Acute kidney injury (AKI) is a growing concern in Intensive Care Units. The advanced age of our patients, with the increase in associated morbidity and the complexity of the treatments provided favor the development of AKI. Since no effective treatment for AKI is available, all efforts are aimed at prevention and early detection of the disorder in order to establish secondary preventive measures to impede AKI progression. In critical patients, the most frequent causes are sepsis and situations that result in renal hypoperfusion; preventive measures are therefore directed at securing hydration and correct hemodynamics through fluid perfusion and the use of inotropic or vasoactive drugs, according to the underlying disease condition. Apart from these circumstances, a number of situations could lead to AKI, related to the administration of nephrotoxic drugs, intra-tubular deposits, the administration of iodinated contrast media, liver failure and major surgery (mainly heart surgery). In these cases, in addition to hydration, there are other specific preventive measures adapted to each condition.
La lesión renal aguda (LRA) constituye un problema de importancia creciente en las unidades de cuidados intensivos. La mayor edad de nuestros pacientes, con el aumento de la morbilidad asociada, y la complejidad de los tratamientos realizados favorecen su desarrollo. Puesto que la LRA carece de tratamiento eficaz, todos los esfuerzos se dirigen a la prevención y a su detección precoz con el fin de establecer medidas de prevención secundaria que impidan su progresión. En el paciente crítico, las causas más frecuentemente implicadas son la sepsis y las situaciones que provocan hipoperfusión renal, por lo que las medidas preventivas irán encaminadas a mantener un estado de hidratación y hemodinámico correcto mediante perfusión de fluidos y el uso de fármacos inotrópicos o vasoactivos en función de la enfermedad subyacente. Además de estas circunstancias, existen distintas situaciones que pueden favorecer la LRA, relacionadas con la administración de fármacos nefrotóxicos, los depósitos intratubulares, la administración de contrastes iodados, el fallo hepático y la cirugía mayor, fundamentalmente cirugía cardiaca. En estos casos, además de la hidratación, se dispone de otros aspectos preventivos específicos de cada entidad.
Acute kidney injury (AKI) is a common problem in Intensive Care Units (ICU) with a high level of associated mortality. Several studies had already demonstrated that even small increases in serum creatinine were associated with a poorer prognosis,1 considering acute kidney disease as an independent risk factor of mortality. Therefore, the early identification of patients at risk, the application of preventive strategies and carrying out early diagnosis and treatment are fundamental for reducing its incidence. In this chapter we will revise the situations most commonly related to AKI in critical patients, discussing the most appropriate prevention measures. If, in spite of this, AKI becomes unavoidable, efforts should be made to reduce its duration and to achieve the most complete recovery possible of kidney function (secondary prevention).
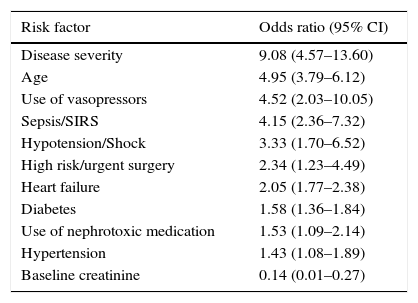
Primary preventionPatients at riskIn patients with intrahospital AKI, the cause is usually multifactorial, with kidney hypoperfusion being the most common (fundamentally related to hypovolemia, heart failure and arterial hypotension), followed by the administration of nephrotoxic drugs and thirdly, contrast associated acute kidney injury (CA-AKI).2 Critical patients, given their differential characteristics, more often experience AKI associated with sepsis and hypovolemia, with nephrotoxic drugs occupying third position.3 Numerous risk factors have been identified in the literature connected with the development of AKI (Table 1), having an age of over 75 years, Diabetes Mellitus (DM) and the presence of chronic kidney disease (CKD) being the most notable. The most significant clinical factors include sepsis, hypovolemia, arterial hypotension, congestive heart failure, time of clamping in patients who undergo heart surgery, complex and prolonged surgery, a high severity index score at hospital admission and recent administration of nephrotoxic medication.4 With all of this, different scales have been proposed to stratify patients at risk, although currently these types of tools have yet to be developed.
Risk factors for the development of AKI.
| Risk factor | Odds ratio (95% CI) |
|---|---|
| Disease severity | 9.08 (4.57–13.60) |
| Age | 4.95 (3.79–6.12) |
| Use of vasopressors | 4.52 (2.03–10.05) |
| Sepsis/SIRS | 4.15 (2.36–7.32) |
| Hypotension/Shock | 3.33 (1.70–6.52) |
| High risk/urgent surgery | 2.34 (1.23–4.49) |
| Heart failure | 2.05 (1.77–2.38) |
| Diabetes | 1.58 (1.36–1.84) |
| Use of nephrotoxic medication | 1.53 (1.09–2.14) |
| Hypertension | 1.43 (1.08–1.89) |
| Baseline creatinine | 0.14 (0.01–0.27) |
Modified from Ref. 4.
With regard to the actual pathologies of the critical patient, sepsis stands out as the main cause of acute kidney dysfunction, with an incidence of 15–20%.3 Regarding the non-septic AKI, it has a higher mortality rate, a longer stay in the ICU, but a better rate of renal recovery, with similar continuous renal replacement therapy (CRRT) needs.5 In this clinical context, hypovolemia and hypotension tend to trigger AKI, and although cases have been reported with normal hemodynamic parameters,6 early resuscitation with fluids7 and vasoactive drugs are the basis for its prevention and treatment, taking into account that water overload can lead to tissue edema, intraabdominal hypertension, multiorgan dysfunction and greater mortality.8
Acute heart failure (HF) is also associated with a higher risk of AKI and a worse prognosis. Type 1 Cardio-renal syndrome (CRS) appears in 27–40% of all acute descompensated heart failure, having a complex physiopathology.9
Traditionally, secondary renal hypoperfusion has been attributed to a low cardiac output, however, the results of the ESCAPE trial10 did not find a correlation between AKI and the cardiac index or values of systemic vascular resistance. To achieve its prevention it is necessary to treat and avoid situations that might decompensate the HF such as anemia, hydro-electrolytic disorders, arrythmia and medication. To prevent AKI, the administration of inotropic intravenous agents such as dobutamine would be indicated in situations of low cardiac output. Loop diuretics would be the pharmacological treatment of choice for the control of water overload. Other treatments, such as vasopressin antagonists, natriuretic peptide and levosimendan have been evaluated for the treatment of acute descompensated HF. Unfortunately, none of these agents have been shown to significantly improve long-term outcomes of this patient population, including renal function.9 Identifying risk factors, improving cardiac function and preventing acute decompensation are the key elements for its prevention. Therefore, traditionally recommended measures include: modifying cardiovascular risk factors; avoiding nephrotoxic agents that could provoke salt retention; and the appropriate pharmacological treatment of heart failure.
Measures to improve renal perfusionUnder normal conditions the kidneys receive 25% of total blood flow to ensure a good oxygen delivery and consumption but they are extremely sensitive to decreases in blood flow with a rapid deterioration of renal function. In the same way to ensure an adequate glomerular filtration rate (GFR) it is important to maintain an adequate mean arterial pressure (MAP); in most cases values of 65–70mmHg are a reasonable threshold, but in patients with chronic hypertension, diabetes or initially impaired renal function, higher MAP values should be considered.11 Therefore, to avoid hemodynamic instability and hypoperfusion it is critical to prevent AKI. When these circumstances exist, volume expansion is the first therapeutic measure to optimize preload and thus to improve cardiac output. Moreover, fluid overload may have adverse outcomes, including increased mortality and a reduced recovery of renal function. Therefore, it is recommended to estimate a patient's volume status and the use of dynamic and ultrasound measures which are reliable for predicting volume response. Regardless of the amount of fluid administered, the type of fluid may also have an impact on the development of AKI and current recommendations suggest the use of isotonic crystalloids. The use of large amounts of 0.9% NaCl is significantly associated with hyperchloremic metabolic acidosis, nevertheless, the role of the chloride in the critical ill patients remains controversial. It has been shown that hyperchloremia is associated with AKI12 and that restriction of chloride-rich fluid is associated with a significant decrease in the incidence of AKI and the need of renal replacement therapy (RRT).13 However, a recent randomized controlled trial found no difference in the use of balanced crystalloid solutions vs saline solution in the development of AKI.14 The use of hydroxyethyl starches (HES) has been associated with adverse effects on kidney function. These products have probably a direct nephrotoxic effect that appears to be dose and time dependent. A recent meta-analysis showed a significant increased risk of RRT and mortality in critically ill HES treated individuals15 and a Cochrane review16 showed that all HES products increase the risk of AKI and the need for RRT so that the authors recommended to avoid its use.
When the worsening of renal function is due to a drop in renal perfusion caused by low cardiac output the short-term administration of selected inotropic agents, especially dobutamine is indicated. In the case of sepsis, the use of inotropic agents is recommended only when there are signs of hypoperfusion, despite achieving adequate intravascular volume and adequate MAP; or when myocardial dysfunction is present.17 Levosimendan, a positive inotropic agent with vasodilator effect, may have a beneficial impact on renal function probably related to an increase in renal perfusion pressure and a decreased need for RRT.18 However, according to the results of a recent clinical trial, in adult patients with septic shock, the addition of levosimendan to standard care is not associated with less severe organ dysfunction, including renal failure, or lower mortality.19
In patients who remain hypotensive and oliguric after adequate fluid resuscitation and with a normal or increased cardiac output, the use of the vasopressor agent is recommended to restore the blood pressure and protect renal function. In septic shock, MAP of at least 65mmHg17 is recommended. Although a recent trial found no significant differences in mortality when two MAP targets (65–70mmHg vs. 80–85mmHg) were compared, in patients with chronic hypertension the need of RRT was lower in the higher MAP target group.20 In septic shock, norepinephrine is the vasopressor of first choice, epinephrine or vasopressin (not available in Europe) being the reasonable second-line vasopressor.17,20 Renal dose dopamine is ineffective for improving kidney function in AKI and its use is not recommended for renal protection.21
Fenoldopam, a selective dopamine D1 receptor agonist, is able to increase renal blood flow (RBF) inducing dose-dependent renal vasodilation in patients with AKI or at a high risk of AKI, although not available in Spain. In a recent meta-analysis it has been suggested that its administration may reduce post-operative AKI,22 slow progression to dialysis-dependent AKI and improve survival.
DiureticsDiuretics are widely used in critical patients when diuresis decreases, in an attempt to prevent the development of AKI, however, the diuretic response to furosemide is only one marker of the grade of residual renal function and less severe kidney failure.23 Loop diuretics have the property of reducing renal oxygen consumption as they reduce the active transport of sodium, reducing the energetic requirements of tubular cells and theoretically protecting them from ischemia, but they can have deleterious effects as they increase urine output fostering a depletion in volume, electrolyte imbalances, nephrotoxicity and ototoxicity, thus perpetuating AKI.
The use of mannitol, although able to protect the kidney, decreasing tissue edema, increasing tubular flow and decreasing intra-tubular obstruction, is also not justified as a preventive measure. Its use has been trialed in kidney transplant surgery24 and in rhabdomyolysis25 without providing conclusive results. Therefore, in light of the available evidence, it is recommended not to use diuretics for preventing AKI, except in the management of water overload.26 Diuretics should be administered over a short period and should never lead to a delay in starting continuous renal replacement therapy (CRRT).
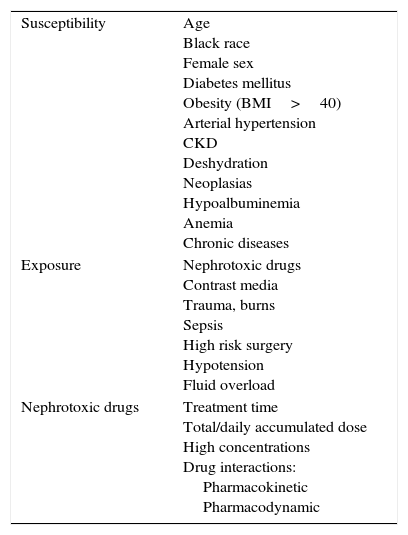
Specific measuresNephrotoxicity by drugsNephrotoxic medication is the third cause of AKI in the ICU,3 due to the high number of prescribed drugs, the potential for adverse drug reactions and the actual nephrotoxicity of the drug. There are increases in risk due to the interrelation of these drugs with factors of susceptibility and exposure (Table 2) and because of its inadequate prescription.27,28 Prognosis and evolution to CKD is usually the same as in all the causes of AKI.28
Nephrotoxic drugs, factors of susceptibility and exposure.
| Susceptibility | Age Black race Female sex Diabetes mellitus Obesity (BMI>40) Arterial hypertension CKD Deshydration Neoplasias Hypoalbuminemia Anemia Chronic diseases |
| Exposure | Nephrotoxic drugs Contrast media Trauma, burns Sepsis High risk surgery Hypotension Fluid overload |
| Nephrotoxic drugs | Treatment time Total/daily accumulated dose High concentrations Drug interactions: Pharmacokinetic Pharmacodynamic |
BDI: body mass index; CKD: chronic kidney disease.
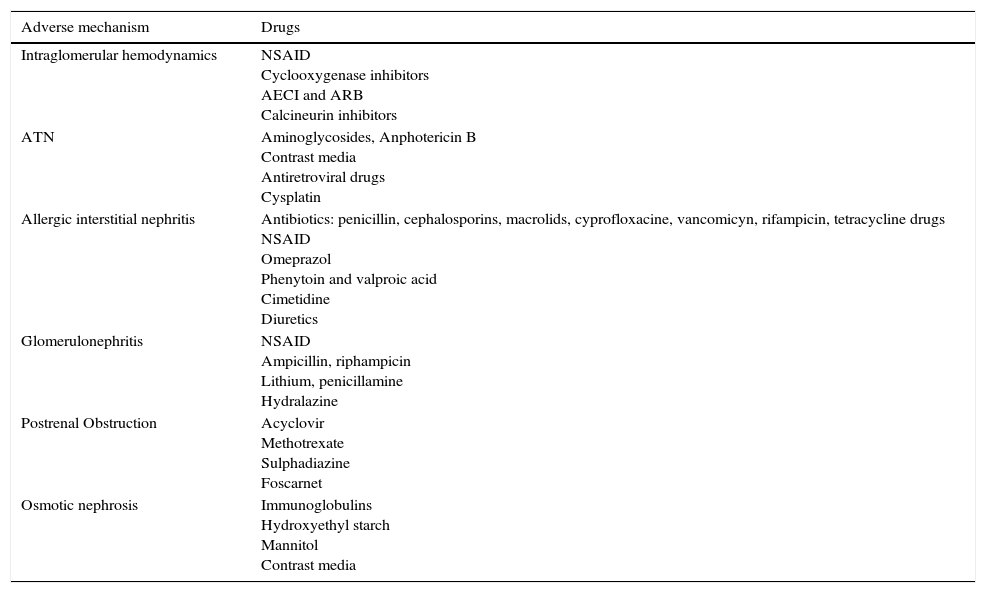
The following are dependent modifiable risk factors: Duration of treatment, daily/total accumulated dose and pharmacodynamic and pharmakinetic interactions. The associations of drugs also constitute a frequent risk,29 such as triple therapy drugs: non-steroid antiinflammatory drugs (NSAIDs)+angiotensin-converting-enzyme (ACE) inhibitors–angiotensin receptor blockers (ARBs)+Diuretics. The toxicity mechanisms are complex, in many cases affecting more than one aspect of kidney function.30 They can be classified according to their kidney injury mechanism, as well as to their histopathological findings (Table 3), many of them interacting on different levels.
Mechanism of kidney injury from nephrotoxic drugs.
| Adverse mechanism | Drugs |
|---|---|
| Intraglomerular hemodynamics | NSAID Cyclooxygenase inhibitors AECI and ARB Calcineurin inhibitors |
| ATN | Aminoglycosides, Anphotericin B Contrast media Antiretroviral drugs Cysplatin |
| Allergic interstitial nephritis | Antibiotics: penicillin, cephalosporins, macrolids, cyprofloxacine, vancomicyn, rifampicin, tetracycline drugs NSAID Omeprazol Phenytoin and valproic acid Cimetidine Diuretics |
| Glomerulonephritis | NSAID Ampicillin, riphampicin Lithium, penicillamine Hydralazine |
| Postrenal Obstruction | Acyclovir Methotrexate Sulphadiazine Foscarnet |
| Osmotic nephrosis | Immunoglobulins Hydroxyethyl starch Mannitol Contrast media |
NSAID: non-steroid anti-inflammatory drugs; AECI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; ATN: acute tubular necrosis.
Prevention is the best method for avoiding AKI, with the early identification of the patients at risk and controlling the potentially modifiable risk factors, including the non-prescription or interruption of nephrotoxic drugs whenever possible.26
Aminoglycoside antimicrobial agents are highly potent, bactericidal antibiotics, its toxicity is multifactorial and strengthened by the susceptibility to and exposure to other factors. The mechanisms involved in nephrotoxicity are31,32: 1) proximal tubular renal toxicity, causing apoptosis and necrosis; 2) mesangial contraction with decreased glomerular filtration rate, and 3) reduction of renal blood flow secondary to increased vascular resistance. Risk factors associated with the treatment include the length of it, trough levels and dosing interval. The pharmacokinetic and pharmacodynamic properties of aminoglycosides favor high dosing strategies with extended intervals between doses, so in patients with normal kidney function in a steady state, aminoglycosides should be administered as a single dose daily rather than multiple-dose daily treatment regimens.26
Therapeutic efficacy is determined by peak blood level divided by minimum inhibitory concentration (MIC) of the infecting organism (Cmax/MIC). The Cmax should be at least 10-fold greater than the MIC of the infecting microorganism, and the sample must be taken within 30min of administration. Nephrotoxicity is associated with high levels of residual concentration (trough level), more than 20h after being administered, hence, the importance of the determination of peak/trough concentration for titration and dosing interval. It is recommended monitoring aminoglycoside drug levels when treatment with single-daily dosing is used for more than 48h or if multiple daily dosing is used for more than 24h.26
Therefore, preventive strategies are extended-interval dosing, limiting duration of therapy, monitoring serum drug levels and renal function, and manintaining trough levels, measured between 18 and 24h post-dose, at 1μg/ml or less.
Amphotericin B deoxycholate is also an important nephrotoxic agent, with an AKI incidence which can reach 80%. The nephrotoxicity mechanism is multifactorial and is associated with total accumulated dose. Prevention is based on adequate hydroelectrolytic replacement and the administration of lipid-based formulations33,34: liposomal form of Amphotericin B and Amphotericin B Lipid Complex. Lipid-based formulations are less nephrotoxic as observed in several clinical studies and meta-analysis, and some clinical studies suggest that liposomal form of Amphotericin B is less nephrotoxic than the other lipid-based formulations.34
With the increase of infections produced by beta-lactam-resistant Gram-positive bacteria, other potential nephrotoxic antibiotics such as Vancomycin are being used increasingly in the ICU. Its effectiveness is determined by the relationship AUC/MIC≥400, being trough levels 15–20mg/L the clinical substitute for that relationship with MIC≤1mg/L multi-resistant bacteria, in case of MIC>1mg/L an alternative agent should be considered.35
Vancomycin-induced nephrotoxicity is based on their oxidative effects on cells of the proximal renal tubule, which produces tubular renal ischemia. It is related to trough levels ≥15mg/L, duration of therapy, renal dysfunction and previous use of other nephrotoxic drugs. Hence the importance of strict monitoring of levels and dose adjustment, with the possibility of administering a continuous intravenous infusion35,36 as a protective factor of nephrotoxicity.
Colistin (Colistemetato Sodium) is another nephrotoxic drug whose use has increased in ICU due to the increase in multidrug-resistant Gram negative bacteria. Nephrotoxicity occurs due to the alteration of the tubular cell permeability and cell lysis. Oxidative and inflammatory pathways are also implicated. Risk factors are the cumulative dose administered, treatment time, concomitant use of other nephrotoxic and renal dysfunction with CrCl <60ml/min. Dose is, according to current recommendations, carried out according to its Pk/Pd properties, with ascorbic acid being a possible independent protective factor administered at a twice-daily dose of 3 (2–4)g.37,38
AKI due to intra-tubular depositsRhabdomyolysis is characterized by the destruction of skeletal muscle with the subsequent release of intracellular and enzymatic content into the bloodstream, leading to systemic complications, with the most common being AKI.39 It has an incidence of between 10 and 55% and is associated with a poor prognosis when it is part of multiorgan dysfunction syndrome. Apart from genetic predisposition,40 risk groups have been identified such as the morbid obese, patients with chronic hypolipemiant drug consumption, patients in a postoperative stage in some kinds of surgery, etc. Postoperative rhabdomyolysis has increased in recent years, with risk factors related to surgical time, immobility, anesthetic drugs and comorbidities such as obesity and diabetes.41 There are many AKI mechanisms including: hypovolemia, myoglobinuria and metabolic acidosis.
The treatment of the underlying etiology is the first measure to be taken and intensive fluid replacement is the cornerstone of the treatment.42 Electrolytic corrections are fundamental, with hyperkalemia being the only one requiring rapid correction due to the risk of cardiac arrythmia. The use of bicarbonate is based on the concept that an acid setting promotes myoglobin toxicity; therefore, alkaline urine (a pH greater than 6.5), could prevent AKI. There is no consensus about the use of mannitol given that its secondary effects include volume depletion and potential prerenal azotemia. However, the theoretical benefits include an improvement in diuresis, an increase in renal perfusion, myoglobin excretion, and a direct antioxidant effect on the renal parenchyma.25 The CRRT filters the myoglobin in the blood and normalizes creatinine and electrolyte levels, although the mortality rate is unchanged. Therefore, it should only be used when the hydroelectrolytic alterations are life threatening.43
Other forms of AKI associated with intra-tubular deposits that we can observe in critical patients include tumor lysis syndrome and intravenous treatment with Acyclovir, sulfonamides, methotrexate, indinavir and cysplatin. Most of these patients are predisposed to having the following risk factors: volume depletion and the presence of CKD, with volume replacement being the most efficient preventive measure. In the case of the administration of cysplatin, at least 3l of saline solution should be replaced 8h before and after its administration together with a chelating agent such as aminophostine. In order to prevent methotrexate precipitation it is recommended to administer saline solution with urinary alkalinization (pH>6.5), and in the case of tumoral lysis syndrome, prevention is carried out by expanding the volume to maintain an adequate urinary flow associated with the use of uricolytic agents like rasburicase.25
Contrast-associated acute kidney injuryDue to the increase in the use of contrast agents in the diagnosis and in interventionist processes, CA-AKI has become the third most common cause of hospital-acquired kidney failure.2 The broadest definition used is an increase in serum creatinine ≥0.5mg/dl or ≥25% of the baseline level, 48–72h after exploration with contrast, in the absence of any alternative etiology. In the latest study carried out on critical patients, an incidence level of 16.3% has been found.44 This incidence level is higher than that detected in Spanish ICUs, according to the results of the NEFROCON study,45 where an overall incidence of 12.1% was detected. We also found significant differences among coronary patients, with an incidence of 8.2%, and the rest of critical patients, with a higher incidence, of 15.3%.
In spite of the clinical importance of this entity, its pathogenesis is still unclear, and it seems to be multifactorial, including rheological alterations, renal hemodynamic changes, regional hypoxia, auto/paracrine factors (adenosine, endothelin, reactive oxygen species) even direct cytotoxic effects.46
Of the patients who developed CA-AKI, the most frequently identified risk factors were DM and CKD (GFR lower than 60ml/min/1.73m2). Other reported risk factors were HF, dehydration, hyponatremia, previous use of diuretics, nephrotoxic drugs, hypoalbuminemia, advanced age, female sex, anemia, administration of intra-arterial contrast and constrast dose.47 There is also an association between CA-AKI and indicators of hemodynamic instability, such as periprocedural hypotension and the use of intra-aortic balloon counterpulsation (IABC). The effect of the risk factors is additive, and the probability of CA-AKI increases considerably as the number of these increase.48 In critical patients,44 the variables associated with the development of CA-AKI were a higher level of serum creatinine, arterial hypotension, the administration of diuretics and vasoactive drugs. These data have also been confirmed in the study carried out on critical Spanish patients,45 where in addition, level of severity, estimated by APACHE II, and anemia grade are factors related to CA-AKI.
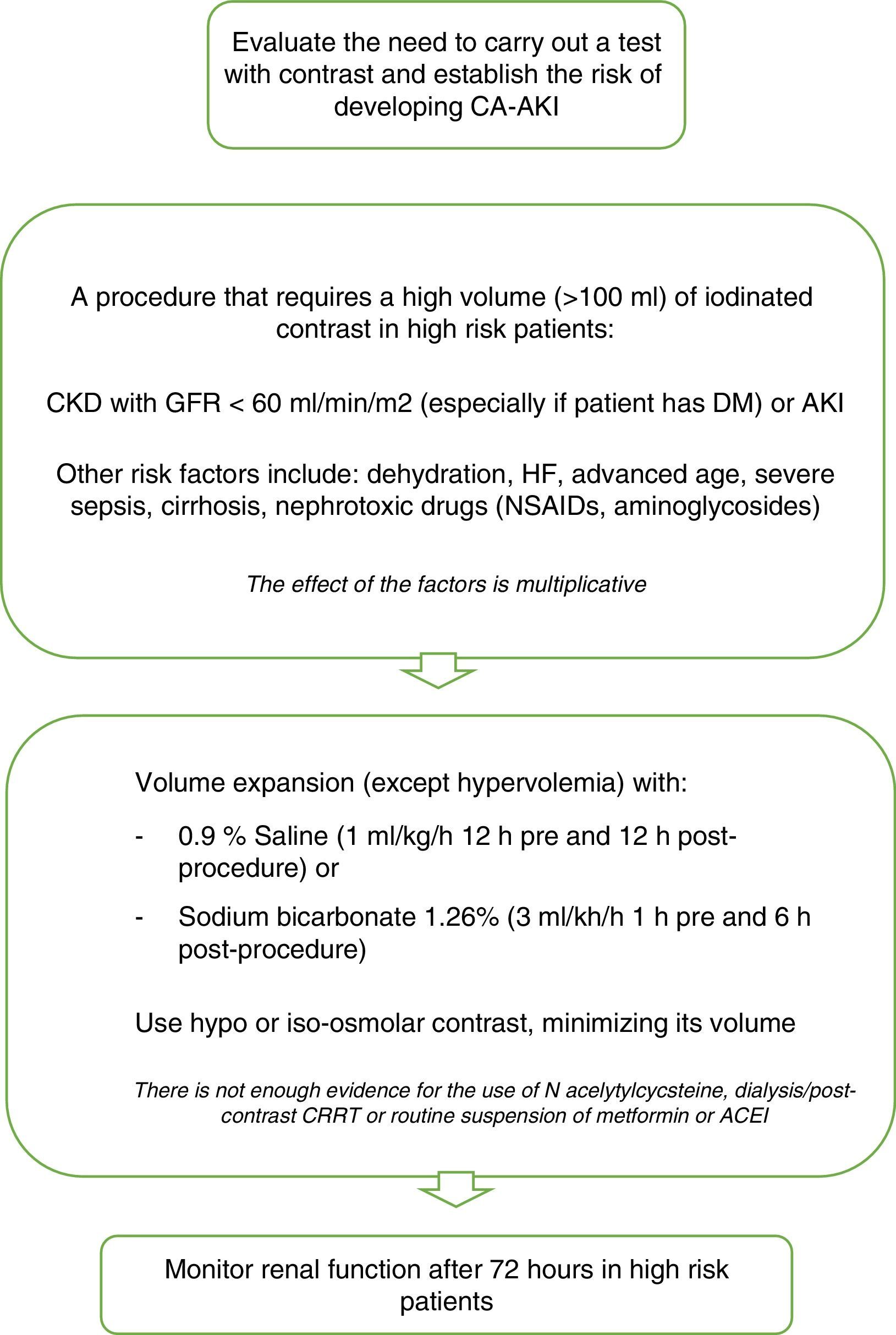
Contrast media can be classified as hypo, iso or hyperosmolar, according to their osmolarity. Several studies49 suggest that in patients with a deterioration in kidney function, hypo-osmolar (500–1000mOsm/kg) and iso-osmolar contrast (290–300mOsm/kg) are less nephrotoxic than hyperosmolar contrast media (1000–2000mOsm/kg). Based on current evidence, their use is recommended in high risk patients; both iso and hypo-osmolar contrast.26 Contrast volume is also an independent predictor of CA-AKI48; as a general rule it should not be more than two times the baseline GFR in milliliters.50
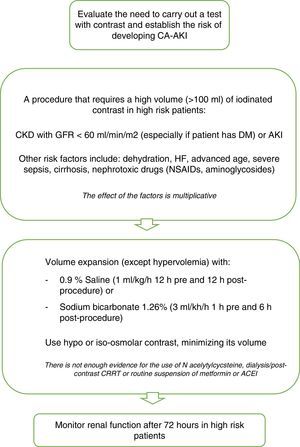
To prevent CA-AKI different kinds of preventive therapies have been trialed. The most adequate approach seems to be to identify patients at risk, administer appropriate peri-procedural hydration and to minimize the quantity of contrast administered51 (Fig. 1).
Recommendations for CA-AKI prevention. Modified from ref. 51. CA-AKI: contrast associated acute kidney injury; CKD: chronic kidney disease; GFR: glomerular filtration rate; DM: diabetes mellitus; HF: heart failure; NSAIDs: non steroidal antiinflammatory drugs; CRRT: continuous renal replacement therapy; AECI: angiotensin converting enzyme inhibitors.
The objective of hydration is to maintain enough intravascular volume in order to be able to increase renal perfusion, dilute the high concentrations of substances in the renal tubules, prevent prolonged contact, establish adequate diuresis prior to the administration of contrast and to prevent hypotension.52 Volume expansion should be intravenous, and one of the most recommended hydration regimes is the administration of 1ml/kg/h of 0.9% Saline 12h before and after the procedure in patients with normal left ventricular ejection fraction (LVEF). For patients with low LVEF (moderate or severe) recommended hydration includes volume replacement according to urinary output to maintain an euvolemic state for 12h pre and post-procedure.
Urinary alkalinization with sodium bicarbonate reduces renal damage by contrast by decreasing the quantity of free radicals dependent on pH, although there are many studies that have assessed its preventive capacity with contradictory results.53 In 2009 probably the most complete systematic review of controlled randomized clinical trials was published, analyzing 23 published and unpublished studies with significant heterogenicity between them, concluding that the effectiveness of treatment with bicarbonate sodium in high risk patients is unclear,54 so that currently it is only used in emergency situations, in which there is not enough time to begin adequate therapy with saline solution.
N-acetylcysteine (NAC) is a powerful antioxidant, evaluated in many studies and meta-analyses, that have been unable to demonstrate its preventive efficacy. Not even the largest randomized multicenter study,55 carried out in 2009, could demonstrate the beneficial effect of N-acetylcysteine for reducing the incidence of CA-AKI. This study included 2308 patients with at least one risk factor for its development (>70 years, CKD, DM, CHF, LVEF ≤45% or shock). The patients were randomized to receive oral NAC: 1200mg twice a day, with two doses before the procedure and two doses after, or a placebo. In both groups similar rates of increase in plasma creatinine were found after 48–96h. Hoffman et al.,56 assessed the efficacy of NAC in 50 healthy volunteers with normal kidney function, finding a small but significant decrease in creatinine and urea in those patients who received it, as well as an increase in glomerular filtrate without any changes in the levels of cystatin C. This is due to its capacity to interfere with the metabolism of the creatinine, bringing into question its efficacy for preventing CA-AKI.
It has also not been possible to demonstrate the protective effect of dopamine, fenoldopam or theophylline57,58 on kidney function and so its use is not currently recommended.
Owing to the capacity of dialysis to eliminate iodinated contrast from the blood flow, several studies have been carried out to assess its role as a potential preventive measure. Nevertheless, it has not been possible to demonstrate a fall in the incidence of CA-AKI. What is more, in a meta-analysis carried out on several studies in which periprocedural extracorporeal techniques are performed it was not possible to demonstrate a beneficial effect either.59
Recently, there is controversy about the toxicity of modern low- or iso-osmolality iodinated contrast material as well as the role of nephroprotection.60,61 The methodology of the studies conducted are being questioned, claiming that the studies included in the meta-analysis are heterogeneous and nonrandomized, assuming a significant bias. Most studies lacked a control group, and in some including it, the incidence is similar in both groups. In addition to methodological issues, many authors suggest that the incidence of AKI in ICU is multifactorial, and the patient subjected to radiological contrast is also exposed to many other risk factors, being the CA-AKI indistinguishable from acute kidney injury that occurs for other reasons.
Perioperative AKIThe risk for its development is the interaction between renal susceptibility and the type and intensity of exposure to renal injury. The knowledge of these risk factors could help in its prevention, especially in the hospital setting, with the most important risks being advanced age and CKD.2,4 The patients who meet the creatinine and diuresis criteria have worse results than the nonoliguric patients. Along similar lines, the FINNAKI study has noted that severe episodes of oliguria are independently associated with the development of AKI.62 However, it should be taken into account that perioperative oliguria is, commonly, secondary to salt and water retention in response to tissue damage, pain and moderate degrees of hypovolemia and hypotension. Nearly 40% of AKI in hospitalized patients occurs in the perioperative period, and is mainly related to the specific surgical procedure.63 Heart surgery has the highest risk at RR: 1.22 (95% CI: 1.17–1.27), with major vascular surgery being another high risk surgical subgroup. These results reinforce the importance of stratification of perioperative risk and the implementation of preventive strategies. In addition, several predictive risk models of AKI have been developed principally in the field of heart surgery.
The main causes of AKI include ischemia, hypoxia, inflammation and nephrotoxicity. Other mechanisms are direct vascular injury or tubular obstruction. Therefore, it is not surprising that the simple restoration of circulating blood volume does not improve the results. Recently, the role of intraoperative hypotension has been related,64 finding an increase in risk when mean blood pressure was <60mmHg during >20min and <55mmHg during >10min. The perioperative administration of levosimendan to patients who have undergone heart surgery reduces the incidence of AKI, CRRT, mortality, mechanical ventilation time and stay in the ICU. Remote ischemic preconditioning (RIPC) has proven cardiac and kidney protection with a possible reduction in mortality when it is used with halogenated anesthetics. However, two recent studies65,66 suggest there is no difference between RIPC and standard treatment, probably due to the use of propofol as a hypnotic drug. Phenoldopam has not demonstrated its utility either, being associated with a higher rate of hypotension. Among the patients undergoing non heart-related major surgery, neither aspirin nor clonidine reduced the perioperative risk of AKI. In fact, aspirin increases the risk of a severe hemorrhage and clonidine does the same to hypotension.67
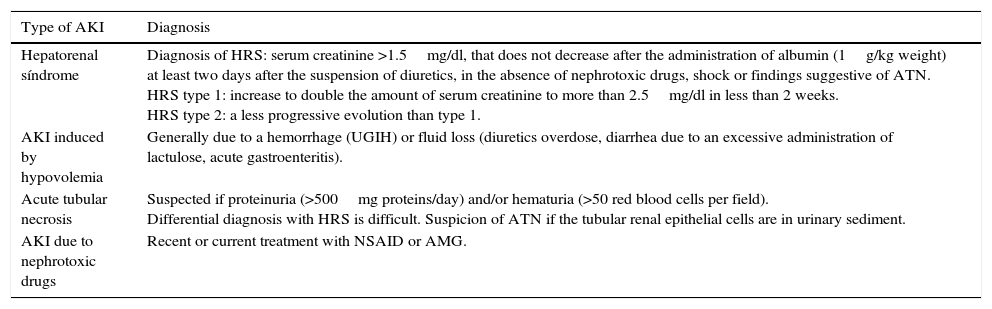
AKI in liver failureAKI is associated with a poor prognosis in cirrhotic patients. The most common causes leading to it are listed in Table 4.68 Splanchnic vasodilatation that is triggered by portal hypertension, seems to induce hemodynamic changes and a deterioration in kidney function due to an increase in the production and activity of vasodilators such as nitric oxide, a derivate of the endothelium, due to bacterial translocation.68 The following preventive measures should be taken: nephrotoxic drugs and NSAIDs should be avoided; early antiviral treatment should be carried out in patients with Hepatitis C virus who fulfill the therapeutic criteria and are in a stable condition; in hypovolemia or sepsis diuretics should be withdrawn; and in the case of hemorrhagic shock, hemoderivates should be administered, variceal ligation should be performed together with preventive treatment of bleeding using propranolol. Water overload should be prevented as this can aggravate hyponatremia and ascites. It should be evacuated with albumin replacement (8g albumin per liter of ascites evacuated). The appearance of Hepatorenal syndrome (HRS) after spontaneous bacterial peritonitis (SBP) can be efficiently prevented through the administration of albumin (1.5g/kg i.v. at the time of diagnosis and 1g/kg i.v. 48h later)68 associated with the administration of norfloxacin (400mg/day). The albumin mechanism is unknown but has beneficial effects for heart function as well as antioxidant properties.69
Main type of AKI in cirrhotic patients.
| Type of AKI | Diagnosis |
|---|---|
| Hepatorenal síndrome | Diagnosis of HRS: serum creatinine >1.5mg/dl, that does not decrease after the administration of albumin (1g/kg weight) at least two days after the suspension of diuretics, in the absence of nephrotoxic drugs, shock or findings suggestive of ATN. HRS type 1: increase to double the amount of serum creatinine to more than 2.5mg/dl in less than 2 weeks. HRS type 2: a less progressive evolution than type 1. |
| AKI induced by hypovolemia | Generally due to a hemorrhage (UGIH) or fluid loss (diuretics overdose, diarrhea due to an excessive administration of lactulose, acute gastroenteritis). |
| Acute tubular necrosis | Suspected if proteinuria (>500mg proteins/day) and/or hematuria (>50 red blood cells per field). Differential diagnosis with HRS is difficult. Suspicion of ATN if the tubular renal epithelial cells are in urinary sediment. |
| AKI due to nephrotoxic drugs | Recent or current treatment with NSAID or AMG. |
HRS: hepatorenal syndrome; ATN: acute tubular necrosis; UGIH: upper gastrointestinal hemorrhage; NSAID: non steroidal antiinflammatory drugs; AMG: aminoglycosides.
Modified from Ref. 66.
The early detection of the disease is the most important secondary prevention, allowing us to detect the disease at its earliest stage to be able to apply all the measures aimed at preventing its progression.
The monitoring of diuresis and creatinine is recommended for the identification of the initial stages of AKI, in accordance with the RIFLE, AKIN or AKI-KDIGO scales.26 In this way, the presence of at least one of the following conditions is required: an increase in creatinine of 0.3mg/dl above the baseline value in less than 48h; an increase in creatinine 1.5 times above the baseline value in less than 7 days; or diuresis of under 0.5ml/kg/h for 6h (urine volume 0.5ml/kg/h for 6h).
The frequency of this monitoring cannot be established generally for all patients. In heart patients undergoing percutaneous coronary interventionist bladder catheterization is not usually used, given that there is a general recommendation not to subject patients to unnecessary invasive procedures to prevent nosocomial infection. This same rule could be applied to non heart-related patients in which bladder catheterization is not considered necessary. Even so, the registration and measurement of spontaneous diuresis can always be used. The same occurs with readings of serum creatinine that could be less frequent in stable patients. In any case, the frequency used should allow for the detection of significant changes in the tendencies.
Once an AKI diagnosis has been established, the patient should be reassessed from an angle more focused on renal function51:
- 1.
History: antecedents, nephrotoxic agents, renal insults, etc.
- 2.
Renal evaluation:
- a.
Urine analysis (ions and sediment).
- b.
Ultrasound in those cases in which the cause of AKI has not been identified,51 will make it possible to rule out obstructive problems, collections, evaluation of renal flow and the ruling out of alterations suggestive of chronicity.
- c.
Estimation of creatinine clearance by measuring urinary creatinine over a time interval (2, 6, 12 or 24h).70 It will allow for the adequate adjustment of drugs and the assessment of evolution.
- a.
- 3.
Hemodynamic evaluation: preload, cardiac output, blood pressure, estimation of peripheral resistance, tissue perfusion, lactate and hemoglobin.
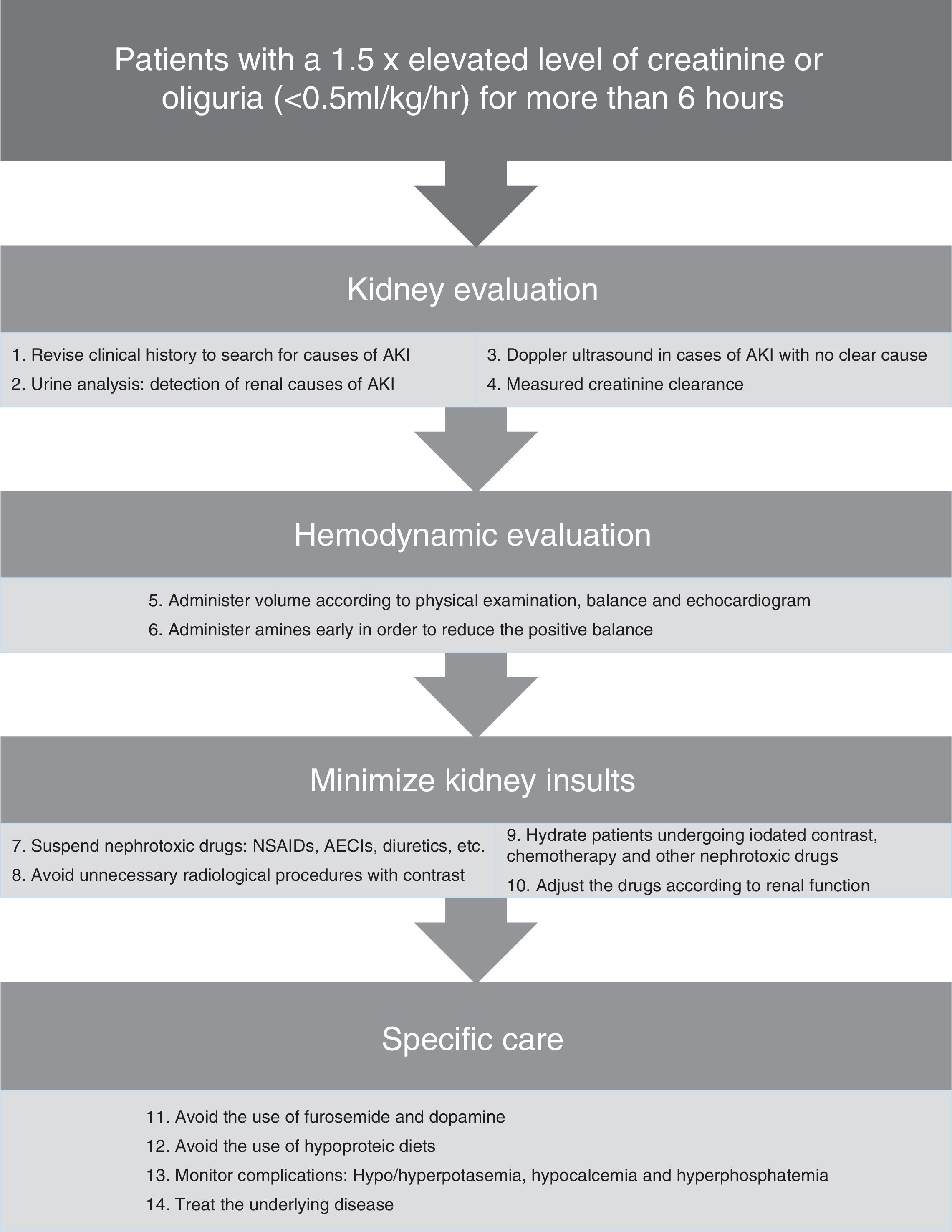
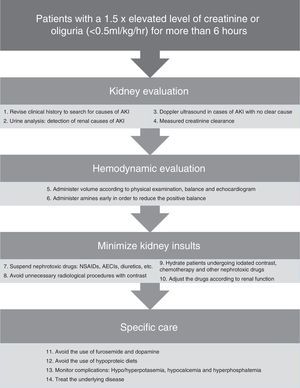
In Fig. 2 there is a summary of recommended measures to be taken in the case of AKI. Clinical evaluation from a renal standpoint, will allow us to take the right measures in each case, that will generally be:
- -
Volume expansion to optimize systolic volume and blood pressure (BP) in the case of shock. Bolus of 250–500ml can be administered until normalization of the preload. It should always be ensured that the patient has a low preload and that there is a favorable response to the volume administration.
- -
Early onset of amines71 may help to improve blood pressure in combination with volume expansion. Although it is controversial, early use of amines can reduce fluid overload and positive balance that may have a deleterious effect. The amine of choice is Norepinephrine.17,22
- -
Daily monitoring of the water balance and measured rapid creatinine clearance (2 or 4h), as well as general monitoring of every critical patient.
- -
Daily monitoring of AKI-related complications such as hyperpotasemia, hypocalcemia and hyperphosphatemia. Hyperpotasemia could be managed using medical measures in most cases. Hypocalcemia should be treated with supplements. Hyperphosphatemia in patients without symptoms can be treated using phosphoric chelates.
- -
Consistently high uremia values can produce changes in platelet aggregation and increase the bledding complications, that can partially improve with the administration of desmopresin.72
- -
In nearly 25% of cases of sepsis-induced AKI the nephrotoxic drugs can contribute to renal deterioration27 and must be discontinued, if not done previously. Only those considered as essential should be continued when they cannot be replaced by another equivalent and with a prior dose adjustment according to the patient's renal function.
- -
Adjustment of the drugs according to renal function because of levels or estimated levels of renal function using the creatinine clearance measurement in urine over short periods of time.
- -
Avoiding the use of furosemide as it can worsen the evolution of AKI, hinder the daily assessment of renal function and delay the start of dialytic techniques. When its use is considered necessary to increase diuresis because of volume overload, and it is considered that it could add a prerenal component, increases in the serum urea value can be used to guide the treatment and interrupt it if necessary.
- -
Avoiding the use of low-dose dopamine; it does not improve the evolution of AKI,21 and it can worsen renal blood flow and produce a higher frequency of supraventricular tachiarrythmias.
- -
Providing adequate nutrition; the basic requirements are usually similar to those of a critical patient according to his or her catabolic state. The protein needs are usually 1–1.5g/kg/day.26 Classic conceptions of hypoproteic diets should be avoided to prevent increases in urea and to delay the start of dialysis.
The majority of the episodes of AKI in patients admitted to the ICU are secondary to another disease that causes renal affectation. The treatment of the cause of admission or the complication that has contributed to the development of AKI is crucial for preventing its progression. In the case of sepsis it is of vital importance to control the focus of the disease using an early start of antibiotics and/or surgical treatment when appropriate.
Funding sourceNone.
Conflict of interestsThe authors declare no conflict of interest.