Exploring infectious diseases (ID) practice in Intensive Care Unit (ICU) to identify gaps and opportunities.
DesignOnline international survey (PRACT-INF-ICU) endorsed by the ESICM and open from July 30, 2019 to October 19, 2019.
SettingInternational study conducted in 78 countries.
ParticipantsPhysicians working in ICU.
InterventionsNone.
Main variables of interestPractice variations were assessed according to respondents’ countries income class, training, and years of practice. Univariate and multivariate ordinal logistic regression were used to estimate associations between respondents’ characteristics and their perceptions regarding adequacy of training.
Results466 intensivists with a median practice of 10 years (interquartile range, 5–19) completed the survey. A third reported no antimicrobial stewardship program and 40% had no regular microbiological rounds in their ICUs. Intensivists were mostly the decision makers for the initial antimicrobial therapy which in 70% of cases were based on guidelines or protocols. Non-ICU expertise were sought more frequently on reviewing (48/72h, culture adjustment and discontinuation in 32%, 39% and 21% respectively) rather than antimicrobial therapy initiation (16%). Only 42% described ID training as adequate. Multivariate ordinal logistic regression showed that low- to middle-income countries (OR: 0.41, 95% CI: 0.28–0.61), ICU practice ≤10 years (OR: 0.55, 95% CI: 0.39–0.79), and dual training with anaesthesia (OR: 0.52, 95% CI: 0.34–0.79) or medicine (OR: 0.49, 95% CI: 0.32–0.76) were associated with less training satisfaction.
ConclusionID practice is heterogeneous across ICUs while antimicrobial stewardship program is not universally implemented. From intensivists’ perspective, ID training and knowledge need improvement.
Explorar la práctica de enfermedades infecciosas (EI) en unidad de cuidados intensivos (UCI) para identificar lagunas y oportunidades.
DiseñoEncuesta internacional online.
ÿmbitoEstudio internacional.
ParticipantesMédicos que trabajan en UCI.
IntervencionesNinguna.
Principales variables de interésLas variaciones de la práctica se evaluaron de acuerdo con la clase de ingresos de los países encuestados, la formación previa y los años de práctica. Se utilizaron análisis de regresión logística ordinal para estimar las asociaciones entre las características de los encuestados y sus percepciones con respecto a la adecuación de la capacitación.
ResultadosCuatrocientos sesenta y seis intensivistas completaron la encuesta. Un tercio de los intensivistas informó que no tenía un programa de optimización de antimicrobianos y el 40% no tenía rondas microbiológicas regulares en su UCI. Los intensivistas eran mayoritariamente los iniciadores de la terapia antibiótica inicial que en el 70% de los casos estaba basada en guías clínicas y protocolos. La regresión logística ordinal multivariante mostró que los países de ingresos bajos a medianos (OR: 0,41; IC del 95%: 0,28-0,61), práctica en la UCI ≤10 años (OR: 0,55; IC del 95%: 0,39-0,79) y entrenamiento dual con anestesiología (OR: 0,52; IC del 95%:0,34–0,79) o medicina (OR: 0,49; IC del 95%: 0,32–0,76) se asociaron con una menor satisfacción con la capacitación en EI.
ConclusionesLa práctica en EI es heterogénea en todas las UCI, mientras que el programa de optimización de antimicrobianos no se implementa universalmente. Desde la perspectiva de los intensivistas, es necesario mejorar la formación y el conocimiento en EI.
Half of patients in Intensive Care Units (ICU) suffer infection while 30% die secondary to it.1 Both ICU-acquired infection and antibiotic resistance are associated with an increased risk of death.1 It follows that 70% of ICU patients are receiving antimicrobial therapy (AMT) representing a burden to healthcare costs, predisposing to antimicrobial resistance (AMR) and worsening patients’ outcome.1,2 AMR is more prevalent in low- to middle-income countries (LMICs) than high-income countries (HICs) and feared to cause millions of deaths by 2050.2
Antimicrobial stewardship programme (ASP) is a mean of limiting AMR while improving patients’ outcome. It is defined as “the coordinated interventions designed to improve and measure the appropriate use of AMT by promoting the selection of the optimal drug regimen including dosing, duration of therapy, and route of administration‿.3 It is recommended as a core competency for intensivists and for all ICUs integrating clinicians, pharmacists, infectious disease (ID) physicians and clinical microbiologists (CM) into one multidisciplinary team.3,4
Understanding ICU ID practice remains essential for identifying gaps and opportunities, but is generally lacking. We conducted an international online survey (PRACT-INF-ICU) to explore from intensivists’ perspective; (1) the practice of ID in the ICU, (2) their satisfaction with ID training, and (3) the impact of defensive medicine on ICU ID practice. Variability in responses were explored regarding settings (HICs vs LMICs), background training and experience.
MethodsSurvey designPRACT-INF-ICU was an international cross sectional descriptive and analytical internet-based open survey of intensivists. The questionnaire was developed by the survey steering committee then pre-tested for clarity, reliability and validity by 2 non-experts and 2 ICU ID experts. It gained approval by the infection section of the European Society of Intensive Care Medicine (ESICM) then endorsed by the ESICM research committee.
The survey consisted of 26 closed and open-ended questions, including Likert scales and adaptive questioning, divided into 4 sections; (1) respondents’ characteristics (questions 1–9), (2) practice of ID management (questions 10–20), (3) respondents’ perceptions regarding adequacy of training (questions 21–24), and impact of defensive medicine on AMT (questions 24 and 25) in addition to a free text annotation (eAppendix-1 in the Electronic Supplementary Material [ESM]).
Survey disseminationThe survey used an online platform (SurveyMonkey®; California, USA) from July 30, 2019 to October 19, 2019 (82 days). It was displayed on the survey page of the ESICM website. ESICM infection section sent 2 invitation emails to all members. Two authors (AR and AE) shared invitations through social media (Facebook and LinkedIn) targeting ICU doctors. All invitations and the ESICM page included a link to the survey page.
The survey was administered anonymously, and the Internet protocol (IP) addresses and identity information were not collected. Unique visitors were determined via IP address but no cookies were used to assign a unique user identifier. IP addresses were not used to identify duplicate entries. The survey consisted of 4 pages (page 1; questions 1–4, page 2; questions 5–9, page 3; questions 10–25 and page 4; question 26). Respondents were able to change their answers on any page until they completed the survey. Completeness check was displayed as question number/total questions. Submitted data were exported as excel spreadsheet and one copy will be kept in ESICM archives for 5 years (accessible only by ESICM employees and protected by its network security system).
The survey was voluntary with no incentives for respondents, and consent was implied by participation. Ethical approval was not required due to the anonymous voluntary nature of the study. This report was constructed according to the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) guidelines (eAppendix-2, ESM).5
Statistical analysisOnly completed surveys were included in the final analyses. Descriptive statistics are presented as either percentage or median (interquartile range [IQR]), as appropriate. Imputation of missing data was not performed. For descriptive statistics, valid percentages (i.e., not including missing data) were used. Comparisons between categorical data were done by chi-square test or Fisher's exact test, as appropriate. Mann–Whitney U test was used for continuous data.
Practice variations were assessed according to respondents’ countries income class (HICs versus LMICs), background training (critical care only, anaesthesia and critical care, or medicine and critical care), and years of practice (categorized as; more experienced [>10 years] or less experienced [≤10 years]). Respondents’ countries income classes were according to the 2019 gross national income per capita using thresholds defined by the World Bank atlas method.6
To estimate associations between respondents’ characteristics and their perceptions regarding adequacy of training, we utilized the ordinal logistic regression (OLR) where the five-point Likert-type responses (‘strongly agree’ is the highest response) as the dependent variable. Only characteristics that had a p value <0.1 in the univariate analysis were included in the multivariate analyses. OLR results are given as cumulative odds ratios (ORs) and 95% confidence intervals (CIs). Reported p values are 2-sided and a p value <0.05 was considered statistically significance. The statistical analysis was performed using SPSS® version 21.0 (IBM).
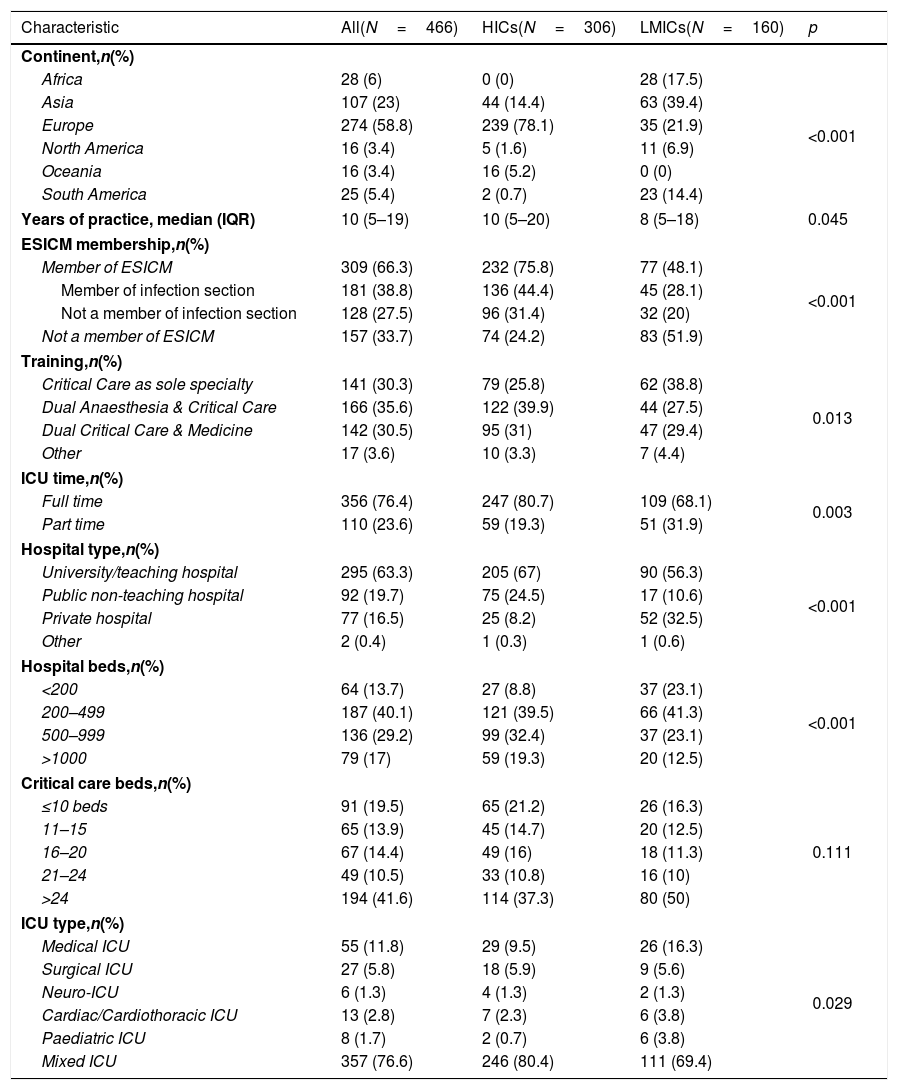
ResultsRespondents’ characteristicsThe survey was initiated by 561 respondents from 82 countries. Four respondents were not ICU physicians and were excluded. Among the remaining 557 respondents, 446 (83.7%) completed the survey (eTable 1, ESM) from 74 countries (eFig. 1) including 26 respondents from Spain. Respondents were from Europe (58.8%), Asia (23%), Africa (6%), South America (5.4%), North America (3.4%), and Oceania (3.4%), including 34.3% from LMICs. They were divided nearly equally into those trained solely in Critical Care Medicine (CCM), or dually along with medicine or anaesthesia (30.3%, 35.6% and 30.5% respectively). The median duration of clinical practice was 10 years (IQR, 5–19), and 77% were working in mixed ICUs (Table 1).
Respondents characteristics.
| Characteristic | All(N=466) | HICs(N=306) | LMICs(N=160) | p |
|---|---|---|---|---|
| Continent,n(%) | ||||
| Africa | 28 (6) | 0 (0) | 28 (17.5) | <0.001 |
| Asia | 107 (23) | 44 (14.4) | 63 (39.4) | |
| Europe | 274 (58.8) | 239 (78.1) | 35 (21.9) | |
| North America | 16 (3.4) | 5 (1.6) | 11 (6.9) | |
| Oceania | 16 (3.4) | 16 (5.2) | 0 (0) | |
| South America | 25 (5.4) | 2 (0.7) | 23 (14.4) | |
| Years of practice, median (IQR) | 10 (5–19) | 10 (5–20) | 8 (5–18) | 0.045 |
| ESICM membership,n(%) | ||||
| Member of ESICM | 309 (66.3) | 232 (75.8) | 77 (48.1) | <0.001 |
| Member of infection section | 181 (38.8) | 136 (44.4) | 45 (28.1) | |
| Not a member of infection section | 128 (27.5) | 96 (31.4) | 32 (20) | |
| Not a member of ESICM | 157 (33.7) | 74 (24.2) | 83 (51.9) | |
| Training,n(%) | ||||
| Critical Care as sole specialty | 141 (30.3) | 79 (25.8) | 62 (38.8) | 0.013 |
| Dual Anaesthesia & Critical Care | 166 (35.6) | 122 (39.9) | 44 (27.5) | |
| Dual Critical Care & Medicine | 142 (30.5) | 95 (31) | 47 (29.4) | |
| Other | 17 (3.6) | 10 (3.3) | 7 (4.4) | |
| ICU time,n(%) | ||||
| Full time | 356 (76.4) | 247 (80.7) | 109 (68.1) | 0.003 |
| Part time | 110 (23.6) | 59 (19.3) | 51 (31.9) | |
| Hospital type,n(%) | ||||
| University/teaching hospital | 295 (63.3) | 205 (67) | 90 (56.3) | <0.001 |
| Public non-teaching hospital | 92 (19.7) | 75 (24.5) | 17 (10.6) | |
| Private hospital | 77 (16.5) | 25 (8.2) | 52 (32.5) | |
| Other | 2 (0.4) | 1 (0.3) | 1 (0.6) | |
| Hospital beds,n(%) | ||||
| <200 | 64 (13.7) | 27 (8.8) | 37 (23.1) | <0.001 |
| 200–499 | 187 (40.1) | 121 (39.5) | 66 (41.3) | |
| 500–999 | 136 (29.2) | 99 (32.4) | 37 (23.1) | |
| >1000 | 79 (17) | 59 (19.3) | 20 (12.5) | |
| Critical care beds,n(%) | ||||
| ≤10 beds | 91 (19.5) | 65 (21.2) | 26 (16.3) | 0.111 |
| 11–15 | 65 (13.9) | 45 (14.7) | 20 (12.5) | |
| 16–20 | 67 (14.4) | 49 (16) | 18 (11.3) | |
| 21–24 | 49 (10.5) | 33 (10.8) | 16 (10) | |
| >24 | 194 (41.6) | 114 (37.3) | 80 (50) | |
| ICU type,n(%) | ||||
| Medical ICU | 55 (11.8) | 29 (9.5) | 26 (16.3) | 0.029 |
| Surgical ICU | 27 (5.8) | 18 (5.9) | 9 (5.6) | |
| Neuro-ICU | 6 (1.3) | 4 (1.3) | 2 (1.3) | |
| Cardiac/Cardiothoracic ICU | 13 (2.8) | 7 (2.3) | 6 (3.8) | |
| Paediatric ICU | 8 (1.7) | 2 (0.7) | 6 (3.8) | |
| Mixed ICU | 357 (76.6) | 246 (80.4) | 111 (69.4) | |
HICs, high-income countries; LMICs, low- and middle-income countries; IQR, interquartile range; ESICM, European Society of Intensive Care Medicine; ICU, Intensive Care Unit.
Two-thirds of respondents reported good knowledge of their hospitals’ microbiological diagnostic capabilities, which was lower in LMICs compared to HICs (54% vs 73%, p<0.001), and among less experienced compared to more experienced intensivists (57% vs 76%, p<0.001).
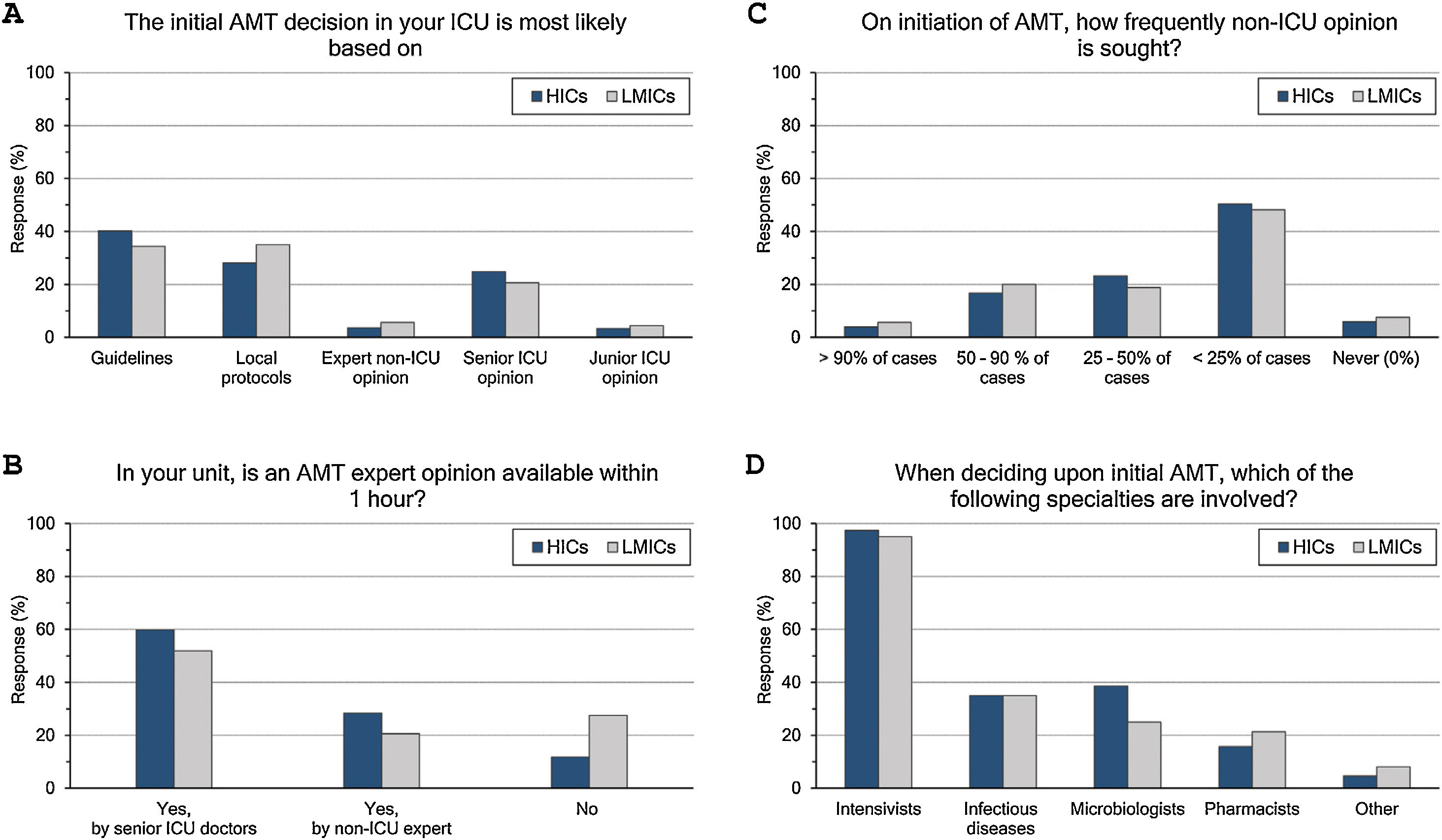
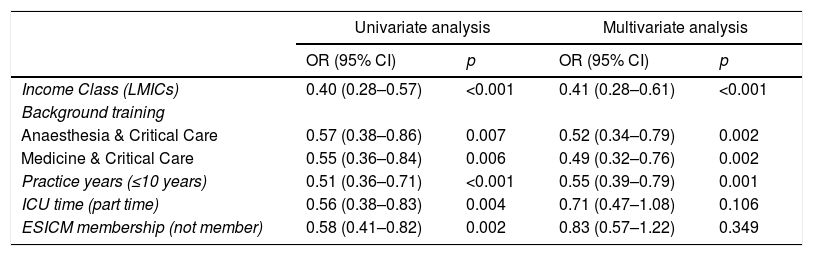
Initial AMT decision: (Fig. 1 and eTable 3, ESM)The majority (70%) of intensivists based their initial AMT decision on guidelines and protocols. An expert opinion within 1 hour was available for 83% of them, yet less frequently in LMICs than HICs (73% vs 88%, p<0.001). Initial AMT decision was almost always done by intensivists while non-ICU specialities were less frequently involved (35%, 33.9% and 17.6% for ID specialities, microbiologists, and pharmacists, respectively). Only 22% of intensivists frequently sought non-ICU opinion (>50% of cases), which was less likely for more experienced than less experienced intensivists (18% vs 26%).
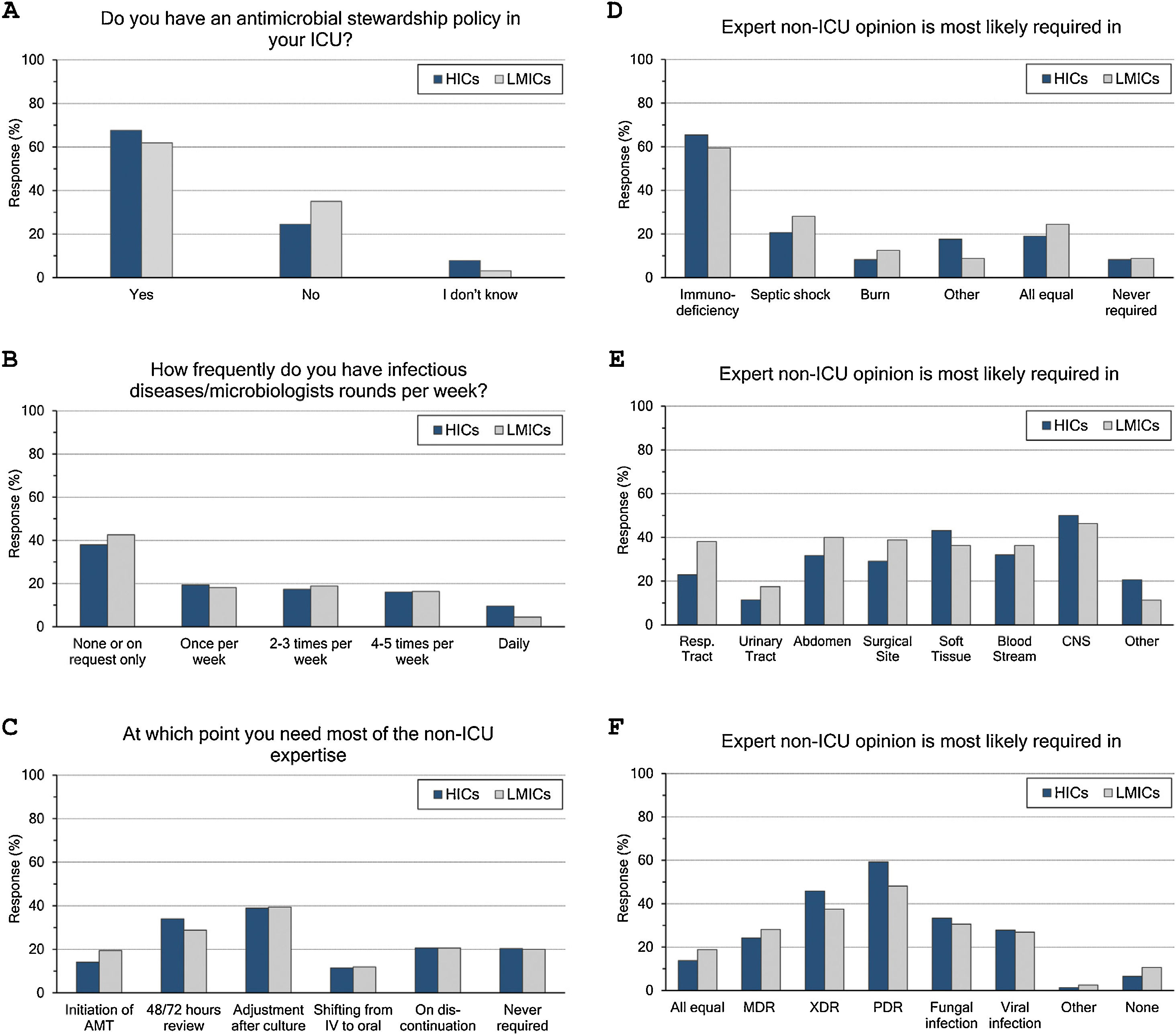
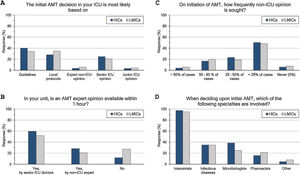
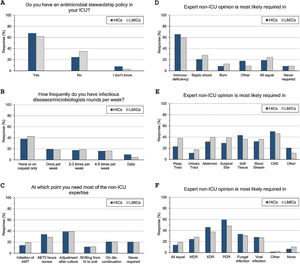
ASP and ID specialists’ rounds: (Fig. 2 and eTable 4, ESM)Two-thirds of intensivists reported having ASP in their units and 40% had no regular rounds by ID specialists’. ASP was less reported in LMICs than HICs (62% vs 68%, p=0.015), and 10% of the less experienced intensivists did not know whether they have ASP in their unit or not.
Antimicrobial stewardship programme implementation (A), infectious diseases specialists’ rounds per week (B), and respondents’ perceptions regarding their need of non-ICU expertise (C–F). ICU, Intensive Care Unit; HICs, high-income countries; LMICs, low- and middle-income countries; IV, intravenous; AMT, antimicrobial therapy; CNS, central nervous system; MDR, multidrug-resistant; XDR, extensively drug-resistant; PDR, Pandrug-Resistant.
Intensivists felt a greater need for non-ICU expertise on reviewing AMT (48/72h review: 32%, adjustment to culture: 39% and AMT discontinuation: 21%), however 20% never requiring non-ICU expertise. More experienced intensivists reported significantly less need for non-ICU expertise and 29% of them never asking for such expertise. Only 11% of intensivists with background training in anaesthesia never required non-ICU expertise compared to 26% with medical training background.
Intensivists were more probably asking for expert non-ICU opinion for immunocompromised patients (63%), central nervous system infections (49%), non-surgical soft tissue infection (41%), followed by abdominal sepsis, blood stream and surgical site infections. For bacterial infections, the more resistant the organism, the more often intensivists asked for external advice (55%, 43% and 25.5% of pan-resistant, extended-resistant and multi-resistant organisms, respectively). In general, intensivists were most likely to ask for secondary opinion when facing resistant bacterial infections than fungal (32%) or viral ones (27%). The more experienced intensivists and those with sole CCM training or dually with medicine were less likely asking for second ID opinion.
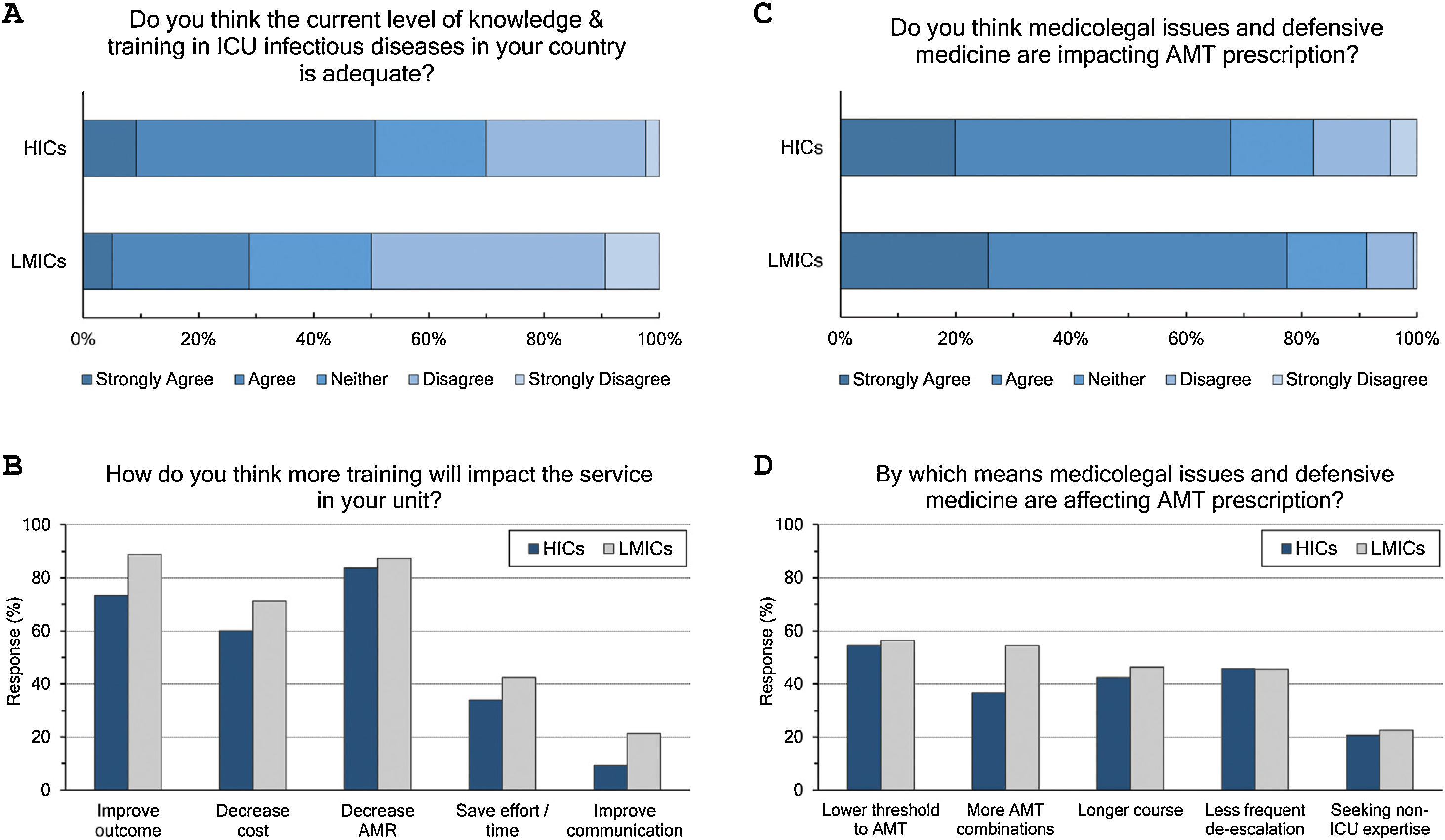
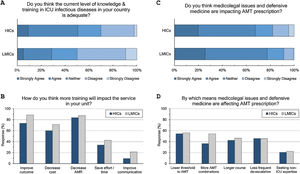
Adequacy of training: (Fig. 3 and eTable 6, ESM)Only 42% of intensivists agreed the level of knowledge and training in ID in their countries is adequate. Satisfaction was significantly lower in LMICs compared to HICs (29% vs 51%, p<0.001) and in less experienced compared to more experienced intensivists (36% vs 51%, p<0.001). Doctors trained in CCM as sole specialty are more satisfied with ID training than doctors trained dually along with anaesthesia or medicine (56%, 39% and 36%, respectively). Almost all respondents (96%) thought there is a need for more training to decrease AMR (85%), improve patients’ outcome (79%), and decrease cost (64%).
Respondents perceptions regarding the adequacy of training in infectious diseases (A and B) and the impact of defensive medicine on AMT (C and D). ICU, Intensive Care Unit; HICs, high-income countries; LMICs, low- and middle-income countries; AMT, antimicrobial therapy; AMR, antimicrobial resistance.
Univariate OLR analysis showed that income class, practice years, background training, CCM working time, and ESICM membership were associated with respondents’ perspective regarding adequacy of training. Multivariate analysis showed that LMICs (cumulative odds ratio [OR]: 0.41, 95% CI: 0.28–0.61), practice years ≤10 years (OR: 0.55, 95% CI: 0.39–0.79), and dual CCM training with anaesthesia (OR: 0.52, 95% CI: 0.34–0.79) or medicine (OR: 0.49, 95% CI: 0.32–0.76) were independently associated with less ID training satisfaction (Table 2).
Ordinal regression analysis for adequacy of infectious diseases training.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Income Class (LMICs) | 0.40 (0.28–0.57) | <0.001 | 0.41 (0.28–0.61) | <0.001 |
| Background training | ||||
| Anaesthesia & Critical Care | 0.57 (0.38–0.86) | 0.007 | 0.52 (0.34–0.79) | 0.002 |
| Medicine & Critical Care | 0.55 (0.36–0.84) | 0.006 | 0.49 (0.32–0.76) | 0.002 |
| Practice years (≤10 years) | 0.51 (0.36–0.71) | <0.001 | 0.55 (0.39–0.79) | 0.001 |
| ICU time (part time) | 0.56 (0.38–0.83) | 0.004 | 0.71 (0.47–1.08) | 0.106 |
| ESICM membership (not member) | 0.58 (0.41–0.82) | 0.002 | 0.83 (0.57–1.22) | 0.349 |
OR, cumulative odds ratio; CI, confidence interval; LMICS, low- and middle-income countries; ICU, Intensive Care Unit; ESICM, European Society of Intensive Care Medicine.
The majority (71%) of respondents agreed that medicolegal issues and defensive medicine are impacting AMT prescription which was higher in LMICs than HICs (77.5% vs.67.6%). Defensive practices reduce the threshold to prescribe antibiotics (55%), and leads to more antibiotic combinations (43%), longer courses (44%), less frequent de-escalation (ADE) (46%) and seeking non-ICU expertise (21%).
DiscussionOur survey highlights shortfalls and heterogeneity in managing infectious diseases in the ICU. ASPs are variously implemented and intensivists reported a need for more ID education.
ASP aims to reduce AMT exposure and to improve patients’ outcomes. Despite valued by most intensivists, ASP implementation remains short.7 In 2012, it was applied in just 58% of hospitals worldwide, with significant variability in policies, strategies, priorities and programmes maturity.8 ASP can vary within the single hospital and while our survey focusses on ICU settings, it draws a similar picture to other hospital settings (1/3 of intensivists reported no ASP).8 Even in developed countries, less than half of French intensivists were aware of ASP, and only 74% of Canadian intensivists reported ASP in their institutions.7,9 A Spanish survey indifferently showed ASP to be implemented nationally in only 37% of ICUs.10
It is becoming crucial to study the barriers impeding ASP in the ICU. While some are shared across all healthcare settings (e.g., funds, staffing, clinicians’ resistance and lack of data technology), others remain ICU specific.7 Most importantly may be the lack of ICU specific ASP studies and guidance.4 Furthermore, conflicts can arise between intensivists’ goals (patient cure) and some perceived ASP targets (AMT use or cost). A local leadership within the ICU can mitigate most conflicts, compensate for staff shortage and provide the support needed for a successful, sustainable and effective ASP. ICU ID champions were shown also to be associated with better ASP implementation.9 In this context, it is noteworthy the high participation of Spanish intensivists in their units’ ASP programmes.10 Such training in addition to participation in audits and research can provide better insight and unify goals (e.g., drug toxicity, AMR and long-term impact).4 However, auditing and feedback are known to be among the least applied ASP measures.7 Last, tele-ASP can be the answer for smaller and remote units suffering staff or expertise shortage.
Heterogeneity in ICU ID/ASP practice is not new to report, and our survey is another confirmation.9,11 LMICs intensivists are less exposed to ASP, have less expert opinion and declared greater knowledge gap and need for training. This may reflect inadequate training or be institutional in origin (e.g., part-time work, smaller or private hospitals, less administrative awareness, lack of data technology and auditing culture).8 ASP was previously shown to be implemented in only 12% of African versus 77% of European hospitals.8 While limited access to AMT risks higher mortality, it also limits choices and proper usage.2 This should raise global concerns as ID and AMR are difficult to contain and a local substandard practice can progress easily across borders.
Despite the availability of expert opinion, intensivists preferred a protocolized over a personalized approach when initiating AMT. An independent AMT prescribers should be able to recognize the need for expertise and co-operate as appropriate.12 However, routine ID consultation is practiced in only 11% of Italian ICUs.13 In Spain, the reference expert is intensivist or anaesthesiologist in 91% of ICUs compared to ID specialist in only 7%.10 Lack of expertise risks prescribing higher tier and broader spectrum antibiotics due to diagnostic uncertainty and higher risk of deterioration in the critically ill.14
While adherence to guidelines is one of the ASP goals, AMR may spread fast and guidelines become outdated.15 Furthermore, guidelines are usually issued in HICs reflecting different resources, staffing, training, and AMR patterns.16 The American Thoracic Society (ATS) acknowledged better AMT optimization through individualization rather than rigid guidelines adherence.4 One observational work suggested adherence to guidelines may be associated with increased mortality.17 If local AMR is monitored, Individualized AMT can be preferred (e.g. hospital acquired infections).4 ICUs are sometimes exempt from AMT restriction (an ASP intervention) while surveillance cultures can further blur the boundaries between colonization and infection.4,8 Novel AMT methods may prove challenging for inexperienced prescribers (e.g., therapeutic drug monitoring [TDM], pharmacokinetics/pharmacodynamics [PK/PD] concept, continuous/extended infusion, nebulized AMT). Holding AMT in stable patients had also been suggested but needs expert support, monitoring and rapid diagnostic tests.18 For all the aforementioned reasons, and while guidelines can provide the minimal satisfactory standard, and unless ICU senior team has a high level of expertise, the role of pharmacist and ID specialists remains indispensable for a tailored management, however difficult to achieve.
Review and ADE usually follow 48–72h after AMT initiation. Such refinement is a precision decision: Individualizing AMT based on microbiological results, imaging and clinical course. In the present survey, intensivists sought non-ICU expertise more frequently on reviewing compared to initiation. However, the practice remained modest in best description (48/72h review, adjustment to culture and discontinuation in 32%, 39% and 21% respectively). The DIANA study already showed ADE to be an exceptional ICU practice (16% of cases).14 Feedback during AMT review represents an ASP learning strategy and we can point up here a lost educational opportunity. However, a meta-analysis demonstrated no impact of feedback on ICU mortality despite reduction of AMT use.19
Traditional microbiological results are usually available 12–72h after AMT initiation. Molecular diagnostic tests provide faster identification of the causative micro-organism and, possibly, antibiotic susceptibility (e.g., genetic testing). If applied along with ASP, such tests can improve mortality while reducing AMT use, costs, toxicity and resistance.20 Only two-thirds of respondents reported good knowledge about hospitals’ microbiological diagnostic capabilities, which was even lower in LMICs, and for the less experienced. Such inadequate understanding, may hinder a proper initial timely testing and make subsequent review harder. Our findings suggest the need for proper orientation of such new technology.4
Collaboration between ID specialists and intensivists may impact patients’ outcome.21–23 A previous survey highlighted the intensivists’ inclination for expert opinion especially where ASP is not in place. However, a considerable percentage of respondents in our work reported the absence of regular ID rounds and variable need for non-ICU expertise according to the settings, background training, and expertise. The involvement of ID specialist in managing Staphylococcus aureus bacteraemia24 and candidemia25 were previously shown to be associated with reduction in AMT duration and mortality. However, only one-third of respondents think blood stream or fungal infections require non-ICU expertise. Again, only a quarter considered consultation for viral infections. Taking into account the diagnostic and therapeutic complexity of those infections, we are not sure if the answers reflect an appropriate level of knowledge. To note that only 20% of UK ICUs had intensivists with special interest in fungal diseases.26 This situation may get worse with the emergence of new infections, increasingly complex AMR and the introduction of novel AMT and microbial tests.
AMR awareness and strengthening knowledge (through training and research) are 2 of 5 objectives of the 2015 WHO global action plan on AMR.27 Both AMR and ASP were recommended as CCM core training competencies.4,27 However, only 42% of intensivists consider their ID training and knowledge adequate. Multivariate analysis showed that LMICs, practice ≤10 years, and dual CCM training with anaesthesia or medicine to be independently associated with less training satisfaction. Nevertheless, the insight of a knowledge gap is positive considering previous reports showing absent acknowledgement of the problem.28,29
Competencies recommended by ICU societies30,31 are becoming short to meet the evolving threat of ID and AMR (e.g., COVID-19) and generally lack an updated detailed cover for ICU ID specificities. The ‘Competency-Based Training in Intensive Care Medicine in Europe’ (CoBaTrICE) comprises 4 ID/AMT competencies addressing microbiological sampling, managing sepsis, AMT and infection control.32 UK, USA and China issued also their national recommendations covering a variety of AMT, microbial testing, infection control and management.31,33,34 However, they remain generally missing in LMICs. Intensivists act usually as independent prescribers and should be able to fulfil the 35 competencies recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID).12 More recently, an ATS workshop recommended ASP as a core ICU competency.4 Pragmatically, 2 tiers of knowledge may be needed: Basic knowledge for every intensivist, and more advanced one for ID-subspecialised intensivists leading ASP, ID research and education. Alternatively, focused ASP training can be implemented when ID expertise is absent (e.g., LMICs). Integrating ID/ASP into daily ICU rounds can improve knowledge and awareness while setting ASP as a daily practice.4 Dual ID and ICM training was also proposed.35
CCM is a multi-disciplinary specialty where diversity and inclusiveness are considered points of strength. Even in developed and closely related countries (e.g., European Union), the training pathways for intensivists are not unified.36 Classically, intensivists are initially trained as anaesthetists or internists (or in any medical subspecialty) before acquiring CCM as super-specialty. Less frequently, CCM is a standalone training. Our data show that intensivists trained solely in CCM are seeking generally less non-ICU expertise and follow more personalized approach on initiating AMT. They are also more confident to manage multi-resistant bacterial and viral infections and slightly more satisfied with their ID training. These findings may reflect more profound practical and theoretical ID training for doctors solely trained as intensivists. In this context, Vidal-Cortés et al. showed that CCM trainees in Spain to have more scheduled time in ID units compared to anaesthetic trainees.10 The different training pathways recently raised concerns about the flexibility of intensivists’ movement across Europe in view of the COVID-19 pandemic.36 We hope our survey by shedding lights on the ICU ID practice can help reforming and unifying ICU ID training.
The fear of missing a causative pathogen leading to adverse outcome is a drive for excessive AMT rather than knowledge deficit.4 Such fear from legal liabilities (i.e., defensive medicine) is emerging to impact practice by the mean of unnecessary referrals, unnecessary tests/prescriptions and avoiding high risk procedures.37 In the AntibioLegalMap survey, 75% of ID and CM specialists reported engagement in some form of defensive behaviour.38 71% of intensivists in our survey admitted a defensive attitude by lowering the threshold to start AMT, or by prescribing longer courses or combinations of AMT (55.2%, 43.8% and 42.7% respectively). Certain defensive behaviours (e.g., over prescription or unnecessary invasive procedures) impose risks to patients, while others (e.g., referral to more specialized physicians) might improve quality of care.37 However, only 21% of respondents think defensive behaviour would lead to seeking non-ICU expertise. Furthermore, AMT may wrongly become a substitute for source control interventions reflecting inadequate awareness of the AMT associated harms.
We admit many limitations of our work. Surveys are subject to “response bias‿ with possible discrepancies between what is self-reported and actual practice. Despite our aim was to explore the intensivists’ insight and opinion, we appreciate microbiologists, ID doctors or pharmacists may have a different point of view and probably better insight for some aspects of the questionnaire. The survey was focused on the practice of ID in the ICU; however, we did not revisit more complex principles like therapeutic drug monitoring and pharmacodynamics/pharmacokinetics which may be of interest as standalone survey.
A point of strength is the number of survey responders; however, the target population remains much bigger. Furthermore, there may be “a participation bias‿ where ID-interested intensivists more likely to participate. Most respondents were from either Europe or Asia and the results might be more representative of these regions. Furthermore, the survey targeted intensivists as persons rather than being institutional, so some respondents may be practicing in the same unit and answers were skewed. Despite these limitations, the diversity of respondents’ countries, settings, experience and training are positive points and we believe our data are clearly highlighting a practice variability and gap of knowledge when managing ID in the ICU worldwide.
ConclusionsInfectious diseases practice is heterogeneous across ICUs underscoring the need for a common set of minimal standards. Intensivists feel the need to improve their ID training and knowledge. Such need is more evident in certain settings such in LMICs, but also for part time and less experienced intensivists. In contrast, doctors with CCM as sole training were more satisfied with ID training. This raises the point for a minimal standard set for ID CCM training. There is also a need to issue ICU specific ASP guidance while encouraging a more precise ID approach.
Authors’ contributionsAR and AE designed and disseminated the survey questionnaire, AS reviewed the questionnaire for intellectual content and is responsible for the statistical analysis. AR and AS wrote the first draft. All authors critically revised the manuscript. All authors read and approved the final manuscript.
Conflict of interestAshraf Roshdy declares owning shares in Astra Zeneca, Pfizer and Merk pharmaceutical companies. He gave also unpaid lecture for Pfizer Company. Ahmad Sabry Saleh and Ahmad Samy Elsayed declares no conflict of interest.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
This survey was endorsed by the European Society of Intensive Care Medicine (ESICM). The participation of intensivists from around the globe in the survey, without financial incentive, is acknowledged gratefully. The authors are indebted to thank Prof. Pedro Povoa and Dr. Andrew Conway Morris (from the infection section of the ESICM) for reviewing the survey questionnaire and for their continued support and guidance. We also thank Ms. Sherihane Bensemmane (ESICM research office) for her valuable support in creating and promoting the online survey; Dr. Ferial Moursi for the critical review of the questionnaire and Dr Jeronimo Moreno-Cuesta for his revision of the final draft.
Parts of this report were presented at the 33rd annual congress of the European society of intensive care medicine (LIVES DIGITAL 2020), 6–9 December 2020.