The highly contagious severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulted in a global outbreak of coronavirus disease 2019 (COVID-19). The manifestations of SARS-CoV-2 infection range from a total absence of symptoms to rapidly progressing life-threatening disease.1 Cardiovascular complications reported in patients with COVID-19 admitted to intensive care units (ICU) include arrhythmias (16%–44%), shock (30.6%), acute heart failure (10%), myocarditis (1%), and myocardial injury (22%–33%); these complications have been associated with mortality as high as 67%.2,3 Patients with COVID-19-related cardiovascular complications have elevated inflammatory markers (e.g., interleukin-6, C-reactive protein (CRP), and cardiac troponin (cTn), among others).4 Notably, hs-cTn, which is indicative of myocardial injury, is associated with severe COVID-19 and worse outcomes in mixed populations of patients with severe and non-severe disease.5,6 Nevertheless, the relationship between hs-cTn and mortality in ICU patients has not been firmly established. Based on current evidence, we hypothesized that myocardial injury defined as hs-cTn>47ng/L.7 could worsen outcomes in patients with severe SARS-Cov-2 pneumonia.
This study was approved by local ethical committee at Joan XXIII University Hospital (IRB#CEIM/066/2020). Written informed consent was waived owing the nature of the study.
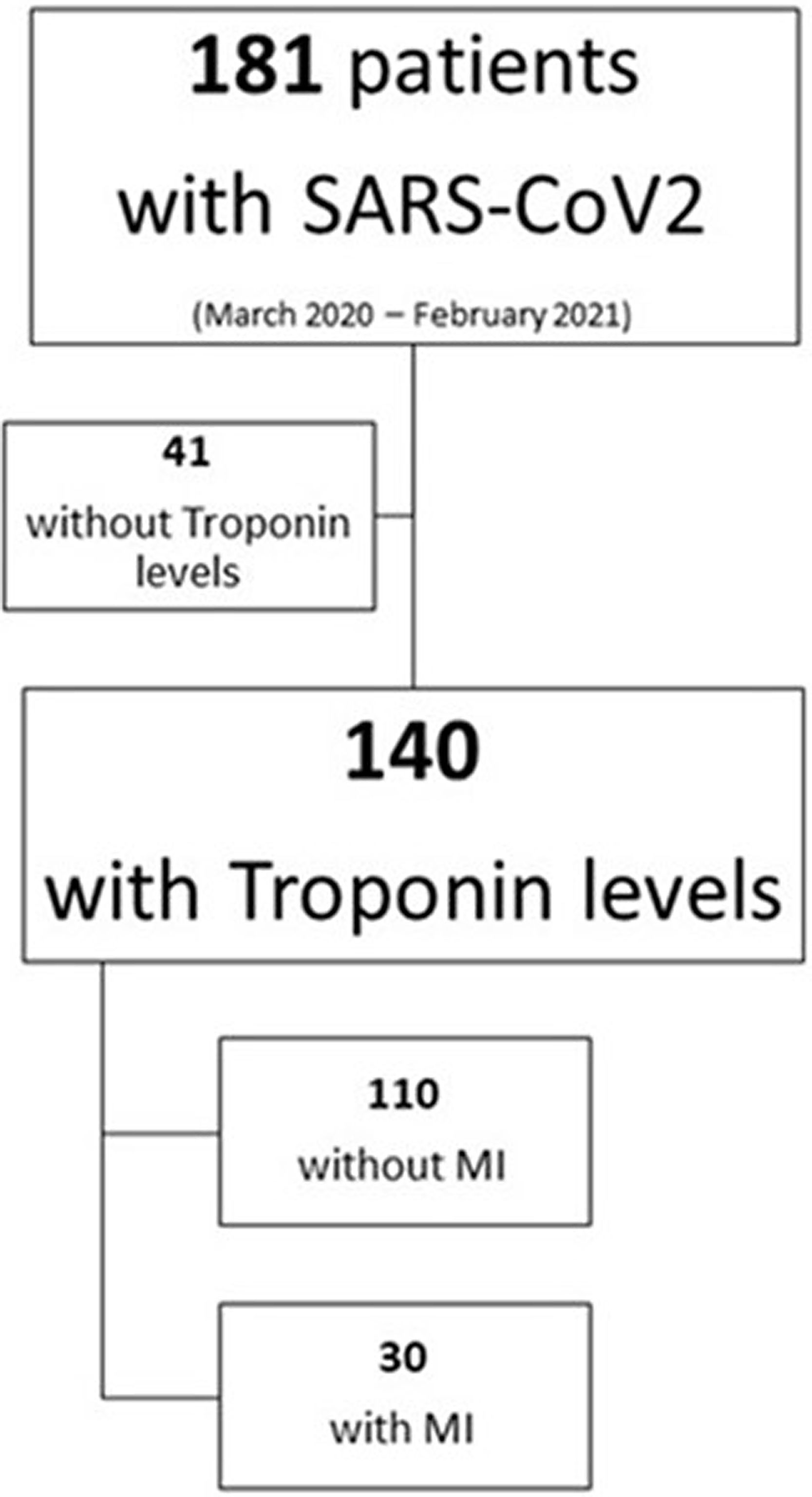
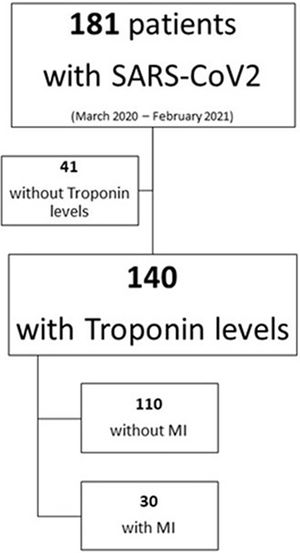
From a total of 181 patients with confirmed severe SARS-CoV-2 pneumonia, we analyzed 140 patients in which hs-cTn levels were available within 72h after admission to the ICU of a university hospital in Spain between March 2020 and February 2021 (Figure 1, Supplementary material).
Table 1 reports patient's baseline characteristics.
Baseline characteristics of patients with and without myocardial injury.
| Cohort population [n 140] | With myocardial injury [n 30] | Without myocardial injury [n 110] | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Female, n (%) | 42 (30%) | 10 (33.3%) | 32 (29.1%) | 0.653 |
| Age, median (IQR) | 66 (54–72) | 65 (55.8–73) | 66 (54–71.2) | 0.415 |
| BMI, median (IQR) | 27.9 (26.1–32.4) | 27.7 (25.9–32.2) | 28.2 (26.1–32.7) | 0.603 |
| Overweight, n (%) | 64 (45.7%) | 15 (50%) | 49 (44.5%) | 0.595 |
| Obesity, n (%) | 39 (28%) | 9 (30%) | 30 (27.3%) | 0.768 |
| Morbid obesity, n (%) | 12 (8.6%) | 1 (3.3%) | 11 (10%) | 0.248 |
| Hypertension, n (%) | 67 (48%) | 15 (50%) | 52 (47.3%) | 0.791 |
| Diabetes mellitus, n (%) | 33 (23.6%) | 8 (23.6%) | 25 (22.7%) | 0.652 |
| Cardiovascular disease | 17 (12%) | 6 (20%) | 11 (10%) | 0.137 |
| Chronic atrial fibrillation, n (%) | 6 (4.3%) | 1 (3.3%) | 5 (4.5%) | 1 |
| Chronic cardiac dysfunction, n (%) | 2 (1.4%) | 2 (6.7%) | 0 (0%) | 0.006 |
| Chronic cardiac ischemia, n (%) | 12 (8.6%) | 5 (16.7%) | 7 (6.4%) | 0.074 |
| Chronic renal disease, n (%) | 7 (5%) | 2 (6.7%) | 5 (4.5%) | 0.637 |
| Severity scores | ||||
| CHARLSON, median (IQR) | 3 (1–4) | 3 (1–5.25) | 2 (1–3) | 0.1 |
| APACHE II, median (IQR) | 16 (12–20) | 17.5 (14.75–21.5) | 15 (12–19.75) | 0.068 |
| SAPS III, median (IQR) | 52 (48–56) | 52.5 (49.5–56) | 52 (48–56) | 0.299 |
| SOFA 24h, median (IQR) | 4 (3–6) | 4 (3.75–7) | 4 (3–6) | 0.134 |
| Analytics | ||||
| Lactate mmol/L, median (IQR) | 1.8 (1.4–2.2) | 1.93 (1.5–2.5) | 1.78 (1.46–2.2) | 0.191 |
| Creatinine mg/dL, median (IQR) | 0.8 (0.63–1) | 1 (0.7–1.5) | 0.77 (0.63–0.98) | 0.012 |
| PCT ng/mL, median (IQR); [n] | 0.19 (0.07–0.5) [124] | 0.25 (0.14–2.2) [29] | 0.15 (0.07–0.44) [95] | 0.012 |
| CRP mg/dL, median (IQR) | 15.5 (7.8–23) | 21.8 (13.1–26.7) | 12.5 (7.38–21.6) | 0.016 |
| Leukocytes×103, median (IQR) | 8.5 (6.5–11.4) | 9 (7.2–12.4) | 8.4 (6.4–11.4) | 0.186 |
| IL-6pg/mL, median (IQR); [n] | 32 (8.2–111) [82] | 38.7 (4.7–135) [15] | 31.5 (8.3–107) [67] | 0.764 |
| Ferritin ng/mL, median (IQR); [n] | 945 (537–1650) [100] | 945 (577–1792) [22] | 956 (526–1552) [78] | 0.934 |
| D-dimer ng/mL, median (IQR); [n] | 1.25 (0.7–2.4) [125] | 2.6 (1.04–15.12) [27] | 1.1 (0.6–1.8) [98] | 0.001 |
| ProBNP ng/mL, median (IQR); [n] | 312 (149–32515) [40] | 2287 (270–17776) [12] | 235 (135–1182) [28] | 0.011 |
| hs-Tn ng/L, median (IQR) | 11 (4.3–30.7) [140] | 162.5 (92–673) | 8.5 (4–14) | 0.000 |
| Use of drugs at 3rd day | ||||
| Corticoids, n (%) | 95 (67.9%) | 19 (63.3%) | 76 (69.1%) | 0.549 |
| Noepinephrine, n (%) | 100 (71.4%) | 24 (80%) | 76 (69.1%) | 0.241 |
| Furosemide, n (%) | 58 (41.4%) | 15 (50%) | 43 (39.1%) | 0.282 |
| Renal | ||||
| Hydric balance 3d ml, mean (±SD) | −788.8 (±1925) | −413.2 (±1636) | −889.7 (±1990) | 0.238 |
| Diuresis 3d ml, mean (±SD) | 4162.9 (±1338.7) | 4231 (±1855) | 4144 (±1172) | 0.813 |
| AKI, n (%) | 39 (27.9%) | 13 (43.3%) | 27 (24.5%) | 0.043 |
| CRRT, n (%) | 7 (5%) | 3 (10%) | 4 (3.6%) | 0.156 |
| Hemodynamic | ||||
| HR on admission, median (IQR) | 79 (70–92) | 84.5 (66–102.8) | 110 (70.8–89) | 0.239 |
| Median BP on admission, mean (±SD) | 91.3 (±17.6) | 88.5 (±21.1) | 92 (±16.5) | 0.408 |
| Cardiovascular complications, n (%) | 122 (87.1%) | |||
| Shock, n (%) | 30 (21.4%) | 13 (43.3%) | 18 (16.4%) | 0.002 |
| LVD, n (%); [n] | 11 (11.8%) [93] | 9 (31%) | 2 (3.1%) | 0.000 |
| RVD, n (%); [n] | 3 (2.6%) [116] | 2 (8%) | 1 (1.1%) | 0.117 |
| Arrhythmias, n (%) | 77 (55%) | 16 (53.3%) | 61 (55.5%) | 0.836 |
| Sinus bradycardia, n (%) | 62 (44.3%) | 10 (33.3%) | 52 (47.3%) | 0.173 |
| Atrial-ventricular block, n (%) | 3 (2.1%) | 0 (0%) | 3 (2.7%) | 0.226 |
| Atrial fibrillation, n (%) | 6 (4.3%) | 4 (13.3%) | 2 (1.8%) | 0.019 |
| Long QT, n (%) | 13 (9.3%) | 4 (13.3%) | 9 (8.2%) | 0.389 |
| MI, n (%) | 30 (21.4%) | |||
| Pulmonary | ||||
| PaO2/FiO2 at intubation, median (IQR); [n] | 91 (75–115) [120] | 82.5 (70–100) [28] | 95 (75–120) [92] | 0.207 |
| MV, n (%) | 124 (88.6%) | 30 (100%) | 94 (85.5%) | 0.026 |
| MV days, median (IQR); [n] | 19 (9–32.3) [130] | 18.5 (8.25–22) | 26 (10–33) [90] | 0.195 |
| Outcomes | ||||
| LOS, median (IQR) | 21 (11–33) | 24 (15.5–32.3) [24] | 14 (9–35) [97] | 0.161 |
| 28-day mortality, n (%) | 23 (16.4%) | 9 (30%) | 14 (12.7%) | 0.024 |
| ICU mortality, n (%) | 36 (25.7%) | 10 (38.5%) | 26 (24.8%) | 0.161 |
Data with [n] is the number of participants in the corresponding cell. BMI=body mass index, APACHE II=acute physiology and chronic evaluation score, SOFA=sequential organ failure assessment, SAPS III=simplified acute physiology score III, PCT=procalcitonin, CRP=C reactive protein, IL-6=interleukine-6, ProBNP=n-terminal pro-B-type natriuretic peptide, hs-Tn=high sensitive cardiac troponin, AKI=acute kidney injury, CRRT=continuous renal replacement therapy, HR=heart rate, BP=blood pressure, LVD=left ventricular systolic dysfunction, RVD=right ventricular systolic dysfunction, MV=mechanical ventilation, LOS=length of stay, and ICU=intensive care unit.
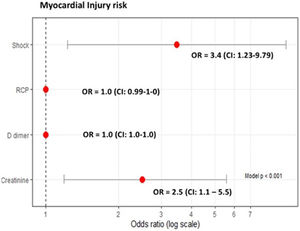
Median hs-cTn was 11ng/L [IQR 4.3–30.7]; hs-cTn was >47ng/L in 30 (21.4%) patients. Compared to patients without myocardial injury, those with hs-cTn>47ng/L had higher median concentrations of procalcitonin (0.25 [IQR 0.14–2.2]mg/dL vs. 0.15 [IQR 0.07–0.44]mg/dL, p=0.016), CRP (21.8 [IQR 13.1–26.7]mg/dL vs. 12.5 [IQR 7.3–21.6]mg/dL, p=0.012), and D-dimer (2600 [IQR 1040–15,120]ng/mL vs. 1100 [IQR 600–1800]ng/mL, p=0.001), and a greater percentage developed shock (43.3% vs. 16.4%, p=0.002), left ventricular systolic dysfunction (31% vs. 3.1%, p=0.000), and acute kidney injury (43.3% vs. 24.5%, p<0.05) (Table 1).
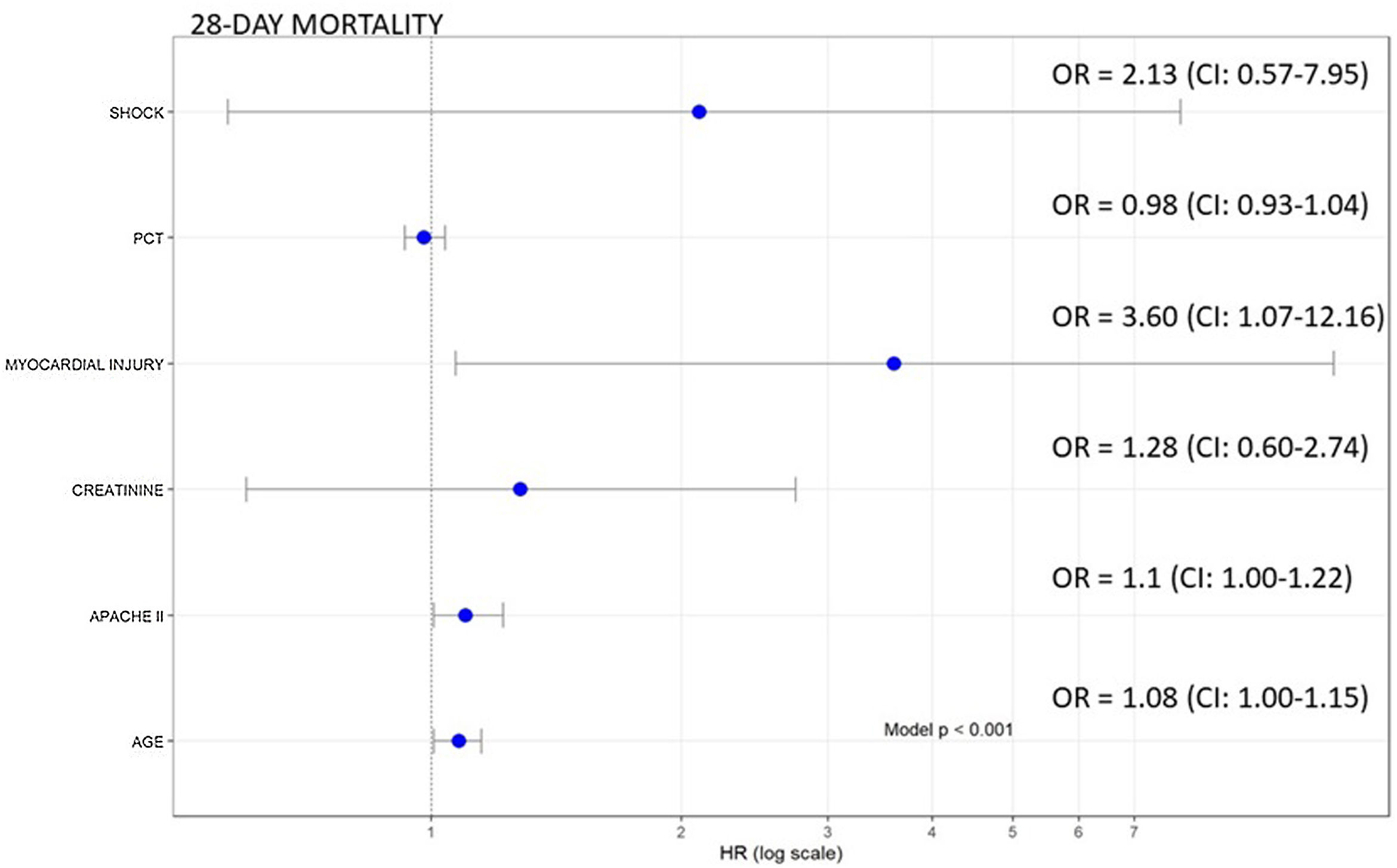
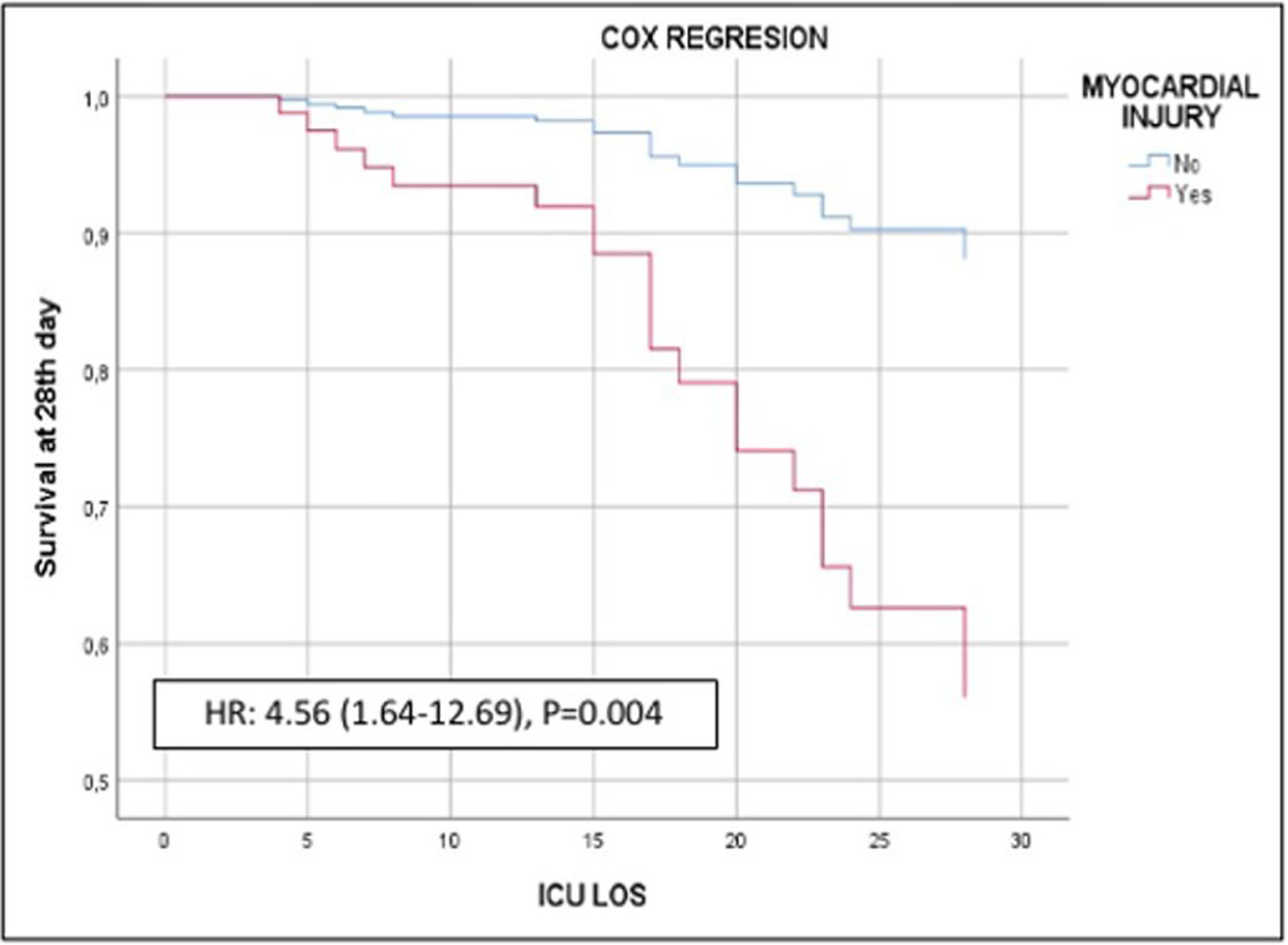
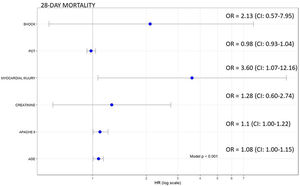
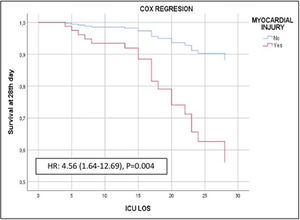
Of the 140 patients admitted to the ICU with severe SARS-CoV-2 pneumonia, 23 (16.4%) died within 28 days. In the bivariate analyses mortality was significantly associated with age, APACHE II, creatinine, procalcitonin, and myocardial injury (Table 1, Supplementary material). In the multivariate analysis myocardial injury was independently associated with 28-day mortality (OR: 3.60, 95%CI 1.07–12.16, p<0.01) (Fig. 1). Cox hazard regression analysis found the risk of death was higher in patients with myocardial injury (HR=4.56, 95% CI 1.64–12.69, p=0.004) (Figure 2, Supplementary material).
Our results suggest that myocardial injury is common in patients with severe SARS-CoV-2 pneumonia and that early development of myocardial injury could increase the risk of death during the ICU stay.
In this sense, in a report of 138 hospitalized patients with COVID-19, Wang et al.,8 found that 46% had at least one coexisting medical conditions and those admitted to the ICU were more likely to have cardiovascular complications, including myocardial injury in 22.2%. Moreover, Zhou et al.9 found that patients with pre-existing cardiovascular disease had higher cardiac markers and higher rates of ICU admission and mortality.
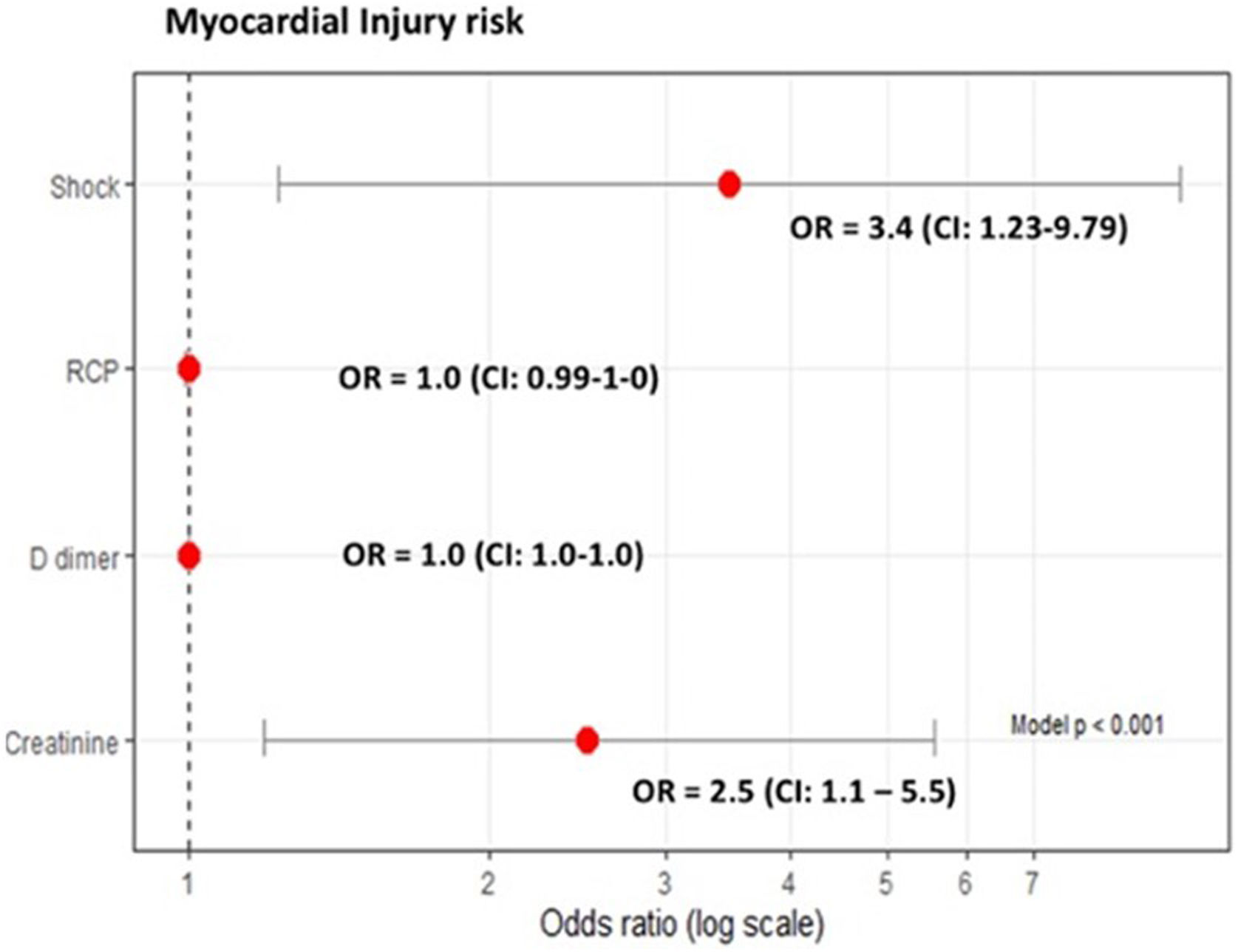
We found that 21.4% of patients with severe COVID-19 developed myocardial injury. Myocardial injury was significantly associated with the development of cardiovascular complications like shock, left ventricular systolic dysfunction, and acute kidney injury. Like in other studies reporting strong correlations between hs-cTn and other inflammatory factors in acute infections (viral or bacterial)10, we found higher values of procalcitonin and CRP in patients with myocardial injury. These findings could be related with pro-inflammatory or pro-coagulable states and could help explain the organic damage and unfavorable prognosis.
Several studies have evaluated the relationship between myocardial injury and mortality in mixed patient populations. In this respect, a recent meta-analysis found that cardiac injury was associated with a greater than sevenfold increase in the risk of mortality, ICU admission, and severe COVID-19.6 Nevertheless, few studies have evaluated the prognostic value of high-sensitivity troponin in critically ill patients. Analyzing 111 ICU patients with confirmed COVID-19, Larcher et al.5 found that hs-cTn>22ng/L at admission was independently associated with in-hospital mortality. Similarly, after adjusting for age, APACHE II score, creatinine, shock, and procalcitonin, we found that myocardial injury was associated with 28-day mortality.
Our study has several limitations. First, it was conducted at a single center and included only 140 patients; data from larger populations at multiple centers are needed to confirm the relation between myocardial injury and high risk of mortality. Second, logistic limitations arising from the urgency of containing the COVID-19 pandemic resulted in incomplete data about some aspects of cardiovascular complications and inflammation (e.g., echocardiography findings, troponin or interleukin-6 levels, or glucocorticoid treatment) in some cases, thus limiting our ability to identify potential mechanisms and patterns associated with myocardial injury. Third, because we evaluated only the first three days after ICU admission, the incidence of myocardial injury and other cardiovascular complications during the course of the disease may have been underestimated; however, by excluding the possible influence of later complications, this approach might also better gauge the direct relationship between SARS-CoV-2 infection and cardiovascular complications. Finally, to attribute the death of an individual patient directly to myocardial injury would require longer follow-up.
We conclude that myocardial injury is common among patients with severe SARS-CoV-2 pneumonia admitted to ICUs and that it seems to be associated with an intense inflammatory response as well as a higher risk of organ failure and ICU mortality.
FundingThe author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.