A study was made of the events occurring in the early post-resuscitation phase that may help to improve the outcomes at hospital discharge.
DesignA retrospective cohort study (2007–2017) of a prospective Utstein type registry database was carried using multivariate logistic regression analysis. Pre- and post-hospital admission events were investigated.
SettingA tertiary cardiac centre.
ParticipantsUnconscious victims of out-of-hospital cardiac arrest (OHCA) with documented ventricular tachycardia or fibrillation.
Main variables of interestEvents occurring before and within 72h after intensive care unit (ICU) admission were recorded. The variables were analyzed to determine their impact on hospital survival and poor neurological outcome. One-year follow-up survival was also considered. Results are presented as odds ratio (OR) and 95% confidence interval (95%CI).
ResultsOf 245 patients admitted to our ICU after OHCA, 152 (62%) were alive and 131 (86.2%) presented good neurological outcomes (cerebral performance categories≤2) at hospital discharge. The one-year follow-up survival rate was 95.9%. Age >70 years (OR 2.0; 95%CI 1.1–4.1), previous myocardial infarction (OR 2.7; 95%CI 1.2–6.1), shock upon hospital admission (OR 2.9; 95%CI 1.3–6.2), time from call to return of spontaneous circulation (ROSC) >25min (OR 3.1; 95%CI 1.6–6.0) and anticonvulsant therapy (OR 18.2; 95%CI 5.5–60) were independent predictors of poor neurological outcome. Immediate admission to the cardiac centre (OR 0.5; 95%CI 0.3–0.9) and lactate clearance reaching plasma levels <2.5mmol/l at 12h (OR 0.4; 95%CI 0.2–0.8) were associated with better outcomes.
ConclusionsUnconscious OHCA patients with documented ventricular tachycardia or fibrillation may benefit from direct admission to a reference cardiac centre. Initial haemodynamic support, urgent coronary angiography and targeted management in the cardiac ICU seem to increase the likelihood of good neurological outcomes.
Llevar a cabo un estudio de los acontecimientos ocurridos en la fase inmediatamente posterior a la reanimación que puedan ayudar a mejorar los desenlaces en el momento del alta hospitalaria.
DiseñoSe realizó un estudio retrospectivo (2007-2017) de cohorte de una base de datos de registro de tipo Utstein prospectivo mediante un análisis de regresión logística multivariable. Se investigaron los acontecimientos previos y posteriores al ingreso hospitalario.
ÁmbitoUn centro de atención cardíaca terciaria.
ParticipantesVíctimas inconscientes de parada cardíaca extrahospitalaria (OHCA) con fibrilación o taquicardia ventricular documentada.
Variables principales de interésSe registraron los acontecimientos ocurridos antes y durante las 72h posteriores al ingreso en la unidad de cuidados intensivos (UCI). Se analizaron las variables para determinar su impacto en la supervivencia hospitalaria y los malos desenlaces neurológicos. También se tuvo en consideración la supervivencia en el seguimiento a lo largo de un año. Los resultados se presentan con valores de oportunidad relativa (OR) e intervalo de confianza del 95% (IC del 95%).
ResultadosDe los 245 pacientes ingresados en nuestra UCI tras una OHCA, 152 (62%) seguían vivos y 131 (86,2%) presentaban unos buenos desenlaces neurológicos (categorías de rendimiento cerebral≤2) en el momento del alta hospitalaria. La tasa de supervivencia en el seguimiento a lo largo de un año fue del 95,9%. La edad>70 años (OR: 2,0; IC del 95%: 1,1-4,1), los antecedentes de infarto de miocardio (OR: 2,7; IC del 95%: 1,2-6,1), el choque en el momento del ingreso hospitalario (OR: 2,9; IC del 95%: 1,3-6,2), el tiempo transcurrido entre la llamada y el regreso a la circulación espontánea (ROSC)>25min (OR: 3,1; IC del 95%: 1,6-6,0) y la administración de tratamiento anticonvulsivo (OR: 18,2; IC del 95%: 5,5-60) fueron factores predictivos independientes de un mal desenlace neurológico. El ingreso inmediato en un centro de cuidados cardíacos (OR: 0,5; IC del 95%: 0,3-0,9) y el hecho de que el aclaramiento de lactato alcanzase unos niveles plasmáticos<2,5mmol/l al cabo de 12h (OR: 0,4; IC del 95%: 0,2-0,8) se asociaron con unos mejores desenlaces.
ConclusionesLos pacientes inconscientes tras OHCA y con fibrilación o taquicardia ventricular documentada podrían beneficiarse del ingreso directo en un centro cardíaco de referencia. El apoyo hemodinámico inicial, la angiografía coronaria urgente y el tratamiento dirigido en la UCI cardíaca parecen aumentar la probabilidad de obtener unos buenos desenlaces neurológicos.
Out-of-hospital cardiac arrest (OHCA) is a sudden disease presentation where survival is tightly related to the rapid intervention delivered through an optimal teamwork. This represents the “chain of survival” concept based on prompt recognition of the cardiac arrest, call for help, bystander cardiopulmonary resuscitation (CPR), early defibrillation and standardized post-resuscitation care.1–6 The implementation of a regional lay rescue programme focused on a rapid coordinated network response7 and easy access to automatic external defibrillator (AED)8 improve OHCA survival.9 The aim is the return of spontaneous circulation (ROSC) in the first minutes following cardiac arrest (CA). In Canton Ticino, a region of Switzerland of about 350,000 inhabitants, most patients suffering from a cardiac-related OHCA are managed in a tertiary cardiac receiver centre in which an interdisciplinary team composed by cardiologists, anaesthesiologists, and intensivists is available on 24h/7 days basis. Since myocardial ischaemia is the predominant aetiology of OHCA,10 early coronary revascularisation restores myocardial perfusion and improves patient outcome.11–13 In fact, numerous observational studies have shown that patients with ST-segment elevation after OHCA benefit of urgent angiography and primary percutaneous transluminal coronary angioplasty (PTCA).14–16 Once the heart is re-perfused, the therapeutic pathway continues with the post resuscitation management in the intensive care unit (ICU). The mitigation of cerebral ischaemia–reperfusion injuries by temperature control has proven to play a role in post-OHCA neuroprotection.17–19 Other supportive strategies include optimal organ perfusion monitored by close lactate analysis,20,21 plasma glucose optimization,22 prompt correction of electrolyte imbalance, and appropriate mechanical ventilation.23 This multi-faceted ICU standardized post-cardiac arrest syndrome management combined with a well-organized extra-hospital rescue programmes have improved the survival after OHCA.9,24–26 Furthermore, some evidence suggests that patients treated in a specialized cardiac arrest centre may benefit of a better outcome.11,27–32 Therefore, a comprehensive post-cardiac arrest approach remains a pivotal step of the chain of survival, as highlighted in the last update of the 2015 ILCOR guidelines and in recent publications.1,33–37 We herewith report the data of our ten years register, collected prospectively in a cohort of post-OHCA patients admitted to our cardiovascular ICU. We analyzed independent risk factors that could have influenced patient's cerebral performance at hospital discharge after CA due to ventricular fibrillation (VF) or tachycardia (VT). The aim of the present study was to investigate survival and cerebral performance categories (CPC) in comatose patients after OHCA. Primary goal was to study the association between neurological outcome and clinical events occurring before ICU admission and during ICU stay.
Patients and methodsData sourceWe conducted an observational cohort study based on a single centre OHCA register between 2007 and 2017. Prospective data collection was performed according to definitions and reporting templates described in Utstein's style recommendations for research.38 All the data were recorded in our electronic institutional CA database. Additional sources of information were rescue medical reports, emergency department chart and hospital records. We included in our registry all victims of OHCA admitted to Cardiocentro Ticino, Lugano, Switzerland. The local Ethical Committee (Comitato etico Canton Ticino, Switzerland) reviewed and approved this study (ID 3218, BASEC 2017-00681). Waiver of consent was allowed using an encoded anonymous database, according to Swiss Human Research Act art.34/HRO.
Study populationConsecutive patients who underwent cardiopulmonary resuscitation (CPR) due cardiac-related OHCA with a shockable rhythm and persistent Glasgow Coma Score <8 after ROSC and age ≥18 years. Exclusion criteria were: early neurological recovery before admission; non-shockable rhythm as pulseless electrical activity (PEA) or asystolia as first electrocardiographic finding; secondary OHCA of non-cardiac origin
Patient managementIn our region rescue help calls are transmitted to a single regional emergency dispatcher call centre, which dispatch the first aid instructions for witnesses, alert the first responders, and active emergency medical service (EMS). Depending on the presumed aetiology, the clinical condition, and the location of OHCA, the victims could be transported either to a tertiary-care specialized cardiac arrest centre or to the nearest local hospital. If on site a cardiac origin is suspected, paramedics transmit 12-lead electrocardiogram via mobile phone application to the attending cardiologist of our centre. Whenever an ECG consistent with potential acute coronary syndrome is detected (ST-segment elevation myocardial infarction (STEMI), new left bundle branch block or N-STEMI) the local hospital could by bypassed and a dedicated emergency team (cardiologist, anaesthetist, and non-medical staff) is alerted and the OHCA patients are directly admitted to our cardiac centre. In case of questionable ECG or a suspicion of non-cardiac origin the patient is transported to the nearest local hospital emergency department (ED) for investigation and transferred secondarily to the catheterisation laboratory if needed.
Once the cardiac procedure was concluded, a transfemoral endovascular cooling catheter for targeted temperature control was placed in the inferior vena cava and the patient then transferred to the ICU. A standardized goal-directed strategy targeting a mean arterial pressure >65mmHg, monitoring of lactate clearance, and urine output >0.5ml/kg/h was pursued. The haemodynamic management included pharmacologic (e.g. continuous i.v. infusion of dobutamine or epinephrine as inotropes, with or without norepinephrine, as vasopressor) or mechanical (e.g.; intra-aortic balloon pump) haemodynamic support, as clinically judged appropriate according to current state-of-the art evidence. Haemodynamic monitoring was conducted with invasive arterial pressure, echocardiography, and in case of pronounced instability, with pulmonary artery catheter or with pulse-contour technique-based devices. Active target temperature body temperature control was promptly initiated at ICU admission with the COOLGARD™ device through the previous placed endovascular catheters. Therapeutic hypothermia entailed a rapid cooling phase favoured also by external ice packs. Shivering was controlled according to a standardized protocol. A target core temperature of 33°C for 24h (measured with a bladder probe) was obtained, followed by a rewarming phase at a speed of 0.2°C/h until 36°C. Strict core temperature control (<37.5°C) was maintained for all patients during the following 72h by means of the intravascular device, as well as acetaminophen.
Events investigatedOur analysis of potential contributors to the final outcome of resuscitated OHCA patients focused on events occurred during two distinct time frames: (A) before ICU admission; (B) within 72h after ICU admission.
Predisposing factors included patient pre-existing features and events occurred before ICU admission (before ICU): (i) patient's co-morbidities; (ii) resuscitation dynamics: witnessed arrest, first responder dispatch, use of first basic life support (BLS), AED shocks delivered by proximal layperson plus number of total of defibrillation performed, location of the CA, and time from call to ROSC. (iii) post-resuscitation factors: cardiogenic shock at hospital admission (defined as arterial systolic blood pressure <90mmHg lasting at least 30min despite pharmacological or mechanical support),39 acute myocardial infarction at admission, immediate admission to a tertiary centre, emergent coronary angiography, need of percutaneous coronary intervention (PCI), and extracorporeal membrane oxygenation (ECMO) support.
The aggravating factors included events occurring during the first 72h of ICU management (in-ICU): (i) pharmacological treatments: vasoactive and inotropic agents, sedatives, neuromuscular blocking agents and antiepileptic drugs; (ii) metabolic derangements: hyperglycaemia (>10mmol/l lasting >4h), or hypoglycaemia (<4mmo/l)40; electrolyte imbalances such as hypokalaemia <3.5mmol/l and hypophosphataemia <0.8 mmo/l, aberrant arterial gas concentration: hypocapnia (PaCO2≤30mmHg), hypercapnia (PaCO2≥50mmHg) at alpha-stat management,41 and hypoxia (PaO2≤80mmHg); (iii) haemodynamic variables: hypotension (MAP<60mmHg over 10min), bradycardia (heart rate <40beats/min), and tachycardia (heart rate>130beats/min); optimal lactate clearance (decreasing trend of serum lactate reaching a value ≤2.5mmol/l in 12±2h after ICU admission).42 The following complications encountered during the whole ICU stay were also reported: (i) haemorrhagic events (>2 blood concentrates needed for Hb <8.0g/l or intra-cranial bleeding); (ii) pneumonia (new or progressive consolidation on the radiograph, fever, leucocytosis, and the presence of purulent tracheobronchial secretions)38; (iii) acute renal failure (RIFLE class F)43 and the need of renal replacement therapy; (iv) manifest myoclonus activity or electroencephalography (EEG) epileptiform electroencephalography (EEG) pattern, requiring the use of anticonvulsants therapy.
Outcome measuresFinal outcome was evaluated at hospital discharge with 5-tiered cerebral performance categories (CPC) scale, assessing neurological disability after cardiac arrest: CPC 1: conscious, alert, able to work, might have mild neurologic or psychological deficit; CPC 2: conscious, sufficient cerebral function to carry out daily life activities in an independent fashion; able to work in a protected environment; CPC 3: severe cerebral disability conscious; dependent on others for daily life because of an impaired brain function; CPC 4: Coma or unawareness, even if the patient appears in an awake vegetative state without interaction with the environment; CPC 5: death during anytime of ICU or hospital stay.44–46 We reported cause of death and we performed a one-year survival follow-up.
Statistical analysisContinuous variables were expressed as mean±standard deviation (SD) and statistical significance of differences was estimates through Mann–Whitney test. All variables (before ICU and in-ICU) were analyzed to determine whether any single factor influenced poor neurological outcome (CPC>2) at hospital discharge. The association was expressed as odds ratio (OR) with 95% confidence interval (CI), and Mantel–Haenszel test for categorical variables was used considering a p-value less than 0.05 as significant. Pre-ICU and ICU risk factors were analyzed separately for each group of variables, the independent predictors of CPC>2 at discharge were estimated using a stepwise multivariable logistic regression. The independent risk factors of the two groups were also run together in a further stepwise multivariable logistic regression. The probability of developing poor neurological outcome at discharge was calculated and interpreted based on a cut-off of 0.50. The performance of the models was assessed through receiver operating characteristics (ROCs) and area under the ROC curve (AUC) was estimated as measure of discriminative ability of the models. Goodness-of-fit statistics for calibration of the models were evaluated through Hosmer–Lemeshow test. Furthermore, Nagelkerke R-square was calculated in order to estimate the proportion of the total variability explained by the models. A Cochran–Armitage test of trend was used to determine whether a linear trend exists between the years and the proportion of patients with poor neurological outcome (CPC>2). Statistical analysis was performed using SAS 9.3 and SPSS 20.0 software.
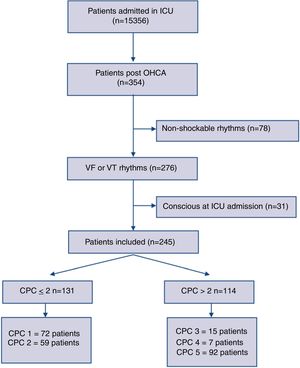
ResultsThree-hundred fifty-four consecutive OHCA adult patients were admitted between 2007 and 2017 to our ICU. We enrolled 276 cases with documented sustained VT or VF as an initial resuscitation rhythm. Thirty-one patients were excluded being already conscious at the ICU admission. Two-hundred forty-five comatose patients were finally included for the analysis. The study flow diagram is showed in Fig. 1.
The average age of the cohort studied was 63.5±13.1 years with a predominance of men (84.5%); demographic features and previous cardiovascular conditions are described in Table 1. The laps from call to EMS crew arrival on OHCA scene was 8.7±4.2min in good outcome group versus 9.0±4.6min in poor outcome group, without statistically difference (p=0.66). OHCA was witnessed in 222 patients (90.6%); first responders arrived on place before EMS in 77 cases (31.4%). AED was applied by bystander in 65 (26.5%) patients, while 180 received the first shock at EMS arrival. Overall number of pre-hospital defibrillations for successful ROSC was similar in the two group, 4.1±3.2 in good outcome patients and 3.7±2.3 in poor outcome patients (p=0.61). In-ICU all patients received at least one vasoactive or inotropic drug during the first 72h. Ischaemia was the leading cause of the cardiac arrest, in fact among 245 patients 170 (69.4) were admitted with suspected diagnosis of acute myocardial infarction (Table 1), whereas in 67 (27.5) patients a culprit lesion was not visible and in 8 (3.2%) the cardiac origin was excluded (six documented hypokalaemia, one pulmonary embolism, one toxic).
Clinical events before intensive care unit admission in relation to poor neurologic outcome, CPC>2.
| Variables | All (N=245) | CPC≤2 (N=131) | CPC>2 (N=114) | Univariate analyses | Multivariable analyses | ||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Pre-arrest patients characteristics | |||||||
| Gender, female | 38 (15.5) | 15 (11.5) | 23 (20.2) | 1.9 (0.9–3.9) | 0.063 | ||
| Age >70 years | 79 (32.2) | 32 (24.4) | 47 (41.2) | 2.2 (1.2–3.7) | 0.005 | 2.03 (1.08–3.81) | 0.028 |
| Previously healthy | 71 (29.0) | 40 (30.5) | 31 (27.2) | 0.8 (0.5–1.5) | 0.565 | ||
| Previous myocardial infarction | 51 (20.8) | 16 (12.2) | 35 (30.7) | 3.2 (1.6–6.1) | 0.001 | 2.36 (1.09–5.10) | 0.029 |
| Hypertension | 103 (42.0) | 50 (38.2) | 53 (46.5) | 1.4 (0.8–2.3) | 0.169 | ||
| Diabetes mellitus | 41 (16.7) | 13 (9.9) | 28 (24.6) | 2.9 (1.4–6.0) | 0.003 | 2.11 (0.86–5.18) | 0.102 |
| Stroke | 14 (5.7) | 7 (5.3) | 7 (6.1) | 1.2 (0.4–3.4) | 0.789 | ||
| Pulmonary disease | 23 (9.4) | 10 (7.6) | 13 (11.4) | 1.6 (0.7–3.7) | 0.316 | ||
| Renal impairment | 17 (6.9) | 3 (2.3) | 14 (12.3) | 5.9 (1.7–21) | 0.006 | 2.29 (0.50–10.09) | 0.291 |
| Resuscitation factors | |||||||
| Witnessed arrest | 222 (90.6) | 124 (94.7) | 98 (86.0) | 0.3 (0.1–0.9) | 0.025 | 0.40 (0.13–1.23) | 0.111 |
| Cardiac arrest in private home | 130 (53.1) | 70 (53.4) | 60 (52.6) | 1.0 (0.6–1.6) | 0.900 | ||
| Bystander CPR | 202 (82.4) | 115 (87.8) | 87 (76.3) | 0.4 (0.2–0.9) | 0.020 | 0.536 (0.24–1.18) | 0.123 |
| First responder | 77 (31.4) | 48 (36.6) | 29 (25.4) | 0.6 (0.3–1.0) | 0.061 | ||
| AED shock delivered | 65 (26.5) | 41 (31.1) | 24 (21.1) | 0.6 (0.3–1.0) | 0.071 | ||
| Time from call to ROSC >25min | 117 (47.8) | 44 (33.6) | 73 (64.0) | 3.5 (2.1–6.0) | 0.001 | 4.58 (2.48–8.45) | 0.001 |
| Post-resuscitation factors | |||||||
| Direct admission in cardiac centre | 122 (49.8) | 73 (55.7) | 49 (43.0) | 0.6 (0.4–0.9) | 0.047 | 0.53 (0.29–0.95) | 0.039 |
| Shock at hospital admission | 58 (23.7) | 19 (14.5) | 39 (34.2) | 3.1 (1.6–5.7) | 0.001 | 3.10 (1.54–6.23) | 0.001 |
| Acute myocardial infarction | 170 (69.4) | 88 (67.2) | 82 (71.9) | 1.3 (0.7–2.2) | 0.421 | ||
| Emergency coronary angiography | 232 (94.7) | 126 (96.2) | 106 (93.0) | 0.5 (0.2–1.7) | 0.272 | ||
| Percutaneous coronary intervention | 163 (66.5) | 94 (71.8) | 69 (60.5) | 0.6 (0.4–1.1) | 0.064 | ||
| ECMO | 9 (3.7) | 3 (2.3) | 6 (5.3) | 2.4 (0.6–9.7) | 0.230 | ||
CPC: cerebral performance category; AED: automated external defibrillator; ROSC: return of spontaneous circulation; CPR: cardiopulmonary resuscitation; ECMO: extracorporeal membrane oxygenation. Values are odds ratio (OR) with low and upper confidence limit at 95% in bracket.
One-hundred fifty-two patients (62%) survived at hospital discharge, 131 (86.2%) of them with favourable neurologic outcome (CPC≤2). The cause of death was circulatory failure 17.2%,16 neurological injury 67.7%,47 multi-organ failure 15.1%.14 Among patients with good neurologic outcome one-year survival rate was 95.9% (117 alive at 1-year, 9 missed, 5 deceased). ICU length of stay (LOS) was respectively 6.4±5.8 days for a good and 5.9±6.7 days for a poor neurologic outcome (p=0.21). Among patients with CPC 1 or 2, the time to the first signs of neurologic recovery was 75±60h after admission (time of “awakening” defined as the best motor response with Glasgow Coma Scale=6).
The patients directly admitted to the tertiary centre had shorter time of access to the catheter laboratory, in comparison to those previously admitted to other hospitals; the time lapsed from ROSC to the beginning of coronary procedure was 67±29min vs 188±52min (p<0.01), nevertheless the lapse was not associated with the outcome (p=0.06). Moreover, this group of patients had earlier target temperature management, since time from ROSC to the activation of the intravascular cooling device was shorter (197±55min vs 220±67min, p=0.04).
All variables (before ICU and in-ICU) related to the primary outcome with a statistically significant value (p<0.05) (Tables 1 and 2), were run in the finally multivariable analysis (Table 3). The final stepwise multivariable logistic regression showed that an age >70 years old (OR 2.0; 95%CI 1.1–4.1), previous myocardial infarction (OR 2.7; 95%CI 1.2–6.1), shock at hospital admission (OR 2.9; 95%CI 1.3–6.2), time from call to ROSC >25min (OR 3.1; 95%CI 1.6–6.0), and anticonvulsant therapy (OR 18.2; 95%CI 5.5–60) were independent predictors of poor neurologic outcome. Direct admission to the cardiac arrest centre (OR 0.5; 95%CI 0.3–0.9) and positive lactate clearance reaching lactate plasma level <2.5mmol/l at 12h (OR 0.4; 95%CI 0.2–0.8), were independent predictors of a more favourable outcome.
Clinical events during intensive care unit in relation to poor neurologic outcome, CPC>2.
| Variables | All(N=245) | CPC≤2(N=131) | CPC>2(N=114) | Univariate analyses | Multivariable analyses | ||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| In-hospital events | |||||||
| Pneumonia | 138 (56.3) | 83 (63.4) | 55 (48.2) | 0.5 (0.3–0.9) | 0.018 | 0.45 (0.2–0.8) | 0.010 |
| Sedatives drug midazolam | 85 (34.7) | 43 (32.8) | 42 (36.8) | 1.2 (0.7–2.0) | 0.510 | ||
| Hypoglycemia <4.0mmol/l | 26 (10.6) | 16 (12.2) | 10 (8.8) | 0.7 (0.3–1.6) | 0.385 | ||
| Hyperglycemia >10mmol/l | 164 (66.9) | 80 (61.1) | 84 (73.7) | 1.78 (1.1–3.1) | 0.037 | 0.8 (0.4–1.6) | 0.531 |
| Anticonvulsants | 34 (13.9) | 4 (3.1) | 30 (26.3) | 11.3 (3.8–33.4) | 0.001 | 17.5 (5.4–56.2) | 0.001 |
| Neuromuscular blockers | 96 (39.2) | 57 (43.5) | 39 (34.2) | 0.7 (0.4–1.1) | 0.138 | ||
| Renal replacement therapy | 16 (6.5) | 4 (3.1) | 12 (10.5) | 3.7 (1.2–11.9) | 0.026 | 3.2 (0.9–11.6) | 0.070 |
| Therapeutic hypothermia | 201 (82.0) | 118 (90.1) | 83 (90.1) | 0.3 (0.1–0.6) | 0.001 | 0.3 (0.2–0.7) | 0.008 |
| Bleeding | 11 (4.5) | 6 (4.6) | 5 (4.4) | 0.9 (0.3–3.2) | 0.942 | ||
| Hypophosphatemia <0.8mmol/l | 32 (13.1) | 20 (15.3) | 12 (10.5) | 0.6 (0.3–1.4) | 0.274 | ||
| Bradycardia <40bpm | 50 (20.4) | 34 (26.0) | 16 (14.0) | 0.5 (0.2–0.9) | 0.023 | 0.8 (0.4–1.7) | 0.539 |
| Tachycardia >130bpm | 30 (12.2) | 16 (12.2) | 14 (12.3) | 1.0 (0.5–2.1) | 0.987 | ||
| Ventricular arrhythmias | 24 (9.8) | 9 (6.9) | 15 (13.2) | 2.0 (0.9–4.9) | 0.104 | ||
| Episode(s) of hypotension | 73 (29.8) | 30 (22.9) | 43 (37.7) | 2.0 (1.2–3.6) | 0.012 | 1.8 (0.9–3.5) | 0.088 |
| Hypocapnia ≤30mmHg | 138 (56.6) | 73 (55.7) | 65 (57.5) | 1.1 (0.6–1.7) | 0.778 | ||
| Hypercapnia ≥50mmHg | 105 (43.0) | 57 (43.5) | 48 (42.5) | 1.0 (0.6–1.6) | 0.871 | ||
| PaO2 <80mmHg | 183 (75.3) | 94 (71.8) | 89 (79.5) | 1.5 (0.8–2.8) | 0.166 | ||
| Hypokalaemia <3.5mmol/l | 128 (52.5) | 72 (55.0) | 56 (49.6) | 0.8 (0.5–1.3) | 0.400 | ||
| Lactate at 12h <2.5mmol/l | 156 (63.7) | 98 (74.8) | 58 (50.9) | 0.3 (0.2–0.6) | 0.001 | 0.3 (0.2–0.7) | 0.003 |
| Haemoglobin <8mg/dl | 26 (10.6) | 10 (7.6) | 16 (14.0) | 2.0 (0.9–4.5) | 0.114 | ||
CPC: cerebral performance category. Values are odds ratio (OR) with low and upper confidence limit at 95% in bracket.
Multivariable analysis showing statistically significant variables (p<0.05) associated with poor neurologic outcome (CPC>2).
| Variables | Multivariable analyses | |
|---|---|---|
| OR (95%CI) | p-Value | |
| Age >70 years | 2.0 (1.1–4.1) | 0.047 |
| Previous myocardial infarction | 2.7 (1.2–6.1) | 0.013 |
| Time from call to ROSC >25min | 3.1 (1.6–6.1) | 0.001 |
| Direct admission in cardiac centre | 0.5 (0.3–0.9) | 0.029 |
| Shock at hospital admission | 2.9 (1.3–6.2) | 0.006 |
| Lactate at 12h <2.5mmol/l | 0.4 (0.2–0.8) | 0.008 |
| Anticonvulsants | 18.2 (5.5–60.2) | 0.001 |
CPC: cerebral performance category; ROSC: return of spontaneous circulation. Values are odds ratio (OR) with low and upper confidence limit at 95% in bracket.
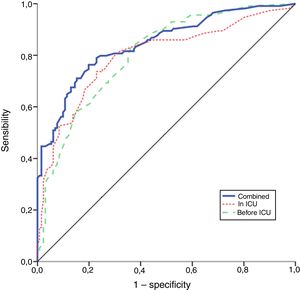
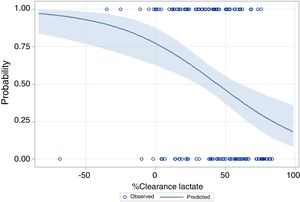
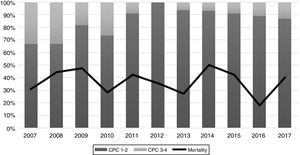
The performance of the models to predict poor neurologic outcome was assessed (Fig. 2). The model considering pre-ICU factors (green dashed line) explained 34.4% of the variance in CPC and correctly classified 71.8% of cases, whereas the model including ICU factors (red dotted curve) explained 34.6% of the variance in CPC, and correctly classified 74.7% of cases. The robustness of the predictive model increased when pre-ICU and ICU variables, from above mentioned analyses, were considered in a combined model (blue continuous line). When all variables (pre-ICU and ICU) were pooled, the Nagelkerke's R2 value increased the variance in neurological outcome to 45.5% and the AUC's predictive value improved up to 77.9%. The better performance of this combined model confirms the importance of a global approach in order to increase the precision in predicting neurological outcome at hospital discharge of OHCA survivors. The pattern of lactate kinetics was also found to correlate to neurological outcome (Fig. 3). A progressive clearance of lactate after ICU admission was associated with an increased probability of CPC ≤2. Conversely, a progressive increase in lactatemia raised the likelihood of poor neurologic outcome. During these ten years the proportion of patients suffering from unfavourable outcome (CPC 3-4 and mortality) were 54% (2007), 63% (2008), 57% (2009), 48% (2010), 47% (2011), 36% (2012), 32% (2013), 54% (2014), 47% (2015), 27% (2016) and 48% (2017), respectively (Fig. 4). The Cochran–Armitage test of trend did not show a statistically significant linear trend between years and the proportion of patients with poor neurological outcome (CPC>2), p=0.08.
This study investigated the impact of continuity of care, on patients presenting OHCA due to shockable rhythm. We assessed the effectiveness of our local chain of survival and the post-resuscitation management in our centre collecting data in conformity to the Utstein register recommendations.48 In our cohort 2007–2017 we found a survival rate of 62% and a favourable neurologic recovery in 86.2% of them (CPC≤2). These encouraging results compared to a survival rate of 33% in 20054 suggest that the continuity of care after OHCA in a dedicated cardiac centre ICU, could be pivotal in increasing the likelihood of good neurologic outcome.27 We investigated factors occurring before ICU admission and during ICU management that can influence the outcome of patients after OHCA. Prolonged call time to ROSC (>25min), previous myocardial infarction, age >75 years and circulatory failure at hospital admission are independent factors associated with poor outcome. While direct admission to a specialized cardiac centre and positive lactate clearance reaching plasma level <2.5mmol/l at 12h are linked with increased survival rate and better neurological performances. The present study and previous publications performed in other environments support the concept that victims of cardiac arrest should be directly admitted to a tertiary centre in where an experienced and multidisciplinary staff is on call 24/7.25,27,29–31,49–52 The large proportion of cardiovascular predisposing factors found in our cohort justifies the direct admission in a cardiac centre with prompt interventional cardiac approach in patients suffering from OHCA due to shockable rhythm. In fact, acute myocardial ischaemia was the primary cause of 69.4% of OHCAs in the studied population. Emergent coronary angiography was thus performed in 94.7% of cases and 66.5% of them were treated by percutaneous coronary intervention (PCI).53–55 After rapid myocardial revascularisation with restoration of an adequate organ driving blood pressure56 ICU treatment should continue with haemodynamic support and neuroprotection.57 This targeted management may improve survival and reduce secondary brain injury.19,25,34,58 Unfortunately, direct admission in a cardiac receiver centre is not yet a reality in several Western countries since two surveys on organization and post-resuscitation care indicate a large discrepancy between published guidelines and the daily clinical practice.35,59–61
In our cohort, a progressive increase of plasma lactates or a negative lactate clearance index in the first hours of ICU stay are likely to be caused by the prolongation of circulatory failure which increases the risk of poor outcome.20,21,42,62,63 The finding of our study suggests that a positive lactate clearance index (i.e. decrease in lactate level) could be an indicator of a proper and timely haemodynamic resuscitation potentially limiting ischaemic damage. Therefore, in our centre, lactate clearance reaching normal plasma levels in the firsts 12h was used as a marker of optimal organ reperfusion and potential favourable outcome.
Myoclonus and sub-clinical epileptic EEG patterns still remain a frequent manifestations of global brain ischaemia.47,64 In our study, we considered the prescription of anticonvulsant therapy as a surrogate marker for post OHCA ischaemic brain injury. From our analysis anticonvulsant therapy appears the strongest indicator of poor neurologic outcome. This result stress the importance of early EEG monitoring to detect clinically silent epileptic activity.65 The time elapsed between OHCA and the first signs of neurological recovery is rarely reported in the literature and therefore in our work we defined the awakening when the patient followed simple verbal orders (best motor response, GCS=6). This finding documents that patients admitted to our ICU after OHCA with a positive neurological prognosis needed approximately 3–6 days of clinical observation.66,67 In our opinion this insight demonstrates the importance to avoid prognostication before a week from OHCA has elapsed.68 The final analysis combining all independent predictors yields a model with a better predictive value. This result suggests that neurological outcome after OHCA depends from a complex multifaceted post-resuscitation pathway based on continuity of care.
Our study has limitations. Firstly, our results report the experience a single cardiac centre experience. In order to reduce the impact of this bias we performed a data collection based on published standards and recommendations in cardiac arrest research. This standardized approach should permit the comparison of our results with other centres using the same criteria for the assessment of quality of care in patients who have survived to OHCA. The second limitation concerns patient's selection since our register includes only shockable rhythms. Thirdly, the outcome after OHCA could be influenced by the local rescue protocols applied in each emergency system. Our study focused on post resuscitation care; therefore, in order to decrease the impact of the pre-hospital phase on our analysis, we adjusted the ICU events for the main unbiased extra-hospital factors. Lastly, we defined CPC <2, as primary end-point, since we considered neurologic outcome as a valid surrogate of survival.
ConclusionsDirect admission to a cardiac referral centre and targeted haemodynamic ICU management monitored by positive lactate clearance during the first 12h seem both to increase the likelihood of good neurological outcome. Additionally, the time between OHCA and the first signs of neurological recovery suggests the need for further investigations focused on the correct timing of prognostication after OHCA.
Authors’ contributions- -
Study design: Tiziano Cassina, Gabriele Casso;
- -
Study conduct: Tiziano Cassina, Sara Clivio, Alessandro Putzu, Michele Villa;
- -
Data analysis: Tiziano Cassina, Sara Clivio, Alessandro Putzu, Michele Villa, Daniela Fortuna;
- -
Data interpretation: Tiziano Cassina, Sara Clivio, Alessandro Putzu, Michele Villa, Tiziano Moccetti, Daniela Fortuna, Gabriele Casso;
- -
Writing and revising paper: Tiziano Cassina, Sara Clivio, Alessandro Putzu, Michele Villa, Tiziano Moccetti, Daniela Fortuna, Gabriele Casso.
- -
All authors read and approved the final manuscript.
The study was supported by department funds only.
Conflicts of interestThe authors declare no conflicts of interests.
The authors wish to thank the entire ICU nurses study group who worked on the Utstein register. We acknowledge the local Emergency Medical System (Federazione Cantonale Ticinese Servizi Autoambulanze) who actively participate to the resuscitation program in the region. The authors are also grateful to Prof. Angelo Auricchio (Fondazione Ticino Cuore) for reviewing the manuscript.