discharge.
A change in the treatment was considered if, based on the pathological analysis of the open lung biopsy, the ICU staff (a) prescribed or discontinued an antimicrobial (dose modification was not considered), (b) indicated a new procedure (e.g. imaging technique), (c) indicated a new medical interconsultation or (d) limited the therapeutic effort.
Side effects and death caused by open lung biopsy were considered if they occurred within the first 48h of the procedure and when a plausible relation exists between both.
The cause of death was determined according to the following criteria: refractory shock if systolic blood pressure was <90mm Hg during 6h prior to death; refractory hypoxemia if oxygen saturation was persistently below 85% during 6h prior to death; refractory shock and hypoxemia if the two causes defined before coexisted.19
Continuous variables were expressed as median and range interquartile; categorical variables as count and percentage. Statistical analysis was performed using R software. p values <0.05 were considered significant.
ResultsDuring the study period 32 open lung biopsies were performed; 17 were ruled out as they did not have ARDS and 15 were considered for further analysis. Of these, 9 were discharged from the hospital and the others were pronounced dead. The reasons of death were refractory hypoxemia (patients 3 and 12), shock (patient 1 and 8) and both refractory hypoxemia and shock (patient 2 and 7).
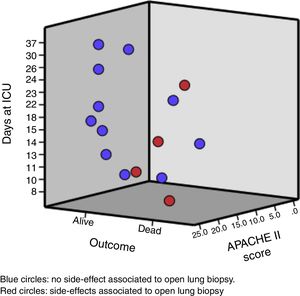
The median age of the patient was 33 (25; 45) years old. Likewise, the median days between hospital admission and ARDS diagnosis was 5 (2; 9) days, and from the ARDS diagnosis to the open lung biopsy 3 (2; 8) days. Clinical and analytical variables of each patient are shown in Table 1 and Fig. 1.
Demographic and clinical variables present at the day of ADRS diagnosis and open lung biopsy perform.
| Patient | Gender | Age | ICU stayed (days) | Hospital stayed (days) | Outcome | APACHE II+ | PeeP | SC | PaC02 | Day of ARDS diagnosis | Creatinine | VS | PeeP | SC | Day of OLB | WBC | Plateletes | Creatinine | VS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pa02/Fi2 | WBC | Plateletes | PaC02 | Pa02/Fi2 | ||||||||||||||||||
| 1 | Female | 82 | 11 | 11 | Dead | 24 | 14 | 20 | 32 | 101 | 14710 | 14100 | 0.84 | NE | 16 | 12 | 41 | 59 | 20010 | 12700 | 0.57 | Dob. NE |
| 2 | Female | 45 | 8 | 31 | Dead | 22 | 16 | 22 | 33 | 108 | 15210 | 15900 | 1.48 | Dop | 14 | 20 | 38 | 177 | 11610 | 160000 | 2.02 | Dop |
| 24 | Dob; | |||||||||||||||||||||
| 3 | Male | 25 | 39 | Dead | 18 | 12 | 17 | 31 | 192 | 15480 | 182000 | 4.23 | NE | 13 | 19 | 24 | 215 | 7660 | 33000 | 5.09 | ||
| 4 | Female | 25 | 26 | 33 | Alive | 23 | 12 | 14 | 42 | 126 | 12820 | 618000 | 1.04 | 18 | 14 | 42 | 109 | 11200 | 780000 | 1.12 | Dob; NE | |
| 5 | Female | 33 | 18 | 31 | Alive | 25 | 14 | 18 | 47 | 142 | 7160 | 84000 | 0.41 | NE | 14 | 20 | 47 | 142 | 7160 | 84000 | 1.11 | NE |
| 6 | Male | 26 | 10 | 24 | Alive | 13 | 16 | 17 | 41 | 64 | 10150 | 361000 | 0.11 | Dop | 16 | 11 | 65 | 73 | 13400 | 355000 | 0.44 | Dob; NE |
| 15 | Dop; | |||||||||||||||||||||
| NE; | ||||||||||||||||||||||
| 7 | Male | 52 | 32 | Dead | 2 5 | 14 | 9 | 38 | 92 | 13700 | 115000 | 1.38 | Vas | 15 | 10 | 33 | 104 | 12130 | 91000 | 1.41 | Dop; Dob; NE | |
| 8 | Male | 54 | 23 | 46 | Dead | 21 | 10 | 24 | 37 | 156 | 3100 | 31000 | 0.51 | NE | 14 | 19 | 43 | 135 | 5520 | 58v000 | 0.81 | NE |
| 9 | Female | 41 | 13 | 19 | Alive | 21 | 14 | 26 | 48 | 65 | 9900 | 176000 | 0.62 | NE | 16 | 30 | 42 | 68 | 14000 | 187000 | 0.67 | |
| 15 | ||||||||||||||||||||||
| 10 | Female | 45 | 28 | Alive | 22 | 12 | 21 | 44 | 110 | 11400 | 138000 | 1.03 | 14 | 16 | 40 | 75 | 12300 | 154000 | 1.1 | NE | ||
| 11 | Female | 22 | 22 | 30 | Alive | 23 | 12 | 18 | 40 | 105 | 15300 | 135000 | 0.5 | 16 | 18 | 38 | 110 | 11500 | 135000 | 0.8 | ||
| 12 | Male | 33 | 14 | 17 | Dead | 14 | 14 | 26 | 29 | 176 | 18300 | 490000 | 0.79 | NE | 14 | NA | 36 | 100 | 13600 | 172000 | 0.41 | |
| 13 | Male | 26 | 30 | 56 | Alive | 15 | 12 | 29 | 30 | 86 | 9900 | 36000 | 1.3 | 12 | 29 | 40 | 102 | 9900 | 36000 | 1.3 | ||
| 14 | Male | 19 | 37 | 56 | Alive | 23 | 14 | 27 | 32 | 177 | 14600 | 322000 | 1 | NE | 10 | NA | 28 | 129 | 6600 | 313000 | 3.6 | |
| 15 | Female | 22 | 10 | 16 | Alive | 16 | 12 | 29 | 38 | 113 | 19800 | 145000 | 1.92 | NE | 10 | 24 | 53 | 175 | 20300 | 136000 | 3.48 | NE; Vas |
SC: static compliance; VS: hemodinamic support. NE: norepinephrine; DoB: dobutamine; DoP: dopamine; VaS: vasoppresine.
WBC: white blood count. NA: not available.
The result of the open lung biopsies allowed us to diagnose a specific histological entity in all the patients except for one that presented lung edema (Table 2). Within the group of patients with a specific histological entity, 12 presented DAD (4 as an isolated pattern and 8 associated with another entity), 1 presented Bronchiolitis Obliterans Organizing Pneumonia and 1 presented a Wegener granumolmatous with alveolar hemorrhage. The presence of DAD did not modify the risk of death (DAD 1 of 3 and non DAD 5 of 12; p=0.792).
Open lung biopsy complications and pathological findings.
| Patient | Suspected diagnosis previous to OLB | Histological diagnosis after OLB | Complications associated to OLB |
|---|---|---|---|
| 1 | Pulmonary tuberculosis, pneumocitis jiroveci | DAD; lung cancer | |
| Bacterial pneumonia, alveolar hemorrage, pulmonary | |||
| 2 | Tuberculosis | DAD; bacterial pneumonia | Hemothorax |
| Bacterial pneumonia, pneumocitis jiroveci, pulmonary | |||
| 3 | Tuberculosis | DAD | Airway leak |
| 4 | Bacterial pneumonia | DAD | |
| Bacterial pneumonia, viral pneumonia, alveolar | |||
| 5 | Hemorrage | DAD | |
| 6 | Bacterial pneumonia, pulmonary tuberculosis | DAD; bacterial pneumonia | Airway leak |
| DAD; bacterial pneumonia; | |||
| 7 | Bacterial pneumonia | alveolar hemorrhage | Airway leak |
| DAD; granuloma with | |||
| 8 | Bacterial pneumonia, pulmonary tuberculosis | caseation necrosis | |
| 9 | Bacterial pneumonia, viral pneumonia (H1N1) | DAD; bacterial pneumonia | |
| 10 | Bacterial pneumonia | DAD; bacterial pneumonia | |
| 11 | Bacterial pneumonia, viral pneumonia (H1N1) | DAD; bacterial pneumonia | |
| 12 | Pneumocitis jiroveci, pulmonary tuberculosis | BOOP | |
| Fungal pneumonia (Histoplasmosis), pumonary | |||
| 13 | Tuberculosis | DAD | |
| Wegener granulomatosis, | |||
| Alveolar hemorrhage | |||
| 14 | Alveolar Hemorrage | Organized pneumonia | |
| Bacterial pneumonia, pneumocitis jirovecci, pulmonary | |||
| 15 | Tuberculosis | Alveolar edema |
The open lung biopsy results determined a treatment optimization in 11 patients; in most of the cases the change was related to anti-tuberculous and steroid treatment (Table 3). Limitation in the therapeutic effort or indication of a new procedure or medical interconsultation was not performed based on the open lung biopsy result.
Impact of open lung biopsy over the treatment.
| Patient | Change in the treatment | Antibiotic (before) | Antibiotic (after) | Anti-TB (before) | Anti-TB (after) | Antifungal (before) | Antifungal (after) | Antiviral (before) | Antiviral (after) | Steroid (before) | Steroid (after) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | TAZ/PIPC | Discontinued | IZN; RFN; EMB; PZA | Discontinued | AmphoB | Discontinued | Hydrocortisone | Discontinued | ||
| 2 | Yes | TAZ/PIPC | Meropenem | IZN; RFN; EMB; PZA | Discontinued | AmphoB | Discontinued | Methylprednisolone | Discontinued | ||
| 3 | Yes | TAZ/PIPC | Discontinued | Discontinued | AmphoB | Continued | Prednisone | MP | |||
| 4 | Yes | Meropenem | Continued | MP | |||||||
| 5 | Yes | TAZ/PIPC; vancomicine | Continued | AmphoB | Discontinued | Ganciclovir | Continues | Prednisone | MP | ||
| 6 | Yes | AMP/SUM; clarithromycin | Continued | IZN; RFN; EMB; PZA | Discontinued | MP | |||||
| 7 | No | AMP/SUM; clarithromycin | Continued | Hydrocortisone | MP | ||||||
| 8 | Yes | Meropenem; Discontinued | Vancomicine | IZN; RFN; EMB; PZA | Continued | AmphoB | Discontinued | Prednisone | Discontinued | ||
| 9 | Yes | AMP/SUM; clarithromycin | Continued | IZN; RFN; EMB; PZA | Continued | Oseltamivir | Discontinue | MP | |||
| 10 | No | AMP/SUM; clarithromycin | Continued | ||||||||
| 11 | Yes | AMP/SUM; clarithromycin | Continued | IZN; RFN; EMB; PZA | Discontinued | Oseltamivir | Discontinue | ||||
| 12 | No | SMZ/TMP | Continued | IZN; RFN; EMB; PZA | Continued | Prednisone | MP | ||||
| 13 | Yes | SMZ/TMP | Continued | IZN; RFN; EMB; PZA | Continued | AmphoB | Continued | Hydrocortisone | Discontinued | ||
| 14 | No | SMZ/TMP | Continued | IZN; RFN; EMB; PZA | Methylprednisolone | Continued | |||||
| 15 | Yes | SMZ/TMP | Continued | IZN; RFN; EMB; PZA | Continued | Hydrocortisone | Discontinued |
TAZ/PIPC: piperacillin/tazobactam; SMZ-TMP: trimethoprim/sulfamethoxazole; AMP/SUM: ampicillin/sulbactam; AmphoB: amphotericin B; MP: methylpredonsolone; IZN: isoniazid; RFN: rifampicin; EMB: ethambutol, PZA; pyrazinamide.
Regarding side effects associated with open lung biopsy, no deaths but 3 airway leaks and 1 hemothorax were registered. All side effect were resolved before ICU discharge.
DiscussionThe main result of our study is the high rate of treatment change (0.73) associated with the pathological result of and the acceptable rate of side effects (0.25) of the open lung biopsy. These results, which are in line with reports from other authors,20–22 confirm the useful information that open lung biopsies provide in patients with ARDS of unknown etiology.
The treatment was changed in roughly 3 of 4 patients based on the open lung biopsy results. The most frequent intervention was to discontinue antimicrobial (antibiotics 3/14; antituberculosis 5/10; antifungal 4/6 and antiviral 1/3) or steroid (5/10) treatment. In only one of the cases the antibiotics were changed, and in 3 of the cases steroid therapy was started after the open lung biopsy results. This is highly relevant because this means that without the information provided by the open lung biopsy, most of the patients would be exposed to an unnecessary risk sourced from the side effects of each drug. In addition, it is well known that drugs can exert their benefit only if the population in which they are tested present their target.29 Most of the preclinical ARDS models used for discovering and testing new drugs exhibit the DAD pattern,30 but only half of ARDS patients present DAD.20,31 This low clinical and pathological correlation may be responsible for the lack of effective pharmacological treatments in ARDS patients.6,15 In our cohort, we found that ARDS with DAD constituted 0.80 of the population, which was a higher proportion than what was reported in a recent meta-analysis (meta proportion of DAD 0.48, 95CI, 0.42–0.53).20 We speculate that this difference could be related to the fact that we only use this procedure in cases of completely unknown ARDS, after the patient has been evaluated by a multidisciplinary team and has undergone an exhaustive work-up diagnosis. Given the small sample, we could not exclude that this discrepancy was explained coincidently. On the other hand, diagnosing pathological patterns different from DAD is also relevant because most of them constitute entities with specific treatments and outcomes.10,20,7–28 For example, patient 15 had a high clinical suspicion of bilateral pulmonary pneumonia but the pathology analysis found alveolar edema, which could be associated with a better outcome. On the contrary, the unexpected discovery of lung cancer in patient 1 undoubtedly darkens her outcome.
In reference to procedure safety, we found that roughly 0.25 of patients presented one side effect but none of these complications resulted in death (the cause of death in the two patients with an air leak was refractory hypoxemia with shock; the other death was from refractory hypoxemia). This proportion is similar to that observed in a recent meta-analysis (metaproprotion 0.23, 95CI 0.16; 0.31).23 Furthermore, the main complication (air leak) in our cohort was also the most frequent complication in the meta-analysis mentioned before.23 This result should be considered cautiously because it is clear that open lung biopsy is an invasive and complex procedure associated with severe side effects.
Another important feature of our study is the fact that all open lung biopsies were performed by a senior thoracic surgeon at bedside without transferring the patient to the operating theater. Similar to the cohort reported on by Charbonney et al.,24 this procedure was not associated with any side effect and could avoid some risks associated with patients’ transference such as transitory disconnection of mechanical ventilation, mobilization in an unstable condition or intravenous access loss.25,26
This study has several limitations, firstly it is a retrospective study that includes a wide period of time (seven years) during which several treatments have changed. Indeed, some clinical parameters that we currently recognize as relevant (e.g. driving pressure, plateau pressure or prone position) were not recorded. This limitation, which is also shared by similar studies (Guerin et al.21: 1998–2013; Kao et al.22: 1999–2014 and Charbonney et al.24: 1993–2005), reflects the difficulty for conducting this type of study. Secondly, it has been carried out in a single center. Thirdly, there is an evident selection bias (e.g. non-resolving ARDS, different time lapsed between ARDS diagnosis and open lung biopsy, non-consecutive patients, etc.). Finally, the size of the cohort is relatively small.
This study also presents several strengths: (a) the review of the lung samples was double blind and (b) all the patients were evaluated by an interdisciplinary group of physicians with well demonstrated experience in ARDS.
To conclude, the information provided from the pathological result of the open lung biopsy performed at bedside in ARDS patients with unknown etiology could be relevant as it may optimize the treatment and outcome. Likewise, this invasive procedure seems to be associated with an acceptable risk. However, a natural question for future studies might be “Are side effects of treatment and procedures that are empirically applied to ARDS patients worse or more dangerous than side effects of open lung biopsy?”
Authors contributionReview concept, design and writing the manuscript: GO, MG, DM, PC
Conflict of interestOn behalf of all authors, the corresponding author declares that we have no conflicts of interest.